Abstract
Objective
To compare the effects of progestin-primed ovarian stimulation (PPOS) protocol and GnRH-a long protocol in infertility patients with normal ovarian reserve function undergoing invitro fertilization and embryo transfer.
Methods
A retrospective cohort study was conducted to analyze the clinical data of 2013 cycles of patients with normal ovarian reserve function who underwent invitro fertilization/intracytoplasmic sperm injection-embryo transfer (IVF/ICSI-ET) in the Department of Human Reproductive Center, Renmin Hospital, Hubei University of Medicine from January 2018 and June 2020. The PPOS protocol group included 679 cycles and GnRH-along protocol group included 1334 cycles, the pregnancy outcomes were compared between the two groups.
Results
The duration of Gn used and total Gn used dosage in the PPOS protocol group were less than those in the GnRH-along protocol group (Duration of Gn used: 10.05 ± 1.48 vs 11.90 ± 1.85 d, p < 0.001; Total Gn used dosage: 1944.49 ± 533.61 vs 2661.34 ± 987.97 IU, p < 0.001); The LH levels were significantly higher on HCG trigger day in PPOS protocol compared to GnRH-a long protocol (2.8 ± 1 ± 1.07 vs 1.01 ± 0.62 IU/L, p < 0.001), the E2 levels on HCG trigger day in PPOS protocol group was lower than that in the GnRH-a long protocol group (2135.92 ± 1387.00 vs 2417.01 ± 1010.70 pg/mL, p < 0. 001). The number of oocytes retrieved in the PPOS protocol group was lower than that in the GnRH-along protocol group (8.03 ± 2.86 vs 9.47 ± 2.64, p < 0.001). No significant differences were found in pregnancy outcome including clinical pregnancy rate, early miscarriage rate and ectopic pregnancy rate between the two group (p > 0.05); In addition, no severe OHSS occurred in the PPOS protocol group during ovulation induction, while 11 patients of severe ovarian hyperstimulation syndrome (OHSS) occurred in GnRH-a long protocol group (p < 0.001).
Conclusion
The clinical efficacy of PPOS protocol combining embryo cryopreservation is comparable to that of GnRH-a long protocol in patients with normal ovarian reserve function, and the PPOS protocol is able to reduce the incidence of severe OHSS significantly.
Introduction
Controlled ovarian hyperstimulation (COH) is an important link in invitro fertilization-embryo transfer (IVF-ET). How to get the right number of high-quality embryos through appropriate ovulation induction protocol, to get an ideal pregnancy outcome and avoid adverse iatrogenic complications at the same time, this is a problem that every reproductive doctor has been paying attention to. Gonadotropin releasing hormone agonist (GnRH-a) long protocol can inhibit endogenous LH surge effectively, GnRH-a protocol has an advantage of promoting the synchronization of follicular development, improving oocyte quality, stabilizing pregnancy rate, and this protocol has been used as a classic ovulation induction protocol in patients with normal ovarian function. However, there are also some disadvantages in this protocol, such as long duration of Gn used, large Gn used dosage, high cost, and high incidence of OHSS [Citation1, Citation2].
Progestin-primed ovulation stimulation (PPOS) in the state of high progestogen can inhibit the premature endogenous LH surge well due to the negative feedback of combined estrogen and progestogen [Citation3]. In recent years, it has been applied more and more in ART. At present, it has been reported that the PPOS protocol could be applied to patients with poor ovarian reserve function, patients with normal ovarian reserve function and patients with PCOS, or as a choice after the poor results with IVF/ICSI-ET in the past [Citation4–7]. The purpose of this study is to compare the efficacy of GnRH-a long protocol and PPOS protocol in patients with normal ovarian reserve function.
Materials and methods
Research object
A retrospective cohort study was conducted to analyze the clinical data of 2013 patients with normal ovarian reserve function who were treated by in vitro fertilization/intracytoplasmic sperm injection-embryo transfer (IVF/ICSI-ET) in Department of Human Reproductive Center, Renmin Hospital, Hubei University of Medicine from January 2018 and June 2020. The PPOS protocol group included 679 cycles and GnRH-along protocol group included 1334 cycles. Inclusion criteria: All patients were in the first ovulation induction cycle, and all the patients had normal ovarian function: the basal serum FSH <10mIU/ml, the antral follicle count >5, and AMH > 1ng/ml. Exclusion criteria: patients with basic diseases, patients with high risk factors of recurrent abortion (such as genetic factors, immune abnormalities, etc.), abnormal uterine environment (previous uterine polyps, intrauterine adhesions, uterine malformations). This study was approved by the Ethics Committee of Renmin Hospital, Hubei University of Medicine and conducted in conformity with the Helsinki Declaration.
Ovulation induction protocol
GnRH-a long protocol group
Triptorelin3.75 mg (Germany Huiling Pharmaceutical Co., Ltd.) is injected of on the third day of menstruation, 30 days later, gonadotropins (rFSH, Gonafen, Merck Serono, or HMG, Livzon Medicine) will be used, and the dosage of Gn will be adjusted according to the follicular growth monitored by B-ultrasound and serum hormone level. When the average follicle diameter measured by ultrasound is more than 18 mm, 250 μg (r-hCG, Merck Serono) is injected. 36 h later, B-ultrasound guided transvaginal aspiration was performed.
PPOS protocol group
On the 3rd day of menstruation, oral medroxyprogesterone acetate tablets MPA10mg/d (2 mg/tablet, Xianju, Zhejiang Province) and 150 ∼ 300 IU/d gonadotropins (HMG, Livzon Medicine) for injection were given to promote ovulation. The dosage of HMG was adjusted according to the follicular growth monitored by B-ultrasound and serum hormone level. When the average follicle diameter measured by ultrasound is more than 18 mm, GnRH-a0.1 mg (dabijia, Huiling, Germany) combined with 2000 IU of chorionic gonadotropin (HCG, Livzon Medicine) is injected, 36 h later, B-ultrasound guided transvaginal aspiration was performed.
Embryo culture, luteal support and diagnosis of clinical pregnancy
The oocytes were cultured after conventional in vitro fertilization. 16 ∼ 18 h later the pronucleus was observed, appearance of two pronucleus was judged as normal fertilization. The embryo scoring method on the 3rd day after oocytes retrieval was referred to Ziebe, etc [Citation8]. Grade I ∼ II are high-quality embryos; Grade I ∼ III is usable embryo; Grade IV embryos are discarded embryos. From the day of oocytes retrieving, 90 mg/d of vaginal progesterone vaginal sustained-release gel (Merck Serono, Switzerland) and 20 mg/d of oral dydrogesterone (Solvay Pharma, Netherlands) were used for luteal support. All the embryo in PPOS group were frozen. After the second normal menstrual cycle, the endometrium was prepared by hormone replacement protocol, and Luteal support was performed as the same as that in fresh embryos transferring.
14 days after embryo transfer, the positive serum HCG was judged as biochemical pregnancy, 30 days after embryo transfer, the original fetal heart beat was detected by B-ultrasound, which was judged as clinical pregnancy, the luteal support medication was continued until 60 days after embryo transfer. Pregnancy loss occurring before 12 weeks of gestation is judged as early spontaneous abortion
Hormone detection
Collect serum samples from the 3rd day of menstrual cycle, the middle stage of ovulation promotion and the day of HCG trigger day, and using BECKMAN COULTERACCESS2 (USA) to detect the serum hormone level (anti-Mullerian hormone AMH, follicle-stimulating hormone FSH, luteinizing hormone LH, estradiol E2, progesterone P).
Observe the outcome measures
Main outcome measures: clinical pregnancy rate (number of pregnancy cycles/number of transplantation cycles ×100%), early spontaneous abortion rate (number of abortion cycles before 12 weeks/number of pregnancy cycles ×100%), live birth rate (number of births of live babies/number of total transplantation cycles ×100%).
Secondary outcome measures: ART cycle characteristics (The duration of Gn used, Total Gn used dosage, E2 on HCG trigger day) and embryologic outcomes (number of oocytes retrieved, 2PN fertilization rate (%), Number of embryos transferred, Number of high-quality embryos)
Statistical methods
Statistical analysis was performed by using SPSS 22.0 software (IBM, Armonk, NY, USA). Kolmogorov–Smirnov test was used to determine normality of parameters. The quantitative data were expressed as mean ± standard deviation (Mean ± SD), the means of two quantitative variables were compared using Student t-test and Mann–Whitney U-test based on the type of distribution. Categorical data are expressed as the rate (%), the chi-square test was used to compare differences between categorical variables. p-value<.05 was considered statistically significant.
Result
Patient characteristics (see for details):
Table 1. Comparison of baseline characteristics between the two groups.
A total of 2013 patients were included, including 1,334 cases in the GnRH-a long protocol group and 679 cases in the PPOS protocol group. The baseline characteristics of the patients are shown in , in all the total 2013 patients, there has an average age of 30.61 ± 2.67 years, an average AMH of 4.69 ± 3.37 ng/ml, an average basal FSH of 6.98 ± 2.14 IU/L and an average antral follicle count 8.20, all those show that the included patients have a normal ovarian reserve function. There have no differences in baseline characteristics of the two groups (p > 0.05).
Comparison of controlled ovarian hyperstimulation and embryologic outcomes (see for details):
Table 2. Comparison of controlled ovarian hyper stimulation and embryologic outcomes.
The duration of Gn used and total Gn used dosage in the PPOS protocol group were less than those in the GnRH-along protocol group (10.05 ± 1.48VS11.90 ± 1.85 d, p < 0.001 and 1944.49 ± 533.61VS2661.34 ± 987.97 IU, p < 0.001, respectively); The LH levels on HCG trigger day were significantly higher and E2 levels on HCG trigger day was significantly lower in PPOS protocol compared to GnRH-a long protocol (2.81 ± 1.07VS1.01 ± 0.62 IU/L, p < 0.001 and 2417.01 ± 1010.70VS2135.92 ± 1387.00 pg/ml, p < 0. 001, respectively). The number of oocytes retrieved in the PPOS protocol group was lower than that in the GnRH-along protocol group (8.03 ± 2.86VS9.47 ± 2.64, p < 0.001) (see ). There is no significant difference in 2PN fertilization rate (see ), number of high-quality embryos (see ) and number of embryos transferred between the two groups (p > 0.05).
Figure 1. Distribution of reproductive outcomes of PPOS protocol group and GnRH-a long group: (A) No. of oocytes retrieved; (B) 2PN fertilization rate; (C) High-quality embryo; (D) Clinical pregnancy rate; (E) Early abortion rate; (F) Severe OHSS rate.
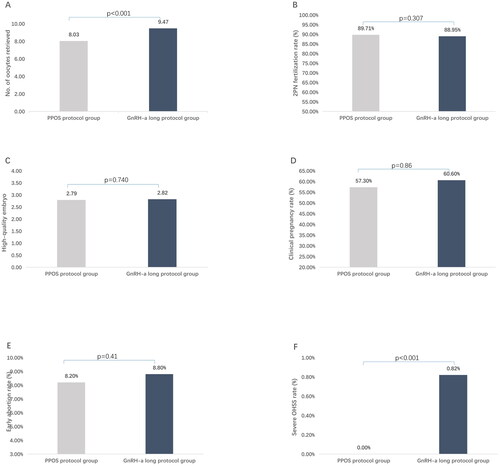
Comparison clinical outcomes (see for details):
Table 3. Comparison of clinical outcomes (per embryo transfer cycle).
No significant differences were found in pregnancy outcome including clinical pregnancy rate(see ), early abortion rate (see ) and ectopic pregnancy rate between the two group (p > 0.05); In addition, no severe OHSS occurred in the PPOS protocol group during ovulation induction, while 11 patients of severe ovarian hyperstimulation syndrome (OHSS) occurred in GnRH-a long protocol group (p < 0.001) (see ).
Discussion
The traditional GnRH protocol has been applied in assisted reproductive technology for a long time, but it may bring some disadvantages, such as high dosage of gonadotropin, high risk of OHSS [Citation1, Citation2]. In recent years, based on the development of embryo freezing technology, there are many new advances in ovulation induction protocol. Kuang et al. Studies have found that high progesterone status can inhibit the endogenous LH peak effectively and does not affect the growth of follicles, therefore, they first proposed the progestin primed ovarian stimulation (PPOS)and applied it to clinical practice in 2015 [Citation9]. Since then, the research on the application of the PPOS protocol has become more and more extensive. The mechanism of PPOS protocol was considered that the progesterone was used to pretreat the hypothalamus before the rise of estrogen, to block the positive feedback induced by estrogen, so as to inhibit the early endogenous LH peak and gain time for follicular development. At present, the PPOS protocol is reported to be suitable for many kinds of patients [Citation4–7].
In this study, patients with normal ovarian reserve function in the first cycle were included in this study. The assisted reproduction outcomes of PPOS protocol group and GnRH-a long protocol group were compared, the results showed that the total dosage of Gn used and duration of Gn used in PPOS group were lower than those of GnRH-a long protocol group (p < 0.05). The mechanism may be that the ovarian was over inhibited by down regulation of GnRH-a in GnRH-a long protocol [Citation10], whereas in PPOS protocol the high progesterone mainly acts on the hypothalamic progesterone receptor without interfering with the function of pituitary GnRH receptor, so the secretion of pituitary gonadotropin is not inhibited [Citation11], which leads to the lower dosage and duration of Gn used in COH in PPOS protocol group. On the other hand, the mechanism of inhibiting endogenous LH surge during ovulation induction is caused by progesterone in PPOS protocol is different from that of GnRH-a long protocol group [Citation12], therefore the change of serum LH level during COH is different from that of GnRH-a long protocol group, this is consistent with our research results: the LH level on the HCG trigger day of PPOS protocol group was higher than that of GnRH-a long protocol group (2.81 ± 1.07 vs 1.01 ± 0.62, p < 0.05). Many studies have shown that an appropriate amount of LH is beneficial to the follicular development and maturation during the process of COH, the reason is that an appropriate amount of LH can act on the granulose cells, it is conducive to maintaining ovarian response to ovulation induction drugs and improving embryo implantation potential on the premise of without inhibiting of pituitary gland excessively [Citation13, Citation14]. Ganor-Paz et al. reported that PPOS protocol can inhibit endogenous LH surge significantly, the fertilization rate, cleavage rate and high-quality embryo rate are not different from those of mini-stimulation protocol [Citation15]. This indicates that high progesterone level would not affect embryo quality and can inhibit endogenous LH surge effectively at the same time.
Our study showed that the number of oocytes retrieved in PPOS protocol group is less than that of GnRH-a long protocol group, however, there was no significant difference in the number of high-quality embryos, blastocysts and clinical pregnancy rate between the two group. Thus, we believe that the embryos obtained by PPOS protocol have the same developmental potential compared with GnRH-a long protocol group. Xi et al. compared the data of PPOS protocol and GnRH-a long protocol in a randomized control clinical trial, the results shown that there is no difference in the clinical pregnancy rate between the two protocols [Citation16], which is consistent with our research results.
In addition, in our study 11 patients in GnRH-a long protocol group developed severe OHSS, whereas no severe OHSS cases occurred in PPOS protocol group. This is in accordance with the previous research reports that PPOS protocol can reduce the incidence of OHSS significantly, especially for people with high response in COH [Citation17, Citation18]. The main reason is that all embryos are frozen after ovulation induction with PPOS protocol, and hormone-lowering therapy with drugs such as letrozole can be used after the oocyte retrieval in patients with a tendency of OHSS so as to avoid the occurrence of severe OHSS in PPOS protocol [Citation19, Citation20]. We think that the risk of OHSS could be lowered for GnRH-a long protocol as well as PPOS if the strategy of freezing all embryos is used in the near future.
To sum up, oral administration route of PPOS protocol is simple, convenient and the treatment costs less for patients in ART. PPOS protocol can reduce the total Gn use dosage and duration of Gn used, there was no significant difference in clinical pregnancy rate compared with GnRH-a long protocol, and the incidence of severe OHSS was significantly reduced in PPOS protocol. So, it is worthy of clinical application of PPOS protocol in patients with normal ovarian reserve function. However, as a new ovulation induction protocol, the influence of PPOS protocol on pregnancy-related factors and the safety of offspring still need more samples and clinical practice to prove.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Toftager M, Bogstad J, Bryndorf T, et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31(6):1–5.
- Jing M, Lin C, Zhu W, et al. Cost-effectiveness analysis of GnRH-agonist long-protocol and GnRH-antagonist protocol for in vitro fertilization. Sci Rep. 2020;10(1):8732.
- Chen Q. Editorial: Recent advances in progestin-primed ovarian stimulation. Front Endocrinol (Lausanne). 2022;13:1004352.
- Kao TC, Hsieh YC, Yang IJ, et al. Progestin-primed ovarian stimulation versus GnRH antagonist protocol in poor responders: Risk of premature LH surge and outcome of oocyte retrieval. J Formos Med Assoc. 2023;122(1):29–35.
- Chen YM, Qi QR, Xie QZ, et al. Effect of progestin-primed ovarian stimulation protocol on outcomes of aged infertile women who failed to get pregnant in the first IVF/ICSI cycle: a self-controlled study. Curr Med Sci. 2018;38(3):513–518.
- Liu A, Li J, Shen H, et al. Progestin-primed ovarian stimulation protocol with or without clomiphene citrate for poor ovarian responders: a retrospective cohort study. BMC Womens Health. 2022;22(1):527.
- Liu Y, Lin J, Yu S, et al. Letrozole cotreatment with progestin-primed ovarian stimulation in women with polycystic ovary syndrome undergoing IVF treatment. Front Physiol. 2022;13:965210.
- Ziebe S, Petersen K, Lindenberg S, et al. Embryo morphology or cleavage state: how to select the best embryos for transfer after in vitro fertilization. Hum Reprod. 1997;12(7):1545–1549.
- Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104(1):62–70.
- Fan Y, Sun YF, Xu YM, et al. Factors affecting clinical pregnancy in controlled ovarian hyperstimulation with GnRH-a long protocol: a retrospective cross-sectional study. J Obstet Gynaecol. 2022;42(6):2486–2491.
- Nathalie M. New stimulation protocols: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23(2):211–220.
- He W, Li X, Adekunbi D, et al. Hypothalamic effects of progesterone on regulation of the pulsatile and surge release of luteinizing hormone in female rats. Sci Rep. 2017;7(1):80–96.
- Drakakis P, Loutradis D, Kallianidis K, et al. Small doses of LH activity areneeded early in ovarian stimulationfor better quality oocytes in IVF-ET. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):77–80.
- Ruan M, Fang L, Yang S, et al. Prolonged pituitary downregulation with gonadotropin-releasing hormone agonist improves the live-birth rate: a retrospective cohort study comparing 3 different protocols. Ann Palliat Med. 2021;10(9):9984–9992.
- Ganor-Paz Y, Friedler-Mashiach Y, Ghetler Y, et al. What is the best treatment for women with polycystic ovarian syndrome and high LH/FSH ratio? A comparison among invitro fertilization with GnRH agonist, GnRH antagonist and in vitro maturation. J Endocrinol Invest. 2016;39(7):799–803.
- Xi Q, Tao Y, Qiu M, et al. Comparison between PPOS and GnRHa-Long protocol in clinical outcome with the first IVF/ICSI cycle: a randomized clinical trial. Clin Epidemiol. 2020;12:261–272. volume
- Huang CY, Chen GY, Shieh ML, et al. Validating the use of corifollitropin alfa in Progestin-Primed ovarian stimulation protocol on normal and high responders by comparing with conventional antagonist protocol: a retrospective study. Life (Basel). 2020;10(6):90.
- Cui L, Lin Y, Wang F, et al. Effectiveness of progesterone-primed ovarian stimulation in assisted reproductive technology: a systematic review and meta-analysis. Arch Gynecol Obstet. 2021;303(3):615–630.
- Zhao J, Xu B, Huang X, et al. Whether letrozole could reduce the incidence of early ovary hyperstimulation syndrome after assisted reproductive technology? A systematic review and meta-analysis. Reprod Health. 2020;17(1):181–181.
- Luo J, Qi Q, Chen Y, et al. Cocktail style treatment of GnRH antagonist, mifepristone and letrozole effectively prevent the progression of ovarian hyperstimulation syndrome in a rat model. Reprod Bio Med Online. 2021;42(2):291–300.
