Abstract
Objective
To assess the effect of cabergoline on endometrial vascular endothelial growth factor receptor-2 (VEGFR-2) immunoexpression in an ovarian hyperstimulation syndrome (OHSS) rat model.
Material and Methods
Twenty-one immature female Wistar rats were assigned into three groups: group 1, the control group; group 2, stimulated with gonadotropins to mimic OHSS; and group 3, in which an OHSS protocol was induced and thereafter treated with cabergoline (100 μg/kg/day). Body weight, ovarian volume, corpora lutea numbers, and endometrial VEGFR-2 expression were compared between the groups.
Results
Weight gain and ovarian volume were highest in the OHSS-placebo group, while cabergoline administration significantly reversed those effects (p = 0.001 and p = 0.001, respectively). VEGFR-2 stained cells were significantly lower in groups 2 and 3 compared to group 1 (p = 0.002). Although VEGFR-2 expression was lowest in group 3, the difference was not statistically significant. Corpora lutea numbers were also similar (p = 0.465).
Conclusion
While successful implantation requires a vascularized receptive endometrium, impaired expression of VEGFR-2 and disrupted endometrial angiogenesis due to cabergoline administration may be associated with IVF failure in fresh OHSS cycles. The insignificant decrease in endometrial VEGFR-2 expression observed in this research needs to be investigated by further studies involving additional techniques such as immunoblotting and/or RT-PCR analyses.
Introduction
Ovarian hyperstimulation syndrome (OHSS) is one of the most severe and life-threatening iatrogenic complications of controlled ovarian hyperstimulation. OHSS is observed at a rate of 0.3% to 5% and can lead to severe morbidities, such as pleural effusion, acute renal insufficiency, and venous thromboembolism [Citation1]. Various vasoactive-angiogenic substances have been implicated in the etiology of OHSS, including vascular endothelial growth factor (VEGF), prostaglandins, cytokines, renin-angiotensin-aldosterone, estradiol, progesterone, kinin-kallikrein, and nitric oxide. VEGF is attributed to be the leading factor in the shift of fluid into the extravascular space due to increased vascular permeability by activating VEGF receptor-2 (VEGFR-2) [Citation2–6]. VEGF also plays a critical role in the stimulation of angiogenesis and endothelial cell mitosis [Citation7–10].
Prevention and treatment strategies for OHSS include cycle cancelation, coasting, intravenous albumin infusion, low doses of hCG for triggering, agonist trigger in antagonist cycle, freeze-all strategy, and the administration of cabergoline which acts as a dopamine receptor-2 agonist [Citation11]. Cabergoline is thought to partially inhibit VEGFR-2 and reduce vascular permeability in order to reduce the incidence and severity of OHSS [Citation12]. While successful implantation requires a vascularized receptive endometrium, impaired expression of VEGFR-2 and disrupted endometrial angiogenesis due to cabergoline administration may be associated with IVF failure in fresh OHSS cycles [Citation13]. Some authors have reported that cabergoline appears to have no adverse impact on implantation and subsequent adverse obstetric outcomes [Citation14]. However, the majority of those studies were retrospective, mainly based on clinical results or involving heterogeneous study groups with low to moderate quality of evidence. The present experimental study is significant in terms of being designed around an objective biomarker within the framework of immunohistochemical analysis, investigating the effect of cabergoline on endometrial VEGFR-2 expression in an OHSS rat model.
Material and methods
Animals
Twenty-one immature female Wistar rats were obtained from the Ankara Education and Research Hospital Animal Research Center Laboratory, Turkey, and housed for one week in the laboratory so that the experiments could begin with animals that were 23 days old and weighed 40-54 g. The animals received a standard diet and were allowed ad libitum access to water in a 12-h light/12-h dark cycle. The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and the Animal Care and Use Committee, and was approved by the Gazi University Institutional Ethics Review Board (reference number:11038).
Interventions
The OHSS model was adopted from a previously published study [Citation15]. The 23-day-old rats were injected subcutaneously (s.c.) with 10 IU of pregnant mare serum gonadotropin (PMSG; Folligon-Intervet; Schering-Plough Animal Health, Pune, India) daily for four days, followed by a single injection of 30 IU human chorionic gonadotropin (hCG; Chorulon-Intervet; Schering-Plough Animal Health, Boxmeer, The Netherlands) (s.c.) on the fifth day. The control group was treated daily with normal saline solution over the same period.
Group 1, the control group (n = 7), received 0.5 cc/day of normal saline solution (s.c.) for four days.
Group 2, the OHSS-placebo group (n = 7), received 10 IU/day of PMSG (s.c.) for four days and 30 IU hCG (s.c.) on the fifth day to mimic OHSS.
Group 3, the OHSS-cabergoline treatment group (n = 7), received 10 IU/day of PMSG (s.c.) for four days and 30 IU hCG (s.c.) on the fifth day to mimic OHSS. Additionally, 100 μg/kg/day cabergoline (Dostinex, Pfizer, Italy) was administered by oral gavage on the fifth and sixth days.
On the 28th day of life, 48 h after hCG injection, all rats were anesthetized via the intramuscular administration of ketamine hydrochloric acid (50 mg/kg; Ketalar; Eczacibasi Warner-Lambert, Levent, Istanbul, Turkey) and xylazine hydrochloric acid (7 mg/kg; Rompun; Bayer, Sisli, Istanbul, Turkey) and then sacrificed. The rats were weighed at the beginning of the study and before surgery to analyze the effects of cabergoline on weight gain. A ventral midline incision was made in order to perform hysterectomy and bilateral salpingo-oophorectomy. Finally, once any adhering tissue had been removed, ovarian volumes were calculated by multiplying the length, width and height of the ovaries.
Histopathological evaluation
Histopathological evaluations were performed by a pathologist blinded to the study groups. The right and left ovaries were first blocked in paraffin. Sections 4–6 µm in thickness were taken from the paraffin blocks using a microtome and then deparaffinized. The sections were then stained with hematoxylin-eosin to examine corpora lutea numbers, as an indicator of ovarian stimulation, under a light microscope. Uterine specimens were fixed in 4% buffered formaldehyde and embedded in paraffin before 5 μm histological sections were obtained. Those were then stained with hematoxylin-eosin to reveal the presence of stroma, the glandular epithelium, and microvessels in endometrial tissue. The immunohistochemical staining was performed using the avidin-biotin- peroxidase complex (ABC) method. To reduce nonspecific background staining due to endogenous peroxidase, the slides were incubated in Hydrogen Peroxide Block for 15 min. They were next microwaved in 10 mM of citric acid at pH 6.0 for 20 min and then incubated for 60 min with anti-VEGF receptor 2 antibody (Abcam PLC, Cambridge, UK) at room temperature. The standard ABC technique was performed using a Lab Vision Secondary Detection Kit (Ultra Vision Detection System Anti-polyvalent, HRP). All slides were counterstained with Mayer’s hematoxylin. For the determination of microvessel density, nine high-power (400×) fields were then randomly selected, and the number of microvessels in the total high-power field (2 mm2) was counted for each sample. The term ‘VEGFR-2 stained cell’ has been referred to the microvessel density throughout the text.
Statistical analyses
Statistical analyses were performed on Statistical Package for the Social Sciences version 15.0 software (SPSS, Chicago, IL, USA). The variables were expressed as mean ± the standard-deviation (SD). Normality of distribution of continuous variables was assessed using the Shapiro–Wilk test. Normally distributed variables were examined using one-way analyses of variance followed by Tukey’s post-hoc tests, and non-normally distributed variables using the Kruskal–Wallis and Mann–Whitney U tests with post-hoc Bonferroni correction. p values <0.05 were regarded as statistically significant.
Results
As expected, ovarian volume and weight gain were highest in group 2, but cabergoline administration significantly reversed those effects (p = 0.001 and p = 0.001, respectively). Corpora lutea numbers were similar between groups 2 and 3 (p = 0.465). Endometrial VEGFR-2 stained cells were significantly lower in groups 2 and 3 compared to group 1 (Group 1 = 342.29 ± 33.63 cells, group 2 = 188.43 ± 78.22 cells, and group 3 = 138.14 ± 22.66 cells) (p = 0.002) (). Although VEGFR-2 stained cell numbers were lowest in group 3, the difference was not statistically significant. The histopathological appearances of the groups are shown in .
Figure 1. Control group; (A) Endometrium, endometrial stromal cell (black arrow) (hematoxylin-eosin × 200) (B) Endometrial stromal cell (black arrow); VEGFR-2 immunostained endothelial cell (yellow arrow); VEGFR-2 immunostained epithelial cell (blue arrow); VEGFR-2 immunostained microvessel (red arrow) (× 200).
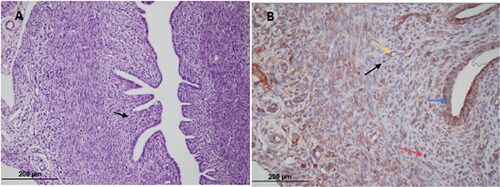
Figure 2. OHSS-placebo group; (A) Endometrium (hematoxylin-eosin × 200) (B) Endometrial stromal cell (black arrow); VEGFR-2 immunostained microvessel (red arrow) (× 200).
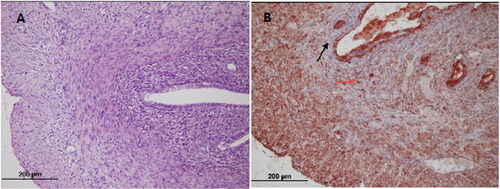
Figure 3. Cabergoline group; (A) Endometrium (hematoxylin-eosin × 200) (B) Endometrial stromal cell (black arrow); VEGFR-2 immunostained microvessel (red arrow) (× 200).
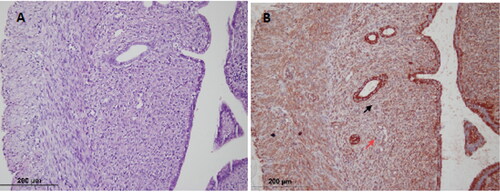
Table 1. A comparison of weight gain, ovarian volume, corpora lutea numbers, and numbers of endometrial VEGFR-2 stained cells between the groups.
Discussion
This study was performed to investigate the impact of cabergoline on implantation through endometrial VEGFR-2 immunoexpression in an OHSS rat model. We observed a decreased number of endometrial VEGFR-2 stained cells in the OHSS group. Cabergoline administration reduced it still further, although the difference was insignificant. To the best of our knowledge, this is the first rodent study to investigate endometrial VEGFR-2 expression using immunohistochemistry in a cabergoline-administered OHSS model.
Numerous animal studies have examined VEGF levels or VEGFR-2 expression in ovarian tissue, blood samples, or peritoneal fluid in OHSS. Gomez et al. reported elevated VEGFR-2 expression in the granulosa cells of rats with OHSS [Citation3]. Saylan et al. and Ozcakir et al. also observed increased VEGF expression in ovarian tissues from OHSS induced rats [Citation16–17]. Kasap et al. suggested that ovarian VEGF expression decreased in the cabergoline group compared to the OHSS group, while Ferrero et al. reported that dopamine agonists inhibited VEGF production post-transcriptionally [Citation18,Citation19]. Atilgan et al. reported a significant decrease in VEGF-2 levels in ovarian tissues after cabergoline administration either with a single dose or long-term supplementation [Citation20].
According to the American Society for Reproductive Medicine, there is good evidence that dopamine agonist administration starting at the time of hCG trigger reduces the incidence of OHSS [Citation21]. However, in the majority of important human clinical studies assessing the effect of cabergoline, the reported evidence was not of high quality in terms of implantation rates, pregnancy rates or miscarriage rates. A review study of 858 women undergoing ART postulated that cabergoline was unlikely to affect clinical pregnancy, although the evidence was low-quality (RR 1.02, 95% CI 0.78–1.34, p = 0.86) [Citation22]. In a Cochrane data analysis involving 16 randomized controlled trials, clinical pregnancy rates were comparable between placebo and intervention groups, the evidence being of moderate quality, although that analysis included not only cabergoline but also other two types of dopamine agonist (bromocriptine and quinagolide) (OR 0.81, 95% CI 0.54 to 1.22) [Citation23].
OHSS is caused by increased vascular permeability through ovarian hypersecretion of VEGF-activating VEGFR-2 [Citation24]. Gomez et al. suggested that the permeability component of the VEGF/VEGFR-2 pathway could be segregated from the angiogenic component in a dose-dependent manner by dopamine agonists via the altered phosphorylation state of this receptor [Citation4]. In that study, mainly involving ovarian tissues, low-dose (100 μg/kg/day) cabergoline only inhibited VEGF-mediated vascular permeability without affecting luteal angiogenesis, whereas a decrease in both vascular permeability and angiogenesis was observed with a higher dosage (500 μg/kg/day). On the other hand, dopamine agonists were found to be effective in preventing endometriotic angiogenesis by blocking the VEGF/VEGFR-2 system in a mouse model of endometriosis in another study [Citation25]. In the present study, we observed a decrease in endometrial VEGFR-2 stained cells following low-dose cabergoline (100 μg/kg/day) administration, which may adversely affect angiogenesis in the endometrium and subsequently compromise implantation. This finding suggested that the effective doses of cabergoline for OHSS prevention may not affect luteal angiogenesis, but unfortunately it is likely to disrupt endometrial vascularization, which may be related to dose-dependent or tissue-specific blockage of angiogenic components of the ovarian and endometrial VEGF/VEGFR-2 pathway.
In a recent OHSS rat study, it was observed that cabergoline administration displayed fewer pinopode-like structures assessing the detrimental effect of cabergoline on endometrial receptivity [Citation26]. In line with this study, our hypothesis about whether cabergoline might negatively affect implantation through influencing endometrial VEGFR-2 expression resulted in insignificant difference.
The limitation of the study was the insignificant decrease in endometrial VEGFR-2 stained cells after cabergoline administration, which may be associated with the limited number of rodents included in the study, which was entirely due to the ethical principles for laboratory animals. Additionally, it is unclear to what extent these findings can be applied to a human model.
In conclusion, further studies involving additional techniques, such as immunoblotting and/or RT-PCR analyses, are needed to evaluate whether cabergoline affects implantation success.
Acknowledgments
None.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Chen CD, Chen SU, Yang YS. Prevention and management of ovarian hyperstimulation syndrome. Best Pract Res Clin Obstet Gynaecol. 2012;26(6):1–5.
- McClure N, Healy DL, Rogers PA, et al. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet. 1994;344(8917):235–236.
- Gomez R, Simon C, Remohi J, et al. Vascular endothelial growth factor receptor-2 activation induces vascular permeability in hyperstimulated rats, and this effect is prevented by receptor blockade. Endocrinology. 2002;143(11):4339–4348.
- Gomez R, Gonzalez-Izquierdo M, Zimmermann RC, et al. Low-dose dopamine agonist administration blocks vascular endothelial growth factor (VEGF)-mediated vascular hyperpermeability without altering VEGF receptor 2-dependent luteal angiogenesis in a rat ovarian hyperstimulation model. Endocrinology. 2006;147(11):5400–5411.
- Nastri CO, Ferriani RA, Rocha IA, et al. Ovarian hyperstimulation syndrome: pathophysiology and prevention. J Assist Reprod Genet. 2010;27(2-3):121–128.
- Brannstrom M, Wang L, Norman RJ. Effects of cytokines on prostaglandin production and steroidogenesis of incubated preovulatory follicles of the rat. Biol Reprod. 1993;48(1):165–171.
- Pauli SA, Tang H, Wang J, et al. The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology. 2005;146(3):1301–1311.
- Smith SK. Angiogenesis and reproduction. BJOG. 2001;108:777–783.
- Heryanto B, Lipson KE, Rogers PA. Effect of angiogenesis inhibitors on oestrogen-mediated endometrial endothelial cell proliferation in the ovariectomized mouse. Reproduction. 2003;125(3):337–346.
- Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153(1):13–19.
- Mathur R, Kailasam C, Jenkins J. Review of the evidence base of strategies to prevent ovarian hyperstimulation syndrome. Hum Fertil (Camb). 2007;10(2):75–85.
- Carizza C, Abdelmassih V, Abdelmassih S, et al. Cabergoline reduces the early onset of ovarian hyperstimulation syndrome: a prospective randomized study. Reprod Biomed Online. 2008;17(6):751–755.
- Douglas NC, Zimmermann RC, Tan QK, et al. VEGFR-1 blockade disrupts peri-implantation decidual angiogenesis and macrophage recruitment. Vasc Cell. 2014;6:16.
- Alvarez C, Alonso-Muriel I, Garcia G, et al. Implantation is apparently unaffected by the dopamine agonist cabergoline when administered to prevent ovarian hyperstimulation syndrome in women undergoing assisted reproduction treatment: a pilot study. Hum Reprod. 2007;22(12):3210–3214.
- Inal HA, Ozturk Inal ZH, Yilmaz N, et al. The effects of formoterol on the serum, peritoneal VEGF, MDA, and VEGF levels in the ovaries and endometrium of rats with OHSS. Clin Exp Obstet Gynecol. 2017;44(1):122–128.
- Saylan A, Arioz DT, Koken T, et al. Prevention of ovarian hyperstimulation syndrome in a rat model: efficacy comparison between cabergoline and meloxicam. Acta Obstet Gynecol Scand. 2010;89(5):692–699.
- Ozcakir HT, Giray SG, Ozbilgin MK, et al. Immunohistochemical detection of transforming growth factor-alpha, epidermal growth factor, and vascular endothelial growth factor expression in hyperstimulated rat ovary. Acta Obstet Gynecol Scand. 2005;84(9):887–893.
- Kasap E, Turan GA, Eskicioğlu F, et al. Comparison between resveratrol and cabergoline in preventing ovarian hyperstimulation syndrome in a rat model. Gynecol Endocrinol. 2016;32(8):634–640.
- Ferrero H, Garcia-Pascual CM, Gaytan M, et al. Dopamine receptor 2 activation inhibits ovarian vascular endothelial growth factor secretion in an ovarian hyperstimulation syndrome (OHSS) animal model: implications for treatment of OHSS with dopamine receptor 2 agonists. Fertil Steril. 2014;102(5):1468–1476 e1.
- Atilgan R, Pala S, Yavuzkir S, et al. What is the impact of short- and long-term supplementation of either cabergoline or clarithromycin on resolving rat ovarian hyperstimulation syndrome (OHSS) model? J Obstet Gynaecol. 2019;39(5):687–694.
- Practice Committee of the American Society for Reproductive Medicine. Electronic address AAO, Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106:1634–1647.
- Leitao VM, Moroni RM, Seko LM, et al. Cabergoline for the prevention of ovarian hyperstimulation syndrome: systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2014;101(3):664–675.
- Tang H, Mourad S, Zhai SD, et al. Dopamine agonists for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev. 2016;11: CD008605.
- Hortu I, Karadadas E, Ozceltik G, et al. Oxytocin and cabergoline alleviate ovarian hyperstimulation syndrome (OHSS) by suppressing vascular endothelial growth factor (VEGF) in an experimental model. Arch Gynecol Obstet. 2021;303(4):1099–1108.
- Delgado-Rosas F, Gómez R, Ferrero H, et al. The effects of ergot and non-ergot-derived dopamine agonists in an experimental mouse model of endometriosis. Reproduction. 2011;142(5):745–755.
- Coban PG, Oruc AS, Ozaksit MG, et al. Comparison of the efficacy of cabergoline and bromocriptine in a rat model of ovarian hyperstimulation syndrome. J Reprod Med. 2018;63:27–32.
