Abstract
Objective
To investigate the efficacy of new endometriosis biomarkers in diagnosis and treatment.
Methods
Thirty women with Stage III-IV endometriosis who were given an indication for surgery and 49 control patients were compared. Preoperative and postoperative serum levels of Annexin A5 (ANXA5), soluble intercellular adhesion molecule-1 (sICAM-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), soluble vascular cell adhesion molecule-1 (sVCAM-1), vascular endothelial growth factors (VEGF) and Ca-125 measurements were compared.
Results
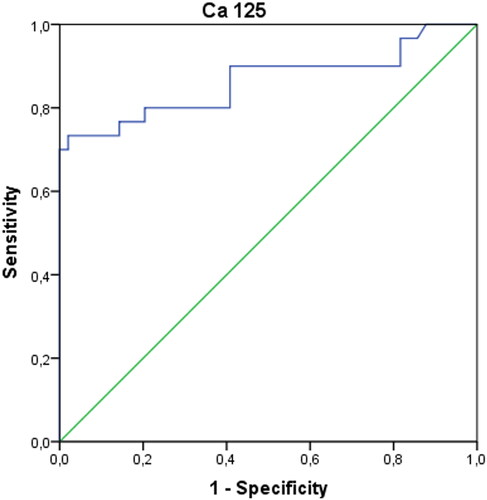
AUCs of ANXA5, sICAM-1, IL-6, TNF-α, VCAM-1, VEGF biomarkers were not found to be significant in diagnosing endometriosis when evaluated alone (p > 0.05). Only the AUC of the Ca-125 biomarker values were found to be significant with 73% sensitivity and 98% specificity (p < 0.001). However, when Ca-125 and ANXA5 were evaluated together, it was concluded that the diagnosis of endometriosis could be made with 73% sensitivity and 100% specificity.
Conclusion
When Ca-125 and ANXA5 are evaluated together, it seems to be more valuable than Ca-125 alone in diagnosing endometriosis.
Introduction
Endometriosis is an estrogen-dependent gynecological disorder characterized by the presence of endometrial-like tissue outside the uterine cavity, associated with pelvic pain and subfertility [Citation1]. Despite its benign character, endometriosis remains a disease that interferes with the quality of life of millions of women. Complex interactions between genetic, immunological, hormonal, and environmental factors in addition to epigenetic changes increase susceptibility to endometriosis [Citation2]. Growth factors, angiogenic factors, cytokines and other specific glycoproteins secreted from immune cells, such as macrophages and monocytes, cause a chronic inflammatory process, leading to pathological growth of endometrial plaque [Citation3]. The time between the appearance of the first symptoms and the final diagnosis can be as long as 8–12 years [Citation4]. Laparoscopy followed by a histopathological examination is the gold standard; however, it is an invasive method for diagnosis [Citation5]. Numerous studies have been conducted to develop noninvasive diagnostic tools, such as ‘biomarkers’, in order to diagnose endometriosis earlier, reduce costs and evaluate the efficacy of treatment [Citation6]. However, it is important to note that no biomarker or biomarker panel for endometriosis has been validated for clinical application in peripheral blood [Citation7] or in the endometriotic lesions [Citation8, Citation9]. For this reason, research is still ongoing to find combinations of various biomarkers that may be more effective in diagnosing the disease [Citation10].
Annexin A5 (ANXA5), soluble intercellular adhesion molecule-1 (sICAM-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), soluble vascular cell adhesion molecule-1 (sVCAM-1) and vascular endothelial growth factors (VEGF), as products of immunocompetent cells, have been suggested to a play role in the progression of this disease by vascularization, promoting the survival and proliferation of ectopic endometrial cells [Citation11]. In this study, these six serum markers were compared before and after operations in women with moderate and severe endometriosis (revised American Fertility Society Score, rAFS Stage III or IV) [Citation12] to have an idea about the pathophysiology of endometriosis and to examine whether it is useful in follow-up and treatment.
Materials and methods
This prospective cohort study was conducted in a tertiary referral hospital from January 2018 to March 2020 (Clinical Trial ID: NCT05312528). Ethical approval was obtained from the local ethics committee of our hospital (approval number: 2018.01.1.01.001). The study was conducted following the Declaration of Helsinki and its later amendments. Seventy-nine patients were included in the study after informed consent was obtained. The study group included 30 women 18–50 years of age, who had an ultrasound and MRI-confirmed mass or deep infiltrating endometriosis or suspected malignancy, who required surgery for severe endometriosis (Stage III-IV). Endometriosis was classified in accordance with the rAFS. Surgery indications (infertility, suspected malignancy and/or pain) of the patients were recorded. The diagnosis of endometriosis was confirmed by histopathological examination. As the control group, 49 healthy patients in the group without endometriosis, who required surgery due to tubal ligation, benign ovarian cysts or uterine fibroids, were included. Exclusion criteria were hormonal treatment (combined oral contraceptive pill or progestins or GnRH analogues) received three months prior to surgery, histories of malignancies, signs of ovarian failure, acute pelvic inflammatory disease, smokers, women with suspected pure adenomyosis, without endometrial mass or nodules, women with a positive pregnancy test or unknown pregnancy status on day of surgery, women who were breastfeeding up to six months prior to the beginning of the study, and chronic autoimmune disease. All patient data, including age, fertility history, treatment history (oral contraceptive and GnRH analogue use), menstrual cycle phase, pain history, histopathology findings and anatomic features of disease lesions, were recorded. Blood samples were taken in the morning prior to surgery into biochemistry tubes with a gel clot activator and kept until coagulation occurred, then centrifuged at 4000 g for 10 min, after which their serums were separated. The separated serums were portioned and taken into Eppendorf tubes without additives and stored in a freezer at −80 °C. When all serums were obtained, they were analized on the same day after thawing at room temperature. Laparoscopy was performed in the follicular phase of the women’s menstrual cycle and all recognizable endometriotic lesions were treated by bipolar coagulation, resectioning of endometriotic nodules and ovarian cystectomy. The serum samples were taken again in the follicular phase of the third postoperative menstrual cycle from all the patients.
Measurements were performed on the Biotek ELx800 (USA) brand Elisa reader using VEGF-D (Catalog no: E-EL-H1601 Elabscience Biotechnology Inc, USA), ANXA5 (Catalog no: E-EL-H0422), IL-6 (Catalog no: E-EL-H6156), TNF-α (Catalog No: E-EL-H0109), VCAM-1/CD106 (Catalog No: E-EL-H5587), sICAM-1/CD54 (Catalog no: E-EL-M0077) as commercial Microeliza kits. CA-125 immunoassay measurements were performed on a Beckman Coulter (USA) Dxl 800 hormone analyzer with following a paramagnetic particle, two-site immunoenzymatic (sandwich), chemiluminescence immunoassay method. The lower limit of detection was 0.374 IU/mL. Intra-laboratory and inter-laboratory coefficient variation values were below 10%. Normal values are 0–35 IU/mL.
Mean standard deviation, median, minimum and maximum values were given in the descriptive statistics for the continuous data, and percentage values were given in the discrete data. The Shapiro-Wilk test was used to examine the conformity of continuous data to the normal distribution. The Mann-Whitney U test was used to compare biomarker values in two groups. The Wilcoxon test was used to examine the differences in preoperative and postoperative biomarker values. The power of biomarker values to distinguish patients with endometriosis was evaluated from the area under the Receiver Operating Characteristic (ROC) curve (AUC). The best cutoff point was calculated using Youden’s Index. The diagnostic performance of the biomarker values was demonstrated using sensitivity, specificity, positive values (PPVs) and negative predictive values (NPVs). The IBM SPSS Statistics 20 program was used in the evaluations with p < 0.05 being accepted as the statistical significance limit.
Results
A total of 79 patients, including 30 with endometriosis and 49 in a control group, were included in the study. The mean age of the women with endometriosis was 38.03 ± 6.74 and 37.67 ± 5.42 for the control group women (p > 0.05). Dyspareunia occurred in 93.3% of the women with endometriosis, dysmenorrhea in 90%, chronic pelvic pain in 83.3%, dyschezia in 76.7% and subfertility in 43.3%.
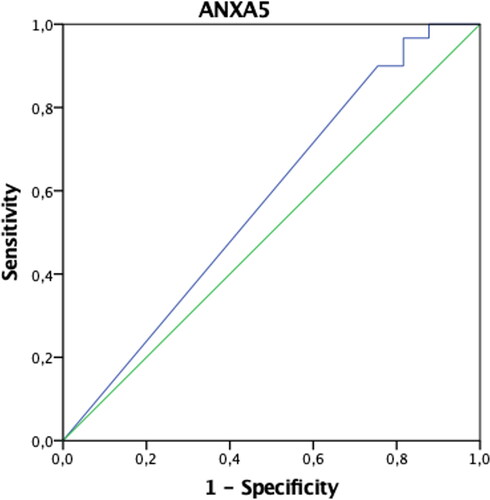
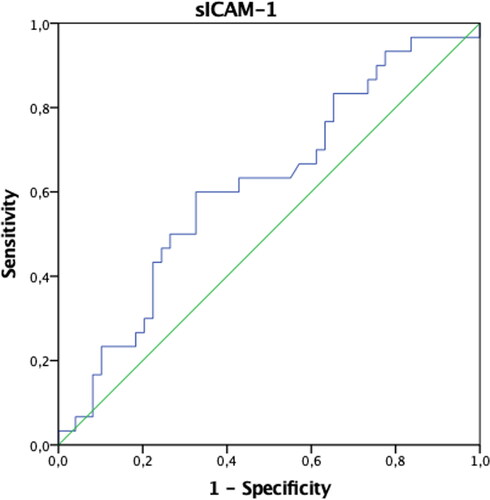
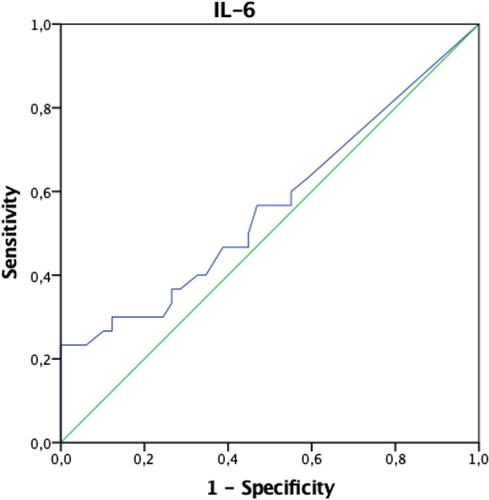
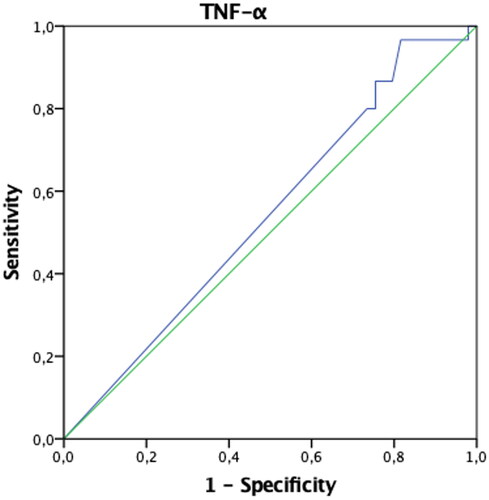
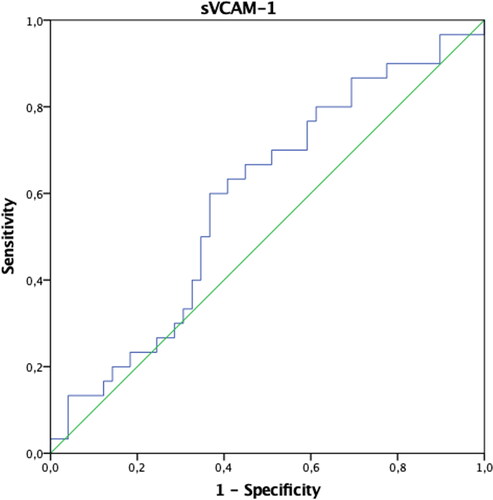
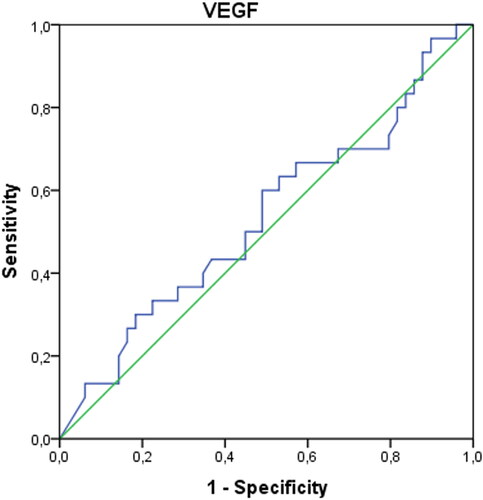
In patients with endometriosis, postoperative ANXA5 and TNF-α values were found to be statistically significantly higher, and VCAM-1 and Ca-125 values were significantly lower compared to the preoperative period (p < 0.05). Preoperative IL-6 values were significantly higher in patients in the control group compared to the postoperative period (p < 0.05). Both preoperative and postoperative Ca-125 values of patients with endometriosis were significantly higher than the control group (p < 0.05) (). The AUCs of ANXA5, sICAM-1, IL-6, TNF-α, VCAM-1, and VEGF biomarkers were found not to be significant when evaluated alone (p > 0.05) (). When the threshold value was accepted as 32.4 IU/mL in separating patients with endometriosis only, only the AUC of the Ca-125 biomarker values were found to be significant with 73% sensitivity and 98% specificity (p < 0.001) () (). When we evaluate the biomarkers together, Ca-125 and ANXA5 can diagnose endometriosis with 73% sensitivity and 100% specificity, Ca-125-TNF-α with 70% sensitivity, 98% specificity, and the ANXA5-TNF-α pair with 93% sensitivity and 31% specificity ().
Table 1. Comparison of preoperative and postoperative biomarker values of patients in the endometriosis group (n = 30) and control group (n = 49).
Table 2. ROC curves and diagnostic performance of biomarker values in distinguishing patients with endometriosis.
Table 3. Diagnostic performance of biomarker combinations in differentiating patients with endometriosis.
Discussion
In this study, it was found that postoperative ANXA5 and TNF-α had increased, but sVCAM-1 and Ca-125 had decreased in endometriosis patients. ANXA5 is one of the members of the Annexin family of membrane binding proteins, an apoptosis marker, and has been reported as a semi-invasive biomarker for the diagnosis of minimal-mild endometriosis [Citation13]. In our study, the ANXA5 level was not found to be significant for the diagnosis of endometriosis alone; however, this study group consisted of severe endometriosis patients. sICAM-1, sVCAM-1, VEGF are glycoproteins that promote cell proliferation and neovascularization, and play an essential role in the promotion of inflammatory and immunological reactions [Citation14, Citation15]. Studies in mice have shown that after implantation of uterine tissue into the peritoneum, macrophage activation and increased VEGF secretion in response to the TNF-α and IL-6 occurred [Citation16], while some studies suggest that VEGF levels following laparoscopic excision of endometriosis foci are reduced [Citation17]. Szubert et al. concluded that following danazol treatment, plasmatic VEGF concentrations significantly increased, therefore implying that this molecule could not be associated with the disease [Citation18]. In this study, VEGF was not found to be a diagnostic marker specific to endometriosis, nor was there any change in blood levels after surgery. IL-6 and TNF-α are proinflammatory cytokines. However, Martinez et al. pointed out that patients with Stage I-II endometriosis had increased levels of IL-6, with a sensitivity of approximately 75% and a specificity of 83.3% [Citation19]. In another study, however, it was shown that there was no significant difference between the endometriosis group and the control group [Citation20]. In this study, the IL-6 level was found to be high preoperatively in the control group patients. This was attributed to the fact that the control group patients were a heterogeneous group, not completely healthy patients. The role of TNF-α as a biomarker for endometriosis is controversial. While several studies proved increased serum levels of TNF-α in women with endometriosis [Citation21], others showed no difference between affected patients and healthy controls [Citation22]. In this study, TNF-α and ANXA5 levels were observed to increase in the postoperative period only in endometriosis patients. Unfortunately, no study could be found in the literature to explain the clinical significance of this condition.
In recent studies, sICAM-1 levels were found to be higher in endometriotic women compared to unaffected patients [Citation14, Citation15]. Kuessel et al. found that the level of sVCAM-1 was significantly higher in patients with endometriosis with lower levels of sICAM-1 when compared with women with endometriosis and control subjects. They concluded that the sVCAM-1/sICAM-1 ratio is a better diagnostic tool for endometriosis than the individual marker [Citation23]. In this study, it was considered promising as the sVCAM-1 level, like Ca-125, decreased significantly after endometriosis surgery.
With the theory that immune system dysfunction plays a role in the pathogenesis of endometriosis, immunological molecules and inflammatory cytokines have been extensively studied as potential biomarkers for endometriosis [Citation7]; however, most results remain controversial. Ca-125 is the most commonly used glycoprotein marker in endometriosis despite its low specificity and sensitivity [Citation24]. Indeed, in 2019 Dorien et al. in their study to validate predefined diagnostic models for endometriosis, were unable to validate any model other than Ca-125 [Citation25], which is mostly used as a prognostic marker [Citation14]. The sensitivity of Ca-125 in endometriosis Stages III and IV was 63.1%, compared to only 24.8% in Stages I and II [Citation26]. In this study, it was concluded that patients with Stage III and IV endometriosis could be diagnosed with a sensitivity of 73% and a specificity of 98% when the threshold value of Ca-125 was taken as 32.4 IU/mL. Vodolazkaia et al. used two panel models containing ANXA5, VEGF, CA-125, glycodelin and sICAM-1 measured in plasma samples obtained during the menstrual phase and determined that it allows detection of ultrasound-negative endometriosis with high sensitivity (82%) and acceptable specificity (63–75%) [Citation14].
However, our aim was to find more effective combinations to catch endometriosis at an earlier stage. It was demonstrated once again that none of the biomarkers in this study alone were predictive of diagnosing endometriosis. However, preoperative and postoperative changes of biomarkers may be helpful in explaining the pathophysiology of endometriosis. While the picture is noisy in the advanced stage, we thought that it could be adapted to the early stage if other biomarkers associated with endometriosis were found. In this study, when Ca-125 and ANXA5 were evaluated together, it was concluded that the diagnosis of endometriosis could be made with 73% sensitivity and 100% specificity. This result seems to be more valuable than Ca-125 alone.
Conclusion
As a result, we could not detect a biomarker as strong as Ca-125 in severe endometriosis. However, the association of ANXA5 and Ca-125 seems to be more effective than Ca-125 alone. In the light of these findings, studies that will investigate the effectiveness of the Ca 125-ANXA5 combination in patients with early-stage endometriosis may help in the early diagnosis of the disease. The pre- and post-operative evolution of these biomarkers can predict whether the disease is progressing and determine the need for additional treatment. Moreover, it can contribute to the development of new treatment modalities by giving an idea about the pathophysiology of the disease.
Some of the limitations of this study were the heterogeneity of the control group in addition to not having included patients with early-stage endometriosis. In addition, considering the frequent coexistence of endometriosis and adenomyosis, the clinical effect of adenomyosis could not be distinguished. The need for further studies in large, well-defined patient groups combining all available methods such as serum, urine markers, glycoproteins, growth factors, peptides, immunological markers, genomic technologies, ultrasonography, and MRI is indisputable.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Tomassetti C, Johnson NP, Petrozza J, International working group of AAGL, ESGE, ESHRE and WES, et al. An international terminology for endometriosis, 2021. J Minim Invasive Gynecol. 2021;28(11):1–6.
- de Fáveri C, Fermino PMP, Piovezan AP, et al. The inflammatory role of Pro-Resolving mediators in endometriosis: an integrative review. IJMS. 2021;22(9):4370.
- La Rosa VL, Barra F, Chiofalo B, et al. An overview on the relationship between endometriosis and infertility: the impact on sexuality and psychological well-being. J Psychosom Obstet Gynaecol. 2020;41(2):93–97.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244–1256.
- Coutinho LM, Ferreira MC, Rocha ALL, et al. New biomarkers in endometriosis. Adv Clin Chem. 2019;89:59–77.
- Horne AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ. 2022; Nov 14379:e070750.
- Kimber-Trojnar Ż, Pilszyk A, Niebrzydowska M, et al. The potential of Non-Invasive biomarkers for early diagnosis of asymptomatic patients with endometriosis. JCM. 2021;10(13):2762. Jun 23
- El Sabeh M, Afrin S, Singh B, et al. Uterine stem cells and benign gynecological disorders: role in pathobiology and therapeutic implications. Stem Cell Rev Rep. 2021;17(3):803–820. Jun
- Nisenblat V, Prentice L, Bossuyt PM, et al. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev. 2016; Jul 137(7):CD012281.
- Kovács Z, Glover L, Reidy F, et al. Novel diagnostic options for endometriosis - Based on the glycome and microbiome. J Adv Res. 2021; Feb 533:167–181.
- Anastasiu CV, Moga MA, Elena Neculau A, et al. Biomarkers for the noninvasive diagnosis of endometriosis: state of the art and future perspectives. IJMS. 2020; Mar 421(5):1750.
- Vermeulen N, Abrao MS, Einarsson JI, International working group of AAGL, ESGE, ESHRE and WES, et al. Endometriosis classification, staging and reporting systems: a review on the road to a universally accepted endometriosis classification. Hum Reprod Open. 2021;2021(4):hoab025. Oct 22
- Sahar T, Nigam A, Anjum S, et al. Differential expression of lumican, mimecan, annexin A5 and serotransferrin in ectopic and matched eutopic endometrium in ovarian endometriosis: a case-control study. Gynecol Endocrinol. 2021;37(1):56–60. Jan
- Vodolazkaia A, El-Aalamat Y, Popovic D, et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum Reprod. 2012; Sep27(9):2698–2711.
- Matalliotakis IM, Vassiliadis S, Goumenou AG, et al. Soluble ICAM-1 levels in the serum of endometriotic patients appear to be independent of medical treatment. J Reprod Immunol. 2001; Jul51(1):9–19.
- Lin YJ, Lai MD, Lei HY, et al. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006; Mar147(3):1278–1286.
- Mohamed ML, El Behery MM, Mansour SA. Comparative study between VEGF-A and CA-125 in diagnosis and follow-up of advanced endometriosis after conservative laparoscopic surgery. Arch Gynecol Obstet. 2013; Jan287(1):77–82.
- Szubert M, Suzin J, Duechler M, et al. Evaluation of selected angiogenic and inflammatory markers in endometriosis before and after danazol treatment. Reprod Fertil Dev. 2014; Mar26(3):414–420.
- Martínez S, Garrido N, Coperias JL, et al. Serum interleukin-6 levels are elevated in women with minimal-mild endometriosis. Hum Reprod. 2007; Mar22(3):836–842.
- Socolov R, Butureanu S, Angioni S, et al. The value of serological markers in the diagnosis and prognosis of endometriosis: a prospective case-control study. Eur J Obstet Gynecol Reprod Biol. 2011; Feb154(2):215–217.
- Cho SH, Oh YJ, Nam A, et al. Evaluation of serum and urinary angiogenic factors in patients with endometriosis. Am J Reprod Immunol. 2007; Dec58(6):497–504.
- Seeber B, Sammel MD, Fan X, et al. Panel of markers can accurately predict endometriosis in a subset of patients. Fertil Steril. 2008 May;89(5):1073–1081.
- Kuessel L, Wenzl R, Proestling K, et al. Soluble VCAM-1/soluble ICAM-1 ratio is a promising biomarker for diagnosing endometriosis. Hum Reprod. 2017; Apr 132(4):770–779.
- O DF, Flores I, Waelkens E, et al. Noninvasive diagnosis of endometriosis: review of current peripheral blood and endometrial biomarkers. Best Pract Res Clin Obstet Gynaecol. 2018; Jul50:72–83.
- O DF, Fassbender A, Van Bree R, et al. Technical verification and assessment of independent validation of biomarker models for endometriosis. Biomed Res Int. 2019; Jul 252019:3673060.
- Hirsch M, Duffy J, Davis CJ, International Collaboration to Harmonise Outcomes and Measures for Endometriosis, et al. Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis. BJOG. 2016; Oct123(11):1761–1768.