Abstract
Purpose
This study explores the effects of endometrial thickness (EMT) before embryo transfer on newborn birth weight after in vitro fertilization–frozen embryo transfer (IVF–FET).
Methods
We collected the medical records related to singleton live births after IVF–FET from June 2015 to February 2019. Pregnant women were aged ≤ 42 years at delivery. Afterward, analyses were performed on outcomes related to newborns (birth weight, gestational age, delivery mode, percentage of newborns with low birth weight, and incidence of macrosomia) and pregnant women (pregnancy-induced hypertension, gestational diabetes mellitus, premature rupture of membranes, and placenta previa).
Results
The birth weight was higher in singleton newborns delivered by patients with EMT > 12 mm before embryo transfer than those delivered by patients with a thinner endometrium. The mean birth weight was 85.107 g higher in the EMT ≥ 12 mm group and 25.942 g higher in the 8–12 mm EMT group than in the EMT < 8 mm group. Independent predictors of newborn birth weight included pregnancy-induced hypertension, premature rupture of membranes, placenta previa, newborn sex, gestational age, delivery mode, number of implanted embryos, follicle-stimulating hormone levels, estradiol levels, and pre-pregnancy body mass index.
Conclusion
The weight of newborn singletons is associated with EMT before embryo transfer in patients undergoing the first FET cycle. Specifically, the birth weight is lower for newborns delivered by patients with a thinner endometrium. Accordingly, it is warranted to increase EMT before embryo transfer for improving neonatal outcomes after fertility treatment.
Introduction
The development of in vitro fertilization (IVF) technology has been a boon for numerous infertile patients, which contributes to increases in pregnancy and live-birth rates for these patients. Nevertheless, concerns are being raised over the safety of IVF because IVF is associated with an elevated risk of pregnancy and neonatal complications. For instance, Geyter et al. observed that women who conceived with assisted reproductive technology (ART) had a higher risk of premature delivery than naturally conceived women, accompanied by lower mean newborn birth weights. Moreover, their data also revealed that the birth weight was reduced markedly for newborns delivered < 37 weeks after embryo transfer [Citation1]. Additionally, mounting studies have reported that various types of ART alter fetal growth dynamics, which may affect the newborn birth weight [Citation2–4].
Reportedly, poor pregnancy outcomes after ART may be attributed to factors related to IVF (including superovulation, hormone supplements, and freezing technology) and the infertility characteristics of parents [Citation4]. Therefore, the effect of IVF on pregnant women and newborns is crucial to optimize the IVF strategy and provide more information on pregnancy and neonatal complications for patients with various characteristics during consultations.
Prior research indicated that endometrial thickness (EMT) might predict endometrial receptivity [Citation5]. Chan et al. found that the overall chance of pregnancy was adversely affected by EMT < 8 mm on the day that human chorionic gonadotropin (hCG) was detected in patients undergoing a fresh-embryo transfer cycle [Citation6]. The research by Yoeli et al. elucidated that EMT was directly proportional to the duration of follicular stimulation and inversely proportional to the age of women and that increased EMT (> 14 mm) was not associated with a reduced rate of implantation or pregnancy [Citation7]. In addition, another study unveiled that after embryo transfer, women with EMT > 12 mm were at a fourfold greater risk of placenta previa than those with EMT < 9 mm [Citation8, Citation9]. From these observations, there is controversy over the impact of EMT before embryo transfer on pregnancy outcomes and related obstetric complications.
Existing studies on the endometrium have focused on the effect of pre-transplant EMT on pregnancy and live-birth rates. For example, Zhang et al. noted that live-birth rates were associated with EMT on the day of oocyte retrieval in fresh-embryo transfer cycles, whereas this association was weaker in freeze–thaw cycles. Furthermore, their results also indicated that EMT > 8.75 mm on the day of oocyte retrieval was the cutoff value for predicting live births [Citation10]. Conversely, the effect of EMT on neonatal weight has been barely studied.
Our study primarily probes the effects of EMT before embryo transfer in freeze–thaw cycles on newborn birth weight and other factors during IVF affecting newborn birth weight, thereby providing a reference for optimizing the IVF strategy and improving neonatal outcomes.
Materials and methods
Ethical approval
Our study was performed in compliance with the Declaration of Helsinki, with written informed consent obtained from all participants for the use of their data in this study.
Exclusion criteria
The exclusion criteria of participants were as follows: (i) patients aged > 42 years at delivery; (ii) patients in the egg donation cycle; (iii) patients with uterine malformations; (iv) patients with uterine submucosal fibroids and endometrial polyps; (v) patients with chronic hypertension, that is, basal blood pressure > 140/90 mmHg before pregnancy or at < 20 weeks of pregnancy; (vi) patients with diabetes mellitus; (vii) newborns with congenital malformations.
Participants and study design
Our study was a retrospective cohort study. We collected the medical records of singleton live births after IVF–frozen-embryo transfer (FET) between June 2015 and February 2019 from Center of Reproductive Medicine, Children’s Hospital of Shanxi and Women Health Center of Shanxi. All patients were transplanted with day-3 embryos. Only the record of the first transfer was collected for patients undergoing multiple freeze–thaw embryo-transfer cycles to obtain a single live birth.
Superovulation and IVF
Routine ovarian stimulation was conducted according to the underlying diseases of patients and the clinical experience of attending gynecologists. Specifically, the short-acting gonadotrophin-releasing hormone agonist (GnRHa) was daily used by women from day 2 or 3 of menstruation until the day hCG was detected. Simultaneously, gonadotropin (Gn) was directly used to promote ovulation from day 3 of menstruation. Next, GnRHa (Cetrorelix [0.25 mg] or Ganirelix [0.25 mg]) was added, one injection (subcutaneous injection) per day, when Gn was used for 4–6 days or the diameter of leading follicles was ≥ 12–14 mm (as observed with ultrasound) until the day of hCG detection. Subsequent to oocyte retrieval, conventional IVF or intracytoplasmic sperm injection was utilized according to semen quality. After being routinely vitrified, cleavage-stage embryos cultured on day 3 were warmed with ready-to-use embryo thawing solutions (VT602 Thawing Media; Kitazato, Tokyo, Japan).
Protocols for endometrium preparation and embryo thawing
The first program was designed based on the natural cycle: the patient had regular menstruation and normal ovulation. First ultrasound monitoring was conducted on day 11 to day 13 of the menstrual cycle, when the follicle is ≥ 14 mm in diameter. Ovulation was induced with hCG, and the thawed cleavage-stage embryos were transferred 48 h after ovulation. The second program was designed based on the hormone-replacement cycle: menstruation was sparse or irregular, with obviously abnormal ovulation. Estrogen (3–10 mg per day) was used by women from day 2 to day 5 of the menstrual cycle. Subsequently, endometrial growth was examined with ultrasound. Then, the secretory transformation of the endometrium was triggered with progesterone, and the cleavage-stage embryo was thawed with VT602 Thawing Media (Kitazato) and transferred. Luteal support was conducted in the artificial cycle after transplantation.For an artificial cycle transplant: corpus luteum support, 90 mg of Crinone is given on the day of transplantation through the vagina (once a day) and 10 mg of Duphaston is taken orally twice a day. Duphaston is discontinued 28 days after transplantation, and Crinone is used until the 8th to 9th week of pregnancy. For a natural cycle transplant, corpus luteum support after transplantation, and 10 mg of Duphaston was taken orally on the day of transplantation (three times a day) until the 8th to 9th week of pregnancy.
EMT evaluation
EMT was measured with vaginal ultrasound. All ultrasound examinations were performed on identical ultrasound instruments by experienced sonographers based on a uniform standard (EMT was measured at approximately 1 cm from the uterine fundus). “EMT” was defined as the maximal distance between the junction of the endometrium and the myometrium in the sagittal plane of the uterus on both sides as detected with vaginal ultrasound. “Natural-cycle EMT” referred to EMT measured on the day ovulation was induced with hCG. EMT in the hormone-replacement cycle represented EMT measured on the day of progesterone level transformation. Based on the literature and our clinical experience, patients were classified into three groups according to EMT before embryo transfer: < 8, 8–12, and > 12 mm groups.
Main outcomes
The analyzed newborn-related outcomes included birth weight, percentage of newborns with low birth weight (number of low-birth-weight newborns/total number of newborns delivered at term; “low birth weight” was defined as birth weight below 2500 g at 37 weeks of gestation), and incidence of macrosomia (number of newborns with macrosomia/total number of newborns delivered at term; “macrosomia” referred to a fetal weight of > 4000 g at any gestational age).
The analyzed outcomes related to pregnant women were the number of days of pregnancy, percentage of pregnant women with preterm delivery (number of cases delivered at 28–37 weeks of gestation/total number of cases), percentage of pregnant women with extremely premature delivery (number of cases delivered at 28–32 weeks of gestation/total number of cases), and the incidence of gestational diabetes mellitus (GDM), pregnancy-induced hypertension (including gestational hypertension, preeclampsia, and eclampsia), placenta previa, and premature rupture of membranes.
Statistical analyses
Data were processed with SPSS 25.0 (IBM, Armonk, NY, USA). Categorical variables were expressed as frequencies (percentages). Differences among the three groups were analyzed with the chi-square test or Fisher’s exact test. The normal distribution of continuous variables was tested with the P–P plot. Continuous variables with normal distribution were summarized as mean ± standard deviation and compared among the three groups with one-way analysis of variance. A difference with p < 0.05 was considered statistically significant.
The variables related to newborn birth weight (dependent variables) were determined with univariate linear regression. The following independent variables were assessed, including age, body mass index (BMI), infertility type, infertility factors, number of years of infertility, endometrium preparation protocol, endocrine values, number of transferred embryos, number of implanted embryos, number of days of pregnancy, newborn sex, delivery mode, presence of GDM, presence of pregnancy-induced hypertension, presence of abnormal placental position, premature rupture of membranes, and EMT. All variables that were considered significant in the univariate analysis were utilized in the multivariate linear regression to establish a regression equation for predicting newborn birth weight. Dummy variables were set for all of the categorical variables. The results of regression analyses were presented as the regression coefficient of the variable and its P value.
Results
Patient characteristics at baseline and FET characteristics
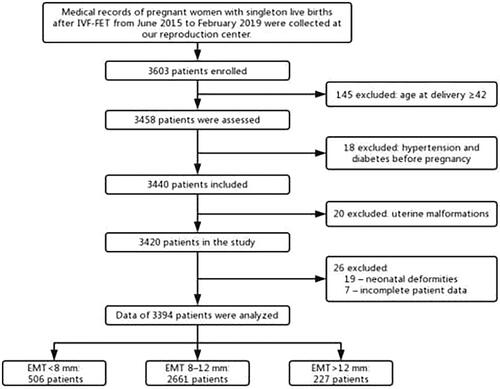
A total of 3394 eligible cases were included in our study with the use of exclusion and inclusion criteria, including 506 patients in the < 8 mm group, 2661 patients in the 8–12 mm group, and 227 patients in the > 12 mm group. The process of case screening is shown in .
Significant differences were found in age, BMI, infertility type, infertility factors, and testosterone levels among the three groups (p < 0.05). Differences were insignificant among the three groups in terms of number of years of infertility, endometrium preparation protocol, and levels of follicle-stimulating hormone (FSH), luteinizing hormone, prolactin, E2, and progesterone (p > 0.05). Likewise, the number of transferred or implanted embryos was not markedly different among the three groups (p > 0.05) ().
Table 1. Characteristics of patients at baseline and characteristics of frozen embryo transfer treatment.
Pregnancy complications and neonatal outcomes
The gestational weeks were converted into days for easy calculations and statistical analyses. The mean duration of pregnancy differed among the three groups, which was the longest in the 8–12 mm group. The incidence of preterm delivery was substantially different among the three groups (p < 0.05), with the highest incidence in the EMT < 8 mm group and the lowest incidence in the 8–12 mm group. Additionally, there was no substantial difference in the incidence of extremely premature delivery and the sex distribution of newborns among the three groups (p > 0.05). As for delivery modes (vaginal delivery and cesarean section), the incidence of cesarean section was the highest in the < 8 mm group (73.3%) and the lowest in the > 12 mm group (63.4%), with a significant difference among the three groups (p < 0.05). The three groups exhibited a substantial difference in the mean newborn birth weight which was the highest in the > 12 mm group and the lowest in the < 8 mm group (p < 0.05). After the birth weights of newborns were further classified, the incidence of low birth weight and macrosomia was calculated, and the results revealed insignificant differences among the three groups (p > 0.05). With regard to pregnancy complications, analyses were conducted on the incidence of GDM, pregnancy-induced hypertension, placenta previa, and premature rupture of membranes. The three groups were markedly different in terms of the incidence of GDM and premature rupture of membranes (p < 0.05) but insignificantly different in terms of the incidence of pregnancy-induced hypertension or placenta previa (p > 0.05) ().
Table 2. Pregnancy complications and neonatal outcomes.
Univariate linear regression analysis of birth weight
The above results illustrated that birth weight might be associated with EMT. Hence, birth weight was used as a dependent variable, and then univariate linear regression was utilized to screen out factors that may affect the birth weight of newborns. displays the regression coefficient of each factor and the P-value of the regression coefficient in the univariate linear regression. The results demonstrated the significance of pre-pregnancy BMI, FSH levels, E2 levels, number of implanted embryos, duration of pregnancy, newborn sex, delivery mode, pregnancy-induced hypertension, placental position, premature rupture of membranes, and EMT (p < 0.05).
Table 3. Univariate linear regression for newborn birth weight.
Multivariate linear regression analysis of birth weight
Based on the results of the univariate linear regression analysis, all significant variables were included in the multivariate linear regression model. Specifically, the Enter method was used to include all significant variables, therefore constructing a multivariate prediction model of newborn birth weight. After dummy variables were established for the EMT groups, EMT < 8 mm was used as the reference group. The mean newborn birth weight in the 8–12 mm group was 25.6 g insignificantly higher than that in the < 8 mm group, with an insignificant difference (p = 0.214). In addition, the mean newborn birth weight in the >12 mm group was 85.1 g higher than that in the < 8 mm group, which was significant (p = 0.013). Contrary to its significant regression coefficient in the univariate regression, the FSH level was not significant in the multivariate regression (p = 0.075). Other variables (pregnancy-induced hypertension, premature rupture of membranes, placental position, newborn sex, number of days of pregnancy, delivery mode, number of implanted embryos, E2 levels, and pre-pregnancy BMI) were significant in the multivariate analysis. The multivariate linear regression equation was listed as follows:
Y (birth weight) = 25.942 × (EMT of 8–12 mm) + 85.107 × (EMT > 12 mm) + 123.483 × (pregnancy-induced hypertension) + 148.859 × (premature rupture of membranes) + 182.342 × (placental position) − 126.242 × (newborn sex) + 23.837 × (number of days of pregnancy) + 130.487 × (delivery mode) − 55.023 × (number of implanted embryos) − 6.215 × FSH level − 1.124 × E2 level + 22.218 × BMI − 4468.101.
The regression coefficients and P-values of the multivariate linear regression model are exhibited in .
Table 4. Multivariate linear regression model for newborn birth weight.
The F value of the regression model was 183.355 (p < 0.001), indicating that the construction of this model was useful for improving the prediction of newborn birth weight. The adjusted R2 value was 0.404, indicating that the model could explain 40.4% of the variance in the newborn birth weight. The summary statistics of the model and the coding of categorical variables in the regression analysis are presented in .
Table 5. Model summary.
Discussion
Our study mainly probed the effect of EMT before embryo transfer on the outcome of singleton live births after freeze–thaw cycles. As exhibited in our result, the mean birth weight of newborns was higher for mothers with EMT > 12 mm before embryo transfer than for mothers with lower EMT. In addition, birth weight was affected by several confounding factors. After confounding variables in the multivariate linear regression were adjusted, EMT still exerted significant effects on newborn birth weight. Accordingly, it can be concluded that a thicker endometrium before embryo transfer in freeze–thaw cycles has clinical implications for increasing newborn birth weight and improving other newborn outcomes.
Guo et al. found insufficient EMT as a risk factor for neonatal small-for-gestational age (SGA) by investigating EMT on the day of hCG detection in fresh-embryo transfer cycles [Citation11]. Combined with our results, it was indicated that a thinner endometrium in women was associated with a lower newborn weight and an increased risk of neonatal SGA. Subsequent to the identification of the relationship between EMT and newborn birth weight, we evaluated the relationship between EMT and the incidence of low birth weight or macrosomia among full-term newborns. The results unraveled no significant difference in the incidence of low birth weight or macrosomia among the three groups. The research by Hwang et al. elucidated that compared with fresh-embryo transfer cycles, FET was associated with higher newborn birth weight and lower risk of low newborn birth weight (adjusted odds ratio: 0.72; 95% confidence interval: 0.59–0.88) [Citation12]. This result illustrates that FET can improve newborn outcomes. Differently, our study exhibited that EMT was only correlated with newborn birth weight but not with the incidence of low birth weight or macrosomia.
Moffat et al. noted that elevated EMT was not a predictor of newborn birth weight in normal pregnancies, whereas EMT before embryo transfer was proportional to newborn birth weight for those with pregnancy complications [Citation13]. Therefore, birth weight is affected by multiple factors. For instance, pregnancy complications, such as pregnancy-induced hypertension, pre-eclampsia, eclampsia, GDM, placenta previa, and premature rupture of fetal membranes, may affect the growth and development of the fetus in the uterus, ultimately resulting in the difference in birth weight. Our study also explored pregnancy complications. GDM incidence was the highest in the > 12 mm group, which was significant. Saito et al. observed that hormone-replacement cycles reduced the risk of GDM in pregnant women [Citation14]. In our study, the number of hormone-replacement cycles was lower in the > 12 mm group than in the other two groups. Accordingly, the > 12 mm group had a higher proportion of patients with GDM. He et al. explored the relationship between EMT and perinatal outcomes and noted that pre-implantation EMT of < 8 mm was substantially associated with the increased risk of premature rupture of membranes [Citation15]. In our research, the incidence of premature rupture of membranes was higher in the > 12 mm group than in the 8–12 mm group. This difference may be explained by the higher incidence of pregnancy-induced hypertension among pregnant women in this group. In the research by Oron et al. the cutoff value of EMT was set as 7.5 mm, and the results demonstrated that obstetric complications had a markedly increased incidence among patients with EMT < 7.5 mm, including premature delivery, pregnancy-induced hypertension, placenta previa, and premature rupture of membranes [Citation16].
After the adjustment of confounding factors, EMT grouping remained important for predicting newborn birth weight, and increased EMT enhanced the newborn birth weight. Specifically, the mean newborn birth weight was 85.107 g higher for the > 12 mm group than for the < 8 mm group, with a significant regression coefficient. This observation is consistent with the results of a similar study by Zhang et al. on freeze–thaw cycles [Citation17] that EMT < 8 mm was associated with lower mean newborn birth weight.
It was also found in our results that the E2 level was an independent predictor of newborn birth weight, with a regression coefficient of −1.124, suggesting that the predicted weight of newborns could decrease by 1.124 g corresponding to each unit change in E2 levels. Of note, a study by Pereira et al. exhibited that high estrogen levels produced during superovulation in fresh-embryo transfer cycles affected the environment of embryo implantation, thereby leading to low newborn birth weight [Citation18]. In addition, another study showed that the superovulation environment was detrimental to the growth and development of fetal mice [Citation19]. Similarly, our study elucidated the absence of the effects of high estrogen levels during superovulation in freeze–thaw cycles and that high E2 levels negatively regulated newborn birth weight. This finding is consistent with the low birth weight caused by a high-estrogen environment during fresh-embryo transfer cycles. Nevertheless, little is known about whether there is a definite correlation between the high-estrogen environment and low newborn birth weight, which requires further exploration in studies with a large sample size. Spada et al. noted that BMI of pregnant women in the first trimester was a reliable predictor of newborn birth weight [Citation20]. In our study, the newborn birth weight increased by 22.218 g for each unit increase in BMI of pregnant women, concordant with data from other studies [Citation20, Citation21].
A prior study elaborated that the mean birth weight of male newborns was higher than that of female newborns and that IVF technology might not change sex-dependent differences in birth weight [Citation21]. According to our regression equation, the mean birth weight of female newborns was 126.242 g lower than that of male babies. As reported, gestational age is a confounding factor affecting newborn birth weight. In our study, the newborn birth weight was elevated by 23.837 g for each additional day of pregnancy. Moreover, the blood circulation between the mother and child during pregnancy may be affected due to pregnancy complications and abnormal fetal appendage function, thereby contributing to adverse neonatal outcomes [Citation22]. Our data demonstrated pregnancy-induced hypertension, premature rupture of membranes, and placenta previa as independent risk factors for low newborn birth weight. Additionally, we also observed that the delivery mode affected newborn birth weight. Specifically, the birth weight was relatively low for newborns delivered by cesarean section, which may be attributable to common pregnancy complications among women who delivered with cesarean section and emergency pregnancy termination for other reasons when the fetus is not yet mature. In addition, the number of implanted embryos can also affect the early development of the newborn [Citation23]. Of note, our finding also indicated the number of implanted embryos as a predictor of newborn birth weight. The increased number of implanted embryos was associated with a higher probability of twins and an increased risk of pregnancy complications.
In the early stages of pregnancy, a hypoxic environment in the endometrium is a prerequisite for the normal development of an embryo [Citation24]. A thin endometrium elevates the concentration of oxygen from the mother, thus producing reactive oxygen species, which affects the intrauterine environment during early embryonic development and eventually impairs fetal growth [Citation25, Citation26]. Accordingly, excessive reactive oxygen species interfere with early embryonic implantation and development to reduce newborn birth weight [Citation24, Citation27].
Conclusions
There were four main strengths in our study. First, strict inclusion and exclusion criteria were used. Second, we excluded patients with underlying diseases (such as hypertension and diabetes mellitus) that may affect fetal development. Third, the treatment regimens were conducted as per uniform standards to ensure treatment consistency. Fourth, EMT was measured by experienced sonographers based on a uniform standard, which diminished measurement variability.
Nevertheless, limitations also existed in our study. First, our study was a single-center and retrospective study. Therefore, a large-scale prospective study with multicenter collaboration is warranted for more accurate analyses of the relationship between EMT and newborn birth weight. Second, this study did not explore the mechanism by which EMT affects newborn birth weight.
Authors’ contributions
XiuPing Zhang performed the experiments, collected the results and wrote the manuscript. LiXia Liang contributed to data analysis and manuscript revision. Zhi Ping contributed to revise the manuscript. YuanXia Wu conceived the study and contributed to editing the manuscript. BingBing Chen collected the results. Xue Qing Wu critically revised the drafts of the manuscript and approved the final version of the manuscript. All of authors agree to publish.
Consent for publication
We had obtained from the patient for written informed consent and any accompanying images for publication
Ethical approval of the study protocol
The study protocol was performed in accordance with the Helsinki Declaration. All women provided written informed consent for their data to be used in this study.The project number: 2021028 and The ethics number: IRB-KYYN-2021-001.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Data availability statement
All authors had full access to the data and materials. The data supporting the conclusions of this article are included within the article.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- De Geyter C, De Geyter M, Steimann S, et al. Comparative birth weights of singletons born after assisted reproduction and natural conception in previously infertile women. Hum Reprod. 2006;21(3):1–8.
- Ahlborg L, Ek S, Fridstrom M, et al. Is fetal growth impaired after in vitro fertilization. Acta Obstet Gynecol Scand. 2006;85(2):195–199.
- Ginod P, Choux C, Barberet J, et al. Singleton fetal growth kinetics depend on the mode of conception. Fertil Steril. 2018;110(6):1109–1117 e2.
- Henningsen AK, Pinborg A, Lidegaard O, et al. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: danish national sibling-cohort study. Fertil Steril. 2011;95(3):959–963.
- Craciunas L, Gallos I, Chu J, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(2):202–223.
- Chan JM, Sukumar AI, Ramalingam M, et al. The impact of endometrial thickness (EMT) on the day of human chorionic gonadotropin (hCG) administration on pregnancy outcomes: a 5-year retrospective cohort analysis in Malaysia. Fertil Res Pract. 2018;4:5.
- Yoeli R, Ashkenazi J, Orvieto R, et al. Significance of increased endometrial thickness in assisted reproduction technology treatments. J Assist Reprod Genet. 2004;21(8):285–289.
- Rombauts L, Motteram C, Berkowitz E, et al. Risk of placenta praevia is linked to endometrial thickness in a retrospective cohort study of 4537 singleton assisted reproduction technology births. Hum Reprod. 2014;29(12):2787–2793.
- Liu X, Qu P, Bai H, et al. Endometrial thickness as a predictor of ectopic pregnancy in 1125 in vitro fertilization-embryo transfer cycles: a matched case-control study. Arch Gynecol Obstet. 2019;300(6):1797–1803.
- Zhang T, Li Z, Ren X, et al. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: a retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine (Baltimore). 2018;97(4):e9689.
- Guo Z, Xu X, Zhang L, et al. Endometrial thickness is associated with incidence of small-for-gestational-age infants in fresh in vitro fertilization-intracytoplasmic sperm injection and embryo transfer cycles. Fertil Steril. 2020;113(4):745–752.
- Hwang SS, Dukhovny D, Gopal D, et al. Health outcomes for Massachusetts infants after fresh versus frozen embryo transfer. Fertil Steril. 2019;112(5):900–907.
- Moffat R, Beutler S, Schotzau A, et al. Endometrial thickness influences neonatal birth weight in pregnancies with obstetric complications achieved after fresh IVF-ICSI cycles. Arch Gynecol Obstet. 2017;296(1):115–122.
- Saito K, Kuwahara A, Ishikawa T, et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod. 2019;34(8):1567–1575.
- He L, Zhang Z, Li H, et al. Correlation between endometrial thickness and perinatal outcome for pregnancies achieved through assisted reproduction technology. J Perinat Med. 2019;48(1):16–20.
- Oron G, Hiersch L, Rona S, et al. Endometrial thickness of less than 7.5 mm is associated with obstetric complications in fresh IVF cycles: a retrospective cohort study. Reprod Biomed Online. 2018;37(3):341–348.
- Zhang J, Liu H, Mao X, et al. Effect of endometrial thickness on birthweight in frozen embryo transfer cycles: an analysis including 6181 singleton newborns. Hum Reprod. 2019;34(9):1707–1715.
- Pereira N, Elias RT, Christos PJ, et al. Supraphysiologic estradiol is an independent predictor of low birth weight in full-term singletons born after fresh embryo transfer. Hum Reprod. 2017;32(7):1410–1417.
- Weinerman R, Ord T, Bartolomei MS, et al. The superovulated environment, independent of embryo vitrification, results in low birthweight in a mouse model. Biol Reprod. 2017;97(1):133–142.
- Spada E, Chiossi G, Coscia A, et al. Effect of maternal age, height, BMI and ethnicity on birth weight: an italian multicenter study. J Perinat Med. 2018;46(9):1016–1021.
- O'Neill KE, Tuuli M, Odibo AO, et al. Sex-related growth differences are present but not enhanced in in vitro fertilization pregnancies. Fertil Steril. 2014;101(2):407–412.
- Wennerholm UB, Bergh C. Perinatal outcome in children born after assisted reproductive technologies. Ups J Med Sci. 2020;125(2):158–166.
- Zemet R, Haas J, Bart Y, et al. Pregnancy outcome after multifetal pregnancy reduction of triplets to twins versus reduction to singletons. Reprod Biomed Online. 2020;40(3):445–452.
- Schoots MH, Gordijn SJ, Scherjon SA, et al. Oxidative stress in placental pathology. Placenta. 2018;69:153–161.
- Ribeiro VC, Santos-Ribeiro S, De Munck N, et al. Should we continue to measure endometrial thickness in modern-day medicine? The effect on live birth rates and birth weight. Reprod Biomed Online. 2018;36(4):416–426.
- Kelley RL, Gardner DK. Individual culture and atmospheric oxygen during culture affect mouse preimplantation embryo metabolism and post-implantation development. Reprod Biomed Online. 2019;39(1):3–18.
- Miwa I, Tamura H, Takasaki A, et al. Pathophysiologic features of “thin” endometrium. Fertil Steril. 2009;91(4):998–1004.