Abstract
Background
Menstrual cycle has a significant impact on women’s health from different perspectives, both physically and psychologically. The assessment of menstrual-related distress is of pivotal clinical interest, especially in women with chronic exposure to abnormal bleeding or pain. The Menstrual Distress Questionnaire (MEDI-Q) is a new tool originally developed in Italian that comprehensively evaluates menstrual-related distress.
Objective
To validate the English version of the MEDI-Q in an English-speaking population.
Methods
The study consisted of two phases: an initial translation phase of the original Italian version of the MEDI-Q, and a data collection phase to validate the new English version among 288 native English-speaking women.
Results
The English version of MEDI-Q showed excellent psychometric properties, with high internal consistency (Cronbach’s alpha = 0.84) and test-retest reliability (intraclass correlation coefficient = 0.95). Construct validity was supported by significant correlations between MEDI-Q scores and scores on measures of psychological distress and premenstrual symptoms.
Conclusions
The English version of the MEDI-Q is a valid and reliable instrument for the assessment of menstrual distress and its impact on psychological well-being. This tool can be utilized in research and clinical settings to comprehensively investigate the impact of menstruation on various populations, identify and monitor menstruation-related disorders promptly and effectively, and to evaluate the effectiveness of targeted treatments for menstrual distress.
Introduction
The menstrual cycle has a relevant impact on women’s health from different perspectives, including both physical and psychological well-being [Citation1–3]. Indeed, ovarian sex steroid hormones (estradiol, progesterone) interact with different functions/organs (e.g. bone, cardiovascular, gastrointestinal), as well as with the central nervous system (modulating mood and cognition) [Citation1–4]. Accordingly, the menstruation is a critical physiological event characterized by uterine bleeding and pain (via inflammatory mechanisms) and these changes are subjectively perceived by women with different degrees of stress response [Citation5, Citation6]. The assessment of menstrual-related distress is of pivotal clinical interest, especially in women with chronic exposure to abnormal bleeding (such as heavy menstrual bleeding) or pain (such as dysmenorrhea), as these symptoms are associated with the development of menstruation-related disorders such as endometriosis, adenomyosis, and uterine fibroids [Citation7]. In addition to these symptoms, other associated ones, such as mood changes and gastrointestinal disorders, can also have a significant impact on both reproductive and psychological health.
The Menstrual Distress Questionnaire (MEDI-Q) is a newly developed tool that has been validated for comprehensive evaluation of menstrual-related distress in an Italian population and is available in the Italian language [Citation8]. It consists of 25 items that cover different areas of menstruation-related symptoms, such as pain, discomfort, psychic or cognitive changes, and gastrointestinal disturbances [Citation8]. The level of distress caused by each symptom is assessed by considering not only its impact on functioning and quality of life during the menstrual phase compared to the intermenstrual and premenstrual phases, but also its frequency. In addition to scores related to individual symptom areas, the MEDI-Q provides four general indices that allow for a comprehensive assessment of menstrual distress [Citation8].
The MEDI-Q has shown excellent psychometric properties. In particular, it showed excellent test-retest reliability and good internal consistency [Citation8]. Furthermore, both convergent validity and concurrent validity were appropriate. The first one was determined through the evaluation of the association of MEDI-Q indices with questionnaires evaluating premenstrual syndrome and general psychopathology, whereas concurrent validity was assessed using the data of a clinical evaluation to identify pathologically distressful menstruation [Citation8]. A receiver operating characteristic (ROC) analysis was performed to evaluate the predictive power of the questionnaire, and the cutoff value of 20 was identified as the best to identify clinically distressful menstruation [Citation8].
Several instruments have been created to measure menstrual symptoms, but each has its limitations and shortcomings in terms of development and validation [Citation9]. The Menstrual Distress Questionnaire (MDQ) [Citation10] is another widely recognized 47 items tool that evaluates symptom severity during the premenstrual, menstrual, and intermenstrual phases, examining eight symptom categories (pain, concentration, water retention, behavior change, negative affect, autonomic reactions, arousal, and control). However, despite its widespread use, the MDQ has faced criticism for being complex and lacking internal consistency and validity [Citation9]. A revised MDQ was introduced, but it only measures symptoms during the premenstrual phase [Citation11]. Another widely used questionnaire for the assessment of menstrual symptoms is the Menstrual Bleeding Questionnaire [Citation12], which, however, exclusively evaluates menstrual bleeding without taking into account the broader impact of menstruation on the individual.
The strengths of MEDI-Q are represented by the fact that it offers a comprehensive evaluation of menstrual-related symptoms and assesses their impact on a person’s functioning, including quality of life, recreational activities, work, and social relationships. It also takes into account the frequency of symptoms, distinguishing between those related to the menstruation and those of the intermenstrual or premenstrual phase. Its psychometric properties have been tested in various age groups and were not affected by the use of oral contraceptives [Citation8]. Finally, a recent study conducted on patients with uterine fibroids evaluated with MEDI-Q found a significant correlation between menstrual distress and abnormal uterine bleeding [Citation13].
Given its potential research and clinical usefulness, the present study aimed at validating the English version of the MEDI-Q in an English-speaking population in order to facilitate its international dissemination and use.
Materials and methods
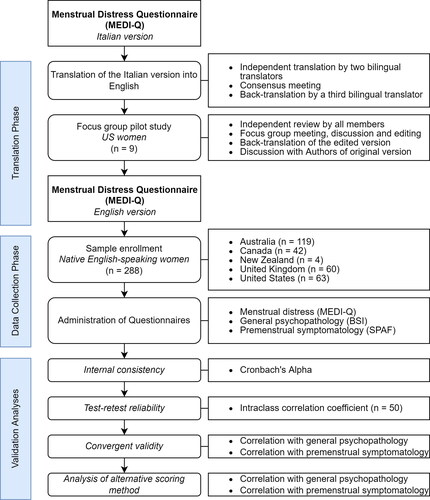
The study consisted of two phases: an initial translation phase of the original Italian version of the MEDI-Q, and a data collection phase to validate the new English version among native speakers. The study flow-chart is reported in .
Figure 1. Study flow-chart.
BSI = Brief Symptom Inventory; SPAF = Shortened Premenstrual Assessment Form
Translation process
The initial phase involved the translation of the original Italian version into English by two independent bilingual (Italian/English) translators, followed by a consensus meeting to reconcile any discrepancies between the translations. The resulting version was then back-translated into Italian by another bilingual translator to ensure the accuracy and consistency of the translation.
This initial English draft was piloted on a sample of 9 US women (ages 19 to 21 years) that were part of a focus group. These women were volunteers recruited from a college population (University of Vermont, USA) and were naïve to the scope of the study and prior research in menstrual and sexual dysfunction.
A research assistant mediated the focus group, ensuring everyone had an opportunity to develop their independent opinions and express their thoughts. First, the research assistant sent out the English draft to all members of the focus group who were asked to set aside at least 1 h and spend as much time as needed to review each question, answer as if it pertained to themselves and note on the side any confusion or uncertainty they experienced while answering the items. Then, all group members met for 2 h and discussed the meaning and potential confusions in the interpretation of each item. This part was crucial to ensure cross-cultural validity of the questionnaire.
The notes from the focus group were combined into an edited version of the English translation which was then back-translated into Italian by a bilingual researcher who was naïve to the original Italian MEDI-Q. During a following step, the original (Italian) and the back-translation versions were compared, and incongruences were discussed between the translators and the authors of the original version. The product of this work was finally incorporated in the ultimate US English version of the questionnaire, which was used for quantitative analysis of the reliability and validity of the measure.
Validation on a population sample
Recruitment occurred through spontaneous participation after the publication of the protocol in university settings via convenience snowball sampling. The enrollment took place at the Western Sydney University and at the University of Florence. In the latter case, the questionnaire was distributed by the study researchers to students, residents and acquaintances who were native English speakers. Participants were recruited according to the following inclusion criteria: female gender, age between 18 and 50 years, being native English speakers and having had at least three menstruations in the past 12 months. Exclusion criteria were as follows: presence of known uterine disorders, known mental disorders and illiteracy or inability to provide informed consent. A total of 288 women was recruited, and a subgroup of participants (n = 50) was asked to complete the questionnaire again two weeks after the first completion, to allow for the study of test-retest reliability. The pairing between the two assessments was carried out anonymously through a unique unidentifiable code entered directly by the participants and not known to the investigators.
All recruited individuals participated voluntarily, did not receive any compensation and provided informed consent. The study protocol was approved by the Ethics Committee of the Institution (Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana, Sezione Area Vasta Centro, register number 14558_oss).
Measures
Basic sociodemographic and anamnestic data were collected, including age, age at menarche, general characteristics of the menstrual cycle in the last year (interval, duration), current use of hormonal contraceptives, presence of gynecological or psychiatric diseases diagnosed by medical professionals. Similarly to the original validation study, the validation of the English version involved an evaluation of convergent validity with administration of tests for general psychopathology and premenstrual symptomatology. Therefore, all participants were asked to complete the English versions of the following self-administered questionnaires:
Menstrual Distress Questionnaire (MEDI-Q): a new tool that assesses and evaluates the global distress experienced by women during their period. It consists of 25 items that cover different areas of menstruation-related distress, such as pain, discomfort, psychic or cognitive changes, gastrointestinal symptoms, and changes in physiological functions. The questionnaire provides a total score (MEDI-Q Total Score), which is a synthetic index of the global menstruation-related distress, and three sub-scales: Menstrual Symptoms (MS), the total number of symptoms that generate more distress during menstruation than in intermenstrual days; Menstrual Symptoms Distress (MSD), which measures the average distress related to menstrual symptoms; and Menstrual Specificity Index (MESI), which represents the proportion of symptoms for which the participant has reported an exacerbation of distress during the menstrual phase as compared to intermenstrual days and premenstrual days. The full questionnaire, together with more details on the scoring procedures, can be found in the Supplementary Materials. The original Italian version of the MEDI-Q showed good internal consistency (Cronbach’s = 0.85) and overall excellent validity [Citation8].
Brief Symptom Inventory (BSI) [Citation14]: a questionnaire that measures psychological distress and general psychopathology. It consists of 53 items that assess nine symptom dimensions: somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. The BSI provides three global indices of distress: the Global Severity Index (GSI), the Positive Symptom Distress Index (PSDI), and the Positive Symptom Total (PST). The BSI is widely used in clinical and research settings to screen for mental health problems and psychological functioning.
Shortened Premenstrual Assessment Form (SPAF) [Citation15]: a self-report questionnaire that measures the severity and impact of premenstrual symptoms. It consists of 10 items that cover physical, emotional, and behavioral domains.
Procedures
The questionnaires were administered both in paper and digital form through online dissemination, depending on the preferences of the recruited subjects. Descriptive statistics, such as means, standard deviations, and frequencies were reported for the demographic and clinical variables and scores of the instruments. The intraclass correlation coefficient (ICC) was calculated to assess the test-retest reliability of the instrument, which indicates the degree of agreement among measurements taken two weeks apart, using a single-measurement two-way mixed effects model. Cronbach’s alpha was calculated to assess the internal consistency reliability of the instrument, which indicates the extent to which the items measure the same construct. Both reliability coefficients range from 0 to 1, with higher values indicating better reliability. The acceptable threshold for both coefficients was set at 0.7.
To test the convergent validity of the MEDI-Q, multiple linear regression models were used. The a priori hypothesis predicted a negative association between menstrual distress and age, and a positive association with premenstrual symptoms measured by the SPAF. In addition, it was hypothesized that there would be an association between the MEDI-Q Total Score and general psychopathology measured by the BSI, as already verified in the Italian validation study [Citation8].
Finally, to further justify the presence of symptom frequency sub-items in the MEDI-Q scoring procedure, an alternative scoring method was tested that did not take into account these sub-items, but only the quantitative measurement of the impact of symptoms on quality of life and functioning (sub-items B and D), regardless of the number of menstrual cycles in which they occurred. This alternative total score was tested with the same linear regression models described above: a weakening or nullification of the expected associations according to the a priori hypothesis was interpreted as an indicator of the added value of sub-items A.
Based on the statistical plan described above, the a priori power analysis required a sample of at least 136 individuals to detect a Cronbach’s alpha of at least 0.80 (against a null hypothesis of 0.70) with 90% power, for a questionnaire containing 25 items [Citation16]. This sample size was sufficient to detect, with a power greater than 90%, medium-to-small effect sizes for multiple linear regression analysis (f2 = 0.10, n = 108 for a model with 2 predictors).
All analyses were performed using R Statistical Software v.4.2.1 and the “psych” package [Citation17, Citation18].
Results
The final sample consisted of 288 women: 62 were aged 25 years or younger, 119 were between the ages of 25 and 35, and 107 were aged 35 years or older. Of the total sample, 63 women were from the United States, 60 from the United Kingdom, 119 from Australia, 42 from Canada and 4 from New Zealand. General and psychometric characteristics of the sample are reported in . presents the mean distress scores for each symptom assessed by the questionnaire. The results showed that the highest mean distress scores were reported for symptoms such as abdominal and muscle/osteoarticular pain, headaches, and fatigue (). Additionally, aspects such as impact on mood (sadness, emotional lability and irritability/anger), and discomfort due to vaginal bleeding also received high mean distress scores as compared to other items ().
Table 1. General and psychometric characteristics of the sample.
Table 2. Average distress score for each MEDI-Q item.
The ICC between participants’ responses measured seven days apart was 0.95, indicating good consistency and confirming the test-retest reliability of the questionnaire. Internal consistency was high, as indicated by a Cronbach’s alpha of 0.84 (95% CI: 0.81-0.86).
MEDI-Q psychopathological correlates are reported in . The Total Score, the MS and MSD indices negatively correlated with age, and positively with premenstrual symptoms as measured by the SPAF total score (). Moreover, MEDI-Q Total Score was significantly associated with general distress, as measured by all three main indices of the BSI questionnaire (). Finally, the proportion of symptoms showing an exacerbation specifically during the menstrual phase (as measured by MEDI-Q MESI) negatively correlated with premenstrual symptomatology (). These results did not differ if the models were adjusted for the presence of hormonal contraception.
Table 3. Correlates of menstrual distress, as measured by the MEDI-Q.
An alternative scoring method was also tested for the MEDI-Q, where data on symptom frequency in the last year’s menstrual cycles were ignored and the score was solely based on the distress score of each item. The total score calculated using this alternative method showed a slightly weaker correlation with age (βAge = −0.15, p = 0.011) compared to the original scoring method that accounted for symptom frequencies and did not show any correlation with general psychopathology measured by BSI scores (βBSI-PST = 0.16, p = 0.093; βBSI-PSDI = 0.13, p = 0.163; βBSI-GSI = 0.16, p = 0.078).
Discussion
The aim of this study was to generate an English translation of the MEDI-Q questionnaire, originally developed in Italian, and to test its psychometric properties in a sample of English native-speaking women. The results were comparable with those obtained with the Italian version of the questionnaire, confirming its reliability and validity in assessing menstrual-related distress. In particular, the English version of the MEDI-Q showed adequate reliability (ICC between participants’ responses measured seven days apart = 0.95) and internal consistency (Cronbach’s alpha = 0.84, 95% CI: 0.81-0.86). Convergent validity, which measures the association between theoretically related constructs [Citation19], was evaluated by examining the correlations between MEDI-Q scores and premenstrual symptomatology and general psychopathology as measured by the SPAF and the BSI, respectively. In agreement with the Italian study, a positive correlation was found between the MEDI-Q Total Score, MS and MSD and general psychopathology and premenstrual symptoms. This is in line with the hypothesis that the questionnaire is suitable for assessing menstrual distress. Indeed, it was expected that a measure of a chronic stressor, such as menstruation-related distress, would correlate with symptoms related to psychological suffering [Citation20]. Furthermore, the correlation with premenstrual symptoms is in line with the observation that premenstrual symptoms often persist during the first days of the menstrual phase [Citation21]. The absence of such correlations when MEDI-Q was scored using the alternative method, which did not take symptom frequency into account, highlighted the importance of considering this aspect in the computation of distress scores. Finally, the negative correlation of the MESI with premenstrual symptoms confirmed the good properties of the questionnaire, as the MESI is an index that measures the level of discomfort specifically associated with the menstrual phase, excluding any discomfort experienced during premenstrual and intermenstrual phases.
It is important to emphasize how the articulated calculation method of the MEDI-Q scores (see Supplementary Materials) allows to take into account only the distress related to the menstrual period, even if certain symptoms or discomforts are present outside the menstrual phase. This is because the general distress indices (Total Score, MS, MSD and MESI) consider only symptoms that are not present during the intermenstrual period, or if they are, they still show an exacerbation during the menstrual phase. This allows for an accurate evaluation of menstrual distress, especially in women who suffer from chronic conditions and symptoms.
An important strength of the present study is the rigorous methodology used for the translation and validation of the questionnaire. In particular, to ensure the cross-cultural validity of the questionnaire, its translation was discussed through focus groups of US women who reviewed each question and discussed its meaning and potential interpretation. Furthermore, the native English-speaking women who responded to the questionnaire resided in various English-speaking countries to ensure that MEDI-Q could also be understood and completed by people outside the US. In addition, the recruited sample was adequate both in terms of size, as indicated by the preliminary power study, and representation of the various age groups.
Regarding the limitations, unlike the Italian validation study of the MEDI-Q a one-on-one assessment of all participants was not possible. Therefore, the presence (or absence) of gynecological or psychiatric diseases was self-reported, and no concurrent validity assessment was performed. However, considering that the scores reported for both individual items and various subscales were comparable to those of the Italian version [Citation8], it can be hypothesized that even for this translated version of the questionnaire a cutoff of 20 may be adequate to identify clinically distressful menstruation. Future studies are needed to confirm this hypothesis.
In conclusion, the present study showed that the English version of the MEDI-Q is a valid and reliable instrument for the assessment of distress related to the menstruation and its impact on the psychological well-being of women. Therefore, this tool can be utilized in both research and clinical settings to comprehensively describe and study the impact of menstruation on various populations and to evaluate the effectiveness of targeted treatments for menstrual distress. Additionally, it can be added into routine healthcare of women as a tool to help identify and monitor menstruation-related disorders promptly and effectively.
Supplemental Material
Download MS Word (52.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, EC, upon reasonable request.
Additional information
Funding
References
- Messinis IE, Messini CI, Dafopoulos K. Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online. 2014;28(6):1–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1472648314001175. doi: 10.1016/j.rbmo.2014.02.003.
- Farage MA, Neill S, MacLean AB. Physiological changes associated with the menstrual cycle. Obstet Gynecol Surv. 2009;64(1):58–72. Available from: https://journals.lww.com/00006254-200901000-00023. doi: 10.1097/OGX.0b013e3181932a37.
- Sundström Poromaa I, Gingnell M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci. 2014;8:380. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25505380. doi: 10.3389/fnins.2014.00380.
- Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update [Internet]. 2012;18(1):73–91. Available from: http://academic.oup.com/humupd/article/18/1/73/853086/Ovarian-antral-folliculogenesis-during-the-human. doi: 10.1093/humupd/dmr039.
- Iacovides S, Avidon I, Baker FC. Does pain vary across the menstrual cycle? A review. Eur J Pain. 2015;19(10):1389–1405. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ejp.714. doi: 10.1002/ejp.714.
- Schoep ME, Nieboer TE, van der Zanden M, et al. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol. 2019;220(6):569.e1–569.e7.
- Maqbool R, Maqbool M, Zehravi M, et al. Menstrual distress in females of reproductive age: a literature review. Int J Adolesc Med Health. 2021;34(2):11–17. doi: 10.1515/ijamh-2021-0081.
- Vannuccini S, Rossi E, Cassioli E, et al. Menstrual Distress Questionnaire (MEDI-Q): a new tool to assess menstruation-related distress. Reprod Biomed Online [Internet]. 2021;43(6):1107–1116. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1472648321004296.
- Haywood A, Slade P, King H. Assessing the assessment measures for menstrual cycle symptoms. J Psychosom Res. 2002;52(4):223–237. doi: 10.1016/s0022-3999(02)00297-0.
- Moos RH. The development of a menstrual distress questionnaire. Psychosom Med. 1968;30(6):853–867. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5749738. doi: 10.1097/00006842-196811000-00006.
- Ross C, Coleman G, Stojanovska C. Factor structure of the modified moos menstrual distress questionnaire: assessment of prospectively reported follicular, menstrual and premenstrual symptomatology. J Psychosom Obstet Gynaecol. 2003;24(3):163–174. doi: 10.3109/01674820309039670.
- Matteson K, Scott D, Raker C, et al. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689. doi: 10.1111/1471-0528.13273.
- Vannuccini S, Clemenza S, Cassioli E, et al. Uterine fibroids, perceived stress, and menstrual distress: a key role of heavy menstrual bleeding. Reprod Sci. 2023;30(5):1608–1615. https://link.springer.com/10.1007/s43032-022-01126-3. doi: 10.1007/s43032-022-01126-3.
- Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13(3):595–605. Available from: https://www.cambridge.org/core/product/identifier/S0033291700048017/type/journal_article.
- Allen SS, McBride CM, Pirie PL. The shortened premenstrual assessment form. J Reprod Med. 1991;36(11):769–772.
- Bujang MA, Omar ED, Baharum NA. A review on sample size determination for Cronbach’s alpha test: a simple guide for researchers. Malays J Med Sci. 2018;25(6):85–99. doi: 10.21315/mjms2018.25.6.9.
- Revelle W. psych: procedures for psychological, psychometric, and personality research [Internet]. Evanston, IL: Northwestern University; 2020. Available from: https://cran.r-project.org/package=psych.
- Core Team R. R: a language and environment for statistical computing [internet]. Vienna, Austria: R Foundation for Statistical Computing. 2022. Available from: https://www.r-project.org/.
- Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull. 1959;56(2):81–105. doi: 10.1037/h0046016.
- Inayoshi R. The relationship between psychological distress in menstruation-related symptoms and well-being. J Japanese Soc Psychos Obstet Gynecol. 2022;26:338–347.
- King S. Premenstrual syndrome (PMS) and the myth of the irrational female. 2020. In C. Bobel, et al. editors. The Palgrave Handbook of Critical Menstruation Studies. Palgrave Macmillan. p. 287–302.
