Abstract
Aims
To explore the relationship between vitamin D and obesity and abdominal obesity in women with infertility.
Material and methods
We screened the data from National Health and Nutrition Examination Survey (NHANES) 2013–2016. A total of 201 infertile women between the ages of 20 and 40 years were included in our study. To estimate the independent association of vitamin D with obesity and abdominal obesity, we used weighted multivariate logistic regression models and cubic spline analyses.
Results
Among infertile women in the NHANES 2013–2016 database, serum vitamin D levels were significantly and negatively associated with body mass index (ß= −0.96, 95% CI: −1.40, −0.51, p < 0.001)and waist circumference (ß= −0.40, 95% CI: −0.59, −0.22, p < 0.001), respectively. After multivariable adjustment, lower vitamin D levels were found to be associated with a higher prevalence of obesity (OR: 8.290, 95% CI: 2.451–28.039, p for trend = 0.001) and abdominal obesity (OR: 4.820, 95%CI: 1.351–17.194, p for trend =0.037). Spline regression showed linearity of the associations between vitamin D and obesity/abdominal obesity (p for nonlinearity > 0.05).
Conclusion
Our findings suggested that a decreased vitamin D might correspond to a higher prevalence of obesity in infertile women, which reminded us to pay more attention to the supplement of vitamin D in obese infertile women.
Introduction
There is no doubt that obesity and infertility are two major global public health epidemics, and both incidence rates are on an upward trend [Citation1–4]. Obesity now affects more than 600 million adults worldwide and more than 20 percent of American women of childbearing age [Citation5, Citation6]. Obesity and abdominal obesity contribute to a variety of chronic diseases [Citation7]. Meanwhile, obesity and abdominal obesity have multifaceted negative effects on reproductive potential, ranging from menstrual irregularity, endometrial pathology, oocyte, embryo, to infertility [Citation8–10]. Infertility is defined as the inability to conceive after 12 months of unprotected sexual involvement. About 15 percent of the world’s population suffers from infertility [Citation11]. In the United States, approximately 12.7 percent of women of childbearing age seek fertility treatment each year [Citation12]. To reduce the impact of obesity on infertility, weight loss is the first-line treatment for infertile patients.
Fat-soluble vitamin D, a steroid hormone, synthesized primarily by the skin under sunlight, is essential for the maintenance of a normal homeostasis of calcium and phosphorus and the development of bone tissue [Citation13]. Vitamin D insufficiency or deficiency is a worldwide female health problem along with obesity and infertility [Citation14]. It is known that approximately one billion people worldwide have suboptimal levels of vitamin D [Citation15]. Both observational and interventional studies have demonstrated that infertile patients with vitamin D deficiency have lower live birth rates, and vitamin D supplementation is more recommended for PCOS patients [Citation16–18].
However, few studies have determined the association between serum vitamin D levels and obesity/abdominal obesity in female infertility populations. It is also unclear whether there is a linear relationship between serum vitamin D levels and obesity/abdominal obesity. Hence, the purpose of the study was to examine the relationship between serum vitamin D levels and obesity/abdominal obesity using the data from the National Health and Nutrition Examination Survey (NHANES) (2013–2016) of the United States to determine whether there may be a link between these parameters among infertile women.
Materials and methods
Data source and study population
Our study data was from two continuous cycles of a large-scale cross-sectional study called the National Health and Nutrition Examination Survey (NHANES) (2013–2014 and 2015–2016). NHANES is a national cross-sectional study to assess the health and nutritional status of the non-institutionalized U.S. civilian population conducted by the U.S. Centers for Disease Control and Prevention (CDC) (all details of the sampling design and data collection can be obtained through NHANES official website: https://www.cdc.gov/nchs/nhanes/index.htm). The NHANES survey was mainly conducted by questionnaires, physical examinations, and laboratory tests, and was approved by the institutional review board of the National Center of Health Statistics (NCHS). The survey data is released every two years. All participants provided written informed consent.
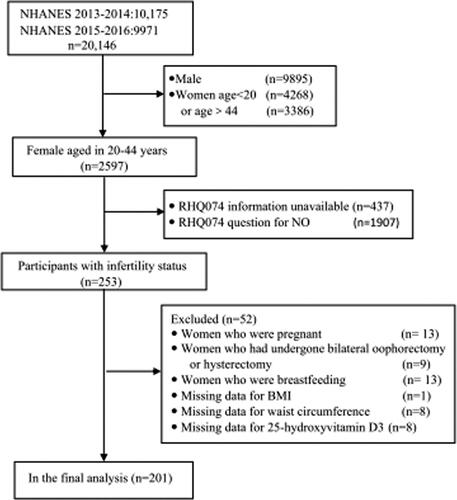
In total, 20,146 people participated in the NHANES 2013–2016. Of these, participants with the following characteristics were excluded from the analysis: (1) males (n = 9895) (2) women aged <20 years (n = 4268) or aged > 44 (n = 3386);(3) those with missing information about fertility(n = 437) and fertile women(n = 1907); (4) women who were pregnant(n = 13), breastfeeding (n = 13), had undergone bilateral oophorectomy or hysterectomy(n = 9); (5) those with missing body mass index (BMI) values (n = 1), waist circumference(n = 8) and 25-hydroxyvitamin D3 (n = 8). Finally, 201 participants were included in our study, and the flow chart was shown in .
Main outcomes
Infertility was defined as those who answered the reproductive health questionnaire question RHQ074 ‘Have you ever tried to conceive for more than a year without becoming pregnant?’ and replied ‘Yes’. Any woman who answered ‘No’ was considered fertile.
Measurement of serum 25(OH)D
In the NHANES 2013–2016, serum 25(OH)D concentrations were measured by ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method. The lower limit of detection (LLOD in nmol/L) for 25-hydroxyvitamin D3 was 2.23 nmol/L, details of which have been documented elsewhere (CDC/National Center for Health Statistics. Analytical Note for 25-Hydroxyvitamin D in Serum NHANES 2013–2014. Accessed 09 February 2010. Available from https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/VID_H_MET.pdf.
Covariates
Firstly, demographic variables were obtained from demographic data in the NHANES database, including age (20–44 years), race/ethnicity, marital status, education level, and the ratio of family income to poverty (PIR). The ages of the participants were divided into three categories with 10-year intervals (20–30, 31–40 and >40 years). The poverty income ratio for the not poor was defined as ≥ 1, and for the poor was defined as < 1.
Secondly, body mass index (BMI), waist circumference, and blood pressure data were extracted directly from examination data. BMI was classified as non-obesity (<30), and obesity (≥30) based on CDC guidelines. Abdominal obesity in women was defined as a waist circumference of > 88 cm [Citation19]. Three or four consecutive blood pressure levels were obtained for every participant. The mean of all available measurements was used to define the systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels. Hypertension was defined as an SBP level greater than or equal to 140, and a DBP level greater than or equal to 90 mmHg [Citation20, Citation21].
Finally, several interesting questionnaire variables were also collected. Sleeping time, PHQ-9 scores, smoking and drinking status, and comorbidities (including diabetes, hypertension, and hyperlipidemia) were obtained from questionnaire data. The PHQ-9 (Patient Health Questionnaire) is a screening tool with 9 questions (each with a score of 0–3, and total scores range from 0 to 27) to assess the frequency of depressive symptoms in the past two weeks.
Statistical analysis
Continuous variables were expressed as mean ± standard error (weighted) and categorical variables were presented as observed frequencies and weighted percentages. Firstly, the baseline characteristics of the participants were simply described statistically. Secondly, weighted univariate and multiple linear regression analyses were used to analyze the relationship between vitamin D and study variables and to identify the underlying factors affecting vitamin D. Thirdly, to analyze the association between vitamin D and obesity/abdominal obesity, the odds ratio (OR) and 95% confidence interval (CI) were calculated using multivariate logistic regression analysis. Three covariate models were evaluated, as follows: no variables were adjusted in Model 1. Age (20–30, 31–40 and >40 years) as a categorical variable was adjusted in Model 2. All the variables were adjusted in Model 3, and the trend in the vitamin D quartiles was evaluated simultaneously. Finally, a spline regression was ultimately used to assess whether there was a linear relationship between vitamin D and obesity/abdominal obesity. Statistical analyses were performed with STATA version 17.0 (Stata Corp LP, College Station, TX, USA) and R version 4.0.5. A p-value < 0.05 (two-tailed) was defined as statistically significant.
Results
General characteristics of the study population
The demographic data, anthropometric data, and disease history of the study population were briefly summarized in . A total of 201 women were eventually included in the study, with an average age of 35.34 ± 0.54 years old, and 30–40 years old accounted for the largest proportion (48.90%). Approximately 88% of the participants had an education level of high school or above. Most participants’ financial situation and sleep hours were good (not poor for 87.06%, mean sleep duration was 7.31 h). Notably, the mean score for PHQ-9 depressive symptoms was just 4.53. Approximately >50% of the participants were obese (BMI ≥ 25), and were abdominal obesity. The average serum vitamin D concentration was 61.05 nmol/L.
Table 1. Descriptions of study individuals’ characteristics.
Associations between vitamin D and study variables
Univariate linear regression analysis showed that age, race, education level, poverty income ratio, BMI, and waist circumference were significantly associated with serum vitamin D levels. Adjusted Multivariate analysis demonstrated that obesity (ß= −17.89, 95% CI: −30.69, −5.09) was significantly negatively associated with serum vitamin D levels ().
Table 2. Linear regression analysis between vitamin D and study variables.
Association between vitamin D and obesity/abdominal obesity
shows the OR value and correlation trends of serum vitamin D with obesity and abdominal obesity after logistic regression modeling. Among obese individuals, the prevalence of obesity was significantly higher in those with the lowest serum vitamin D quartile compared to those with the highest quartile of serum vitamin D in all models (Model 3, OR: 8.290, 95% CI: 2.451–28.039, p for trend = 0.001). A similar association was seen between vitamin D quartile and abdominal obesity. After adjusting for all covariates, we found a significantly higher prevalence of abdominal obesity in women with the lowest serum vitamin D quartile than in those with the highest (Model 3, OR: 4.820, 95%CI: 1.351–17.194, p for trend =0.037). Multivariable adjusted spline curves for relationships between vitamin D and the prevalence of obesity and abdominal obesity were shown in . Lower vitamin D levels were inversely linearly associated with the prevalence of obesity and abdominal obesity (all p for nonlinearity > 0.05).
Figure 2. Multivariable-adjusted spline curves for serum vitamin D levels about obesity (a) and abdominal obesity (b) as assessed by a spline regression model. (Solid lines—OR, dashed lines—95% CI) The model was adjusted for age (20–30, 31–40 and >40 years), race (Mexican American, Non-Hispanic white, Non-Hispanic Black and other race), education level (below high school and high school or above), and poverty income ratios (poor and not poor). P Nonlinear > 0.05 implies a significant linear relationship.
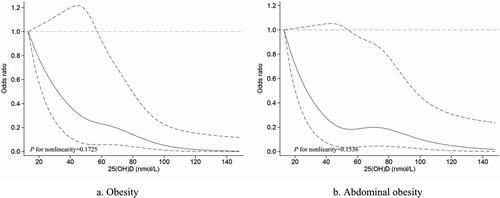
Table 3. Association between serum vitamin D quartiles and obesity/abdominal obesity.
Discussion
Our cross-sectional study aimed to investigate the association between vitamin D and obesity/abdominal obesity in infertile women of childbearing age, as obesity and vitamin D have important effects on human fertility. Our data were extracted from infertile women aged 20–44 years enrolled by NHANES in the United States between 2013 and 2016. After adjusting for other covariates, we found that serum vitamin D levels were inversely associated with BMI and waist circumference. After classifying patients as obese or abdominal obesity based on BMI and waist circumference, we observed that the prevalence of obesity/abdominal obesity was significantly higher in those with the lowest quartile serum vitamin D compared to those with the highest quartile of serum vitamin D in all models. To test whether this relationship was linear, we used multivariate-adjusted spline regression and found a negative linear relationship between vitamin D levels and obesity/abdominal obesity. Our study had similar findings to previous studies. Nutritional deficiencies and obesity in infertility patients were important factors affecting the quality of their fertility. Studying the relationship between vitamin D levels and obesity or abdominal obesity in infertility patients was of great value for their clinical guidance. We selected the NHANES database which included the general population rather than patients in hospitals or clinics, so the study population was representative to a certain extent.
Indeed, data from a bi-directional Mendelian randomization analysis of large cohorts support that obesity may contribute to vitamin D deficiency and vice versa. Specifically, a one-unit increase in BMI (1 kg/m2) was associated with a 1.15% reduction in 25(OH)D, after adjusting for age, sex, season, and other confounding factors [Citation22]. And the negative correlation was stronger in the North American study. However, this relationship has not been proven in infertile women. A prospective study aimed to evaluate the relationship between follicular 25-hydroxyvitamin D levels and body weight in 199 infertility patients who received intracytoplasmic sperm injections (ICSI). Their results showed that patients with lower follicular 25(OH)D3 levels had a greater average weight than those with higher 25(OH)D3 levels [Citation23].
Infertility is a widespread problem that has major implications for individuals, families, and society [Citation2, Citation24]. Modifiable lifestyle factors, such as overconsumption of food and vitamin deficiency can affect pregnancy. It has been well-established that obesity and vitamin D deficiency are two major pandemics undermining global public health [Citation25, Citation26]. Several studies have shown great interest in exploring the association between obesity and vitamin D deficiency and their underlying pathophysiological mechanisms [Citation27, Citation28]. We know that obesity and vitamin D were independently associated with infertility[Citation6, Citation9, Citation29–31]. However, beyond the independent effects of obesity and vitamin D deficiency on female infertility, there is currently a lack of robust evidence from interventional studies as to whether obesity and vitamin D deficiency have antagonistic or synergistic effects. Whether vitamin D deficiency is a consequence or a cause of obesity is a matter of debate. Our study demonstrated a negative linear association between vitamin D levels and obesity/abdominal obesity in infertile women. The possible mechanisms for the inverse relationship between vitamin D and obesity were as follows. On the one hand, when obesity is considered to be the cause of vitamin D deficiency, one possible explanation includes lower sunlight exposure due to a sedentary lifestyle, and decreased participation in outdoor activities [Citation32]. Another hypothesis is that vitamin D is a fat-soluble vitamin that is stored in large quantities in various body fat compartments of obesity, which leads to decreased serum vitamin D levels [Citation33]. Recent evidence suggests that obesity is associated with reduced expression of genes encoding the synthesis of important enzymes involved in vitamin D metabolism [Citation34]. On the other hand, when obesity is considered to be a consequence of vitamin D deficiency, one possible explanation includes vitamin D deficiency or vitamin D receptor (VDR) overexpression can inhibit preadipocyte differentiation, which is implicated in adipose tissue expansion and ultimately leads to obesity [Citation35]. Another hypothesis is that low vitamin D status may increase parathyroid hormone and calcium leading to weight gain and excess fat accumulation [Citation36].
Although the cause-and-effect relationship between vitamin D deficiency and obesity is controversial, evidence from meta-analyses has consistently shown a negative association between vitamin D and obesity [Citation27, Citation37]. Our study in an infertile population also demonstrated this point and further showed that the negative correlation between vitamin D levels and obesity/abdominal obesity was linear, namely the lower vitamin D levels, the greater the chance of obesity, which also suggests that when to treat such patients, doctors should pay special attention to emphasize the importance of weight loss as well as the vitamin D supplements. Certainly, more large sample of clinical and interventional studies are needed to focus on infertility patients either vitamin D supplementation in obese or weight loss in vitamin D-deficient. Considering the results of our study, it is crucial to develop public health education strategies for couples to raise public awareness of the impact of obesity and vitamin D deficiency. In addition, when treating infertility, it is equally important to establish guidelines for clinicians to effectively manage these issues.
There are several limitations to our study. NHANES is a cross-sectional study, and the relationship between vitamin D levels and obesity or abdominal obesity was investigated in infertile women, but not normal fertile controls. Therefore, data should be interpreted carefully, and the study needs to be validated considering these limitations. Secondly, the study designmaked it difficult to establish a definitive causal relationship between vitamin D and the risk of obesity/abdominal obesity. In addition, BMI and waist circumference values cannot be used to distinguish where and how much fat is stored in the body. Nevertheless, our evidence-based study highlights the importance of recognizing the inverse association between vitamin D and obesity/abdominal obesity in female infertility and is used as a reference for developing strategies for infertility treatment guidelines.
In conclusion, Obesity and abdominal obesity are associated with low vitamin D status and are inversely linear. The causal relationship between vitamin D and obesity/abdominal obesity is controversial. Future large-scale prospective observational or interventional studies are needed.
Author contributions
Jinyan Zhao and Shengyu Fu conducted analyses and wrote the draft of the article. Qing Chen conceived the study design. All authors contributed to the collection of clinical data, writing or revising of the manuscript, and approved the final version.
Acknowledgment
We appreciate the efforts of the US National Center for Health Statistics (NCHS) for the creation of the National Health and Nutrition Examination Survey Data and thank all the researchers for their efforts in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- James WPT. Obesity: a global public health challenge. Clin Chem. 2018;64(1):1–7.
- Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356.
- Sun H, Gong TT, Jiang YT, et al. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: results from a global burden of disease study, 2017. Aging. 2019;11(23):10952–10991.
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426.
- Mohammed MS, Sendra S, Lloret J, et al. Systems and WBANs for controlling obesity. J Healthc Eng. 2018;2018:1564748.
- Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. 2017;107(4):840–847.
- Schetz M, De Jong A, Deane AM, et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–769.
- Bond RT, Nachef A, Adam C, et al. Obesity and infertility: a metabolic assessment strategy to improve pregnancy rate. J Reprod Infertil. 2020;21(1):34–41.
- Silvestris E, de Pergola G, Rosania R, et al. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018;16(1):22.
- Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. 2019;158(3):R79–r90.
- Bala R, Singh V, Rajender S, et al. Environment, lifestyle, and female infertility. Reprod Sci. 2021;28(3):617–638.
- Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76.
- Giustina A, Adler RA, Binkley N, et al. Consensus statement from 2(nd) international conference on controversies in vitamin D. Rev Endocr Metab Disord. 2020;21(1):89–116.
- Ota K, Takahashi T, Han A, et al. Effects of MTHFR C677T polymorphism on vitamin D, homocysteine and natural killer cell cytotoxicity in women with recurrent pregnancy losses. Hum Reprod. 2020;35(6):1276–1287.
- Berry S, Seidler K, Neil J. Vitamin D deficiency and female infertility: a mechanism review examining the role of vitamin D in ovulatory dysfunction as a symptom of polycystic ovary syndrome. J Reprod Immunol. 2022;151:103633.
- Fang F, Ni K, Cai Y, et al. Effect of vitamin D supplementation on polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2017;26:53–60.
- Chu J, Gallos I, Tobias A, et al. Vitamin D and assisted reproductive treatment outcome: a systematic review and meta-analysis. Hum Reprod. 2018;33(1):65–80.
- Somigliana E, Sarais V, Reschini M, et al. Single oral dose of vitamin D(3) supplementation prior to in vitro fertilization and embryo transfer in normal weight women: the SUNDRO randomized controlled trial. Am J Obstet Gynecol. 2021;225(3):283.e1–283.e10.
- Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: united States, 2011-2014. NCHS Data Brief. 2015;(219):1–8.
- Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324(12):1190–1200.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572.
- Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10(2):e1001383.
- Deriquehem VA, Antunes RA, Reginatto MW, et al. Body weight and 25-hidroxyvitamin D follicular levels: a prospective study of women submitted to in vitro fertilization. JBRA Assist Reprod. 2016;20(3):127–131.
- Boivin J, Bunting L, Collins JA, et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–1512.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: united States, 2017-2018. NCHS Data Brief. 2020;(360):1–8.
- Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54.
- Karampela I, Sakelliou A, Vallianou N, et al. Vitamin D and obesity: current evidence and controversies. Curr Obes Rep. 2021;10(2):162–180.
- Vranić L, Mikolašević I, Milić S. Vitamin D deficiency: consequence or cause of obesity? Medicina (Kaunas). 2019;55(9):541.
- Voulgaris N, Papanastasiou L, Piaditis G, et al. Vitamin D and aspects of female fertility. Hormones (Athens). 2017;16(1):5–21.
- Cito G, Cocci A, Micelli E, et al. Vitamin D and male fertility: an updated review. World J Mens Health. 2020;38(2):164–177.
- Cozzolino M, Busnelli A, Pellegrini L, et al. How vitamin D level influences in vitro fertilization outcomes: results of a systematic review and meta-analysis. Fertil Steril. 2020;114(5):1014–1025.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693.
- Blum M, Dolnikowski G, Seyoum E, et al. Vitamin D(3) in fat tissue. Endocrine. 2008;33(1):90–94.
- Elkhwanky MS, Kummu O, Piltonen TT, et al. Obesity represses CYP2R1, the vitamin D 25-Hydroxylase, in the liver and extrahepatic tissues. JBMR Plus. 2020;4(11):e10397.
- Ruiz-Ojeda FJ, Anguita-Ruiz A, Leis R, et al. Genetic factors and molecular mechanisms of vitamin D and obesity relationship. Ann Nutr Metab. 2018;73(2):89–99.
- Soares MJ, Murhadi LL, Kurpad AV, et al. Mechanistic roles for calcium and vitamin D in the regulation of body weight. Obes Rev. 2012;13(7):592–605.
- Bosdou JK, Konstantinidou E, Anagnostis P, et al. Vitamin D and obesity: two interacting players in the field of infertility. Nutrients. 2019;11(7):1455.