Abstract
Objectives: Talin1 is a cytoskeletal protein and is localized between cells and the extracellular matrix. This study aimed to investigate the mechanism by which Talin1 affects glucose metabolism and endometrial receptivity via glucose transporter proteins-4 (GLUT-4) in patients with polycystic ovary syndrome (PCOS) and insulin resistance (IR). Methods: We examined the expression of Talin1 and GLUT4 in the receptive endometrium of PCOS-IR and control patients. GLUT4 expression was examined after silencing and overexpression of Talin1 in Ishikawa cells. We validated the interaction between Talin1 and GLUT-4 proteins using a co-immunoprecipitation (Co-IP) assay. After successfully establishing the C57BL/6j mouse model of PCOS-IR, the expression of Talin1 and GLUT-4 were examined in PCOS-IR and control mice. The effect of Talin1 on embryo implantation and the number of live births in mice were examined. Results: Our study found low expression of Talin1 and GLUT-4 in the receptive endometrium of PCOS-IR patients compared to that in control patients (p < 0.01). The level of GLUT-4 expression decreased after silencing Talin1 in Ishikawa cells and increased after overexpression of Talin1. Co-IP results showed that Talin1 interacts with GLUT-4 protein. We successfully established a PCOS-IR C57BL/6j mouse model and found that Talin1 and GLUT-4 expression in the receptive endometrium of PCOS-IR mice were lower than that in control mice (p < 0.05). In vivo experiments confirmed that the knockdown of Talin1 affects embryo implantation (p < 0.05) and live birth rate in mice (p < 0.01). Conclusions: Talin1 and GLUT-4 expression were decreased in the endometrium of PCOS-IR patients, and Talin1 may affect glucose metabolism and endometrial receptivity through GLUT4.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine and metabolic diseases in women of reproductive age, with a prevalence of 4%–8%. PCOS is one of the major causes of infertility in women and is characterized by sporadic ovulation or anovulation, polycystic changes in the ovaries, and hyperandrogenemia [Citation1]. Approximately 44%–70% of patients diagnosed with PCOS have insulin resistance (IR). Clinical characteristics related to the syndrome increase endometrial IR with impaired glucose transport and utilization, leading to chronic low-grade inflammation, immune dysfunction, uterine vascular changes, abnormal endometrial gene expression and cellular abnormalities [Citation2], and even a significantly increased chance of endometrial cancer [Citation3]. Metabolic disorder centered on IR is an important pathophysiological change in PCOS patients [Citation4] and is also the initiating factor for the aggregation of various metabolic disorders [Citation5]. As one of the target organs of insulin, the endometrium of patients with PCOS exhibits a local IR phenomenon. PCOS patients have a lower pregnancy rate and higher abortion rate, which are closely related to reduced endometrial receptivity. Therefore, treating local endometrial IR and improving endometrial receptivity are of great significance for improving the clinical pregnancy rate and reducing the abortion rate of patients with PCOS.
Talin1, a cytoskeletal protein localized between cells and extracellular matrix, is a key regulator of integrin activation [Citation6].Talin1 affects multiple cell signaling pathways and plays a key regulatory role in cell adhesion, migration, thrombus formation, angiogenesis, and tumor cell metastasis. These characteristics are similar to the cellular physiological processes of embryo implantation [Citation7]. This evidence suggests that talin1 regulates multiple signaling networks and thus plays an important role in different cellular signaling pathways. Previous studies have shown that embryos can affect Talin1 expression in the mouse endometrium [Citation8]. Talin1 binding to integrin promotes embryonic ectodermal adhesion and morphogenesis and prevents integrin β1 degradation to a certain extent [Citation9]. To date, Talin1 has not been reported in patients with PCOS.
Glucose transporter proteins-4 (GLUT-4) is an important factor in the downstream pathway of insulin signaling. As an insulin-sensitive glucose transporter, GLUT-4’s decreased expression reduces cellular glucose uptake and utilization, resulting in abnormal glucose metabolism. Endometrial cells require sufficient energy for proliferation, differentiation and maturation, and their energy source is mainly glucose metabolism [Citation10, Citation11]. Therefore, identifying the key molecules regulating GLUT-4 is key to improving endometrial receptivity in patients with PCOS and IR.
Our previous studies found that Talin1 could affect the adhesion function of endometrial epithelial cells by interacting with LASP1 and vitronectin [Citation12], and it could enhance the adhesion of endometrial cells by regulating the Ras signaling pathway, ultimately promoting embryo implantation [Citation13]. Thus, we aimed to explore the molecular mechanism of Talin1 regulation of GLUT-4 in Ishikawa cells and PCOS-IR mouse models and promote new clinical treatment strategies targeting talin1 in patients with PCOS and IR.
Methods and materials
Patient recruitment
We collected the endometrium of infertility patients with PCOS-IR and control patients aged 18-35 years from June 2019 to December 2020 at the Department of Gynecology, Fourth Affiliated Hospital of Guangxi Medical University. The endometrial samples collected were the receptive endometrium seven days after the luteinizing hormone (LH) surge of their natural menstrual cycle [Citation14]. Infertile patients with PCOS-IR meet the following conditions simultaneously: The diagnosis of PCOS met two of the three features of hyperandrogenism, menstrual disturbance, and polycystic ovarian morphology (PCOM) according to Rotterdam criteria [Citation15], and an insulin resistance index homeostatic model assessment of insulin resistance (HOMA-IR) greater than 2.69 is considered IR. Control patients were diagnosed with unexplained infertility or male infertility and did not have a diagnosis of IR or PCOS. Part of the collected endometrium was paraffin-embedded and sliced, and the other part was frozen for RNA and protein extraction. The study was conducted with the consent of all patients and approved by the Ethics Committee of the Fourth Affiliated Hospital of Guangxi Medical University (NO. KY2020003).
Cell lines
Women diagnosed with PCOS are at increased risk of endometrial cancer [Citation16], which may be related to the exposure of the endometrium to abnormally high levels of estrogen due to progesterone deficiency caused by anovulation [Citation17]. Based on the association between PCOS and endometrial cancer, we selected the endometrial cancer cell line Ishikawa for our in vitro study. Ishikawa cells were purchased from Procell Life Technology (Wuhan, China) and stored in 10% fetal bovine serum (Gibco, Auckland, NZ) and PMI-1640 (Gibco, Grand Island, NY, USA) containing 1% penicillin and streptomycin. Cells were cultured in an incubator at 37 °C and 5%CO2.
RNA extraction and real-time quantitative PCR (RT-qPCR)
RNA was extracted from the tissues and cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). We used the first- strand cDNA synthesis kit (Thermo Scientific, Vilnius, LT) to remove DNA from RNA and reverse RNA into cDNA. RT-qPCR was performed using SYBR reagent (Applied Biosystems, Vilnius, LT, USA) on an ABI7500 fluorescent PCR apparatus (10 ng of template cDNA, 1 cycle of 50 °C for 2 min and 95 °C for 2 min, and 40 cycles of 95 °C for 15 s, 55-60°Cfor 15s and 60°Cfor 30 s).GAPDH was used as the reference standard. The primer sequences were as follows: Talin1, 5′-TGCCATTTCCACAGCCTCAA-3′ (forward) and 5′-GGGTCAG AGACACCAACCAGATA-3′ (reverse); GAPDH, 5′-ATGGAAATC CCATCACCATCTT-3′ (forward) and 5′-CGCCCCACTTGAT TTTGG-3′ (reverse). The RT-qPCR results were calculated by the ΔΔCt method, and the mRNA expression of the target gene was expressed by 2-ΔΔCt.
Total protein extraction and western blot
Total protein was extracted from tissues and cells using RIPA lysis buffer (Beyotime, Shanghai, China) containing 1% protease phosphatase inhibitor (Thermo Scientific, Rockford, IL, USA). Different concentrations of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were performed based on the molecular weight of the protein. Proteins were isolated by electrophoresis and transferred to PVDF membranes. The membranes were incubated with primary antibodies against Talin1 (1:2000 14168-1-AP, Proteintech), GLUT4 (1:2000, # PA5-23052, Invitrogen), and GAPDH (1:5000, 10494-1-AP, Proteintech). After the reaction of the membranes with labeled secondary antibodies (1:10000, C31460100, Invitrogen), signals were detected using ECL substrate (Epizme, Shanghai, China) on Bio-Rad ChemiDoc.
Immunohistochemistry (IHC)
The tissues were fixed with 4% paraformaldehyde, dehydrated, embedded, and sliced. The sections were dewaxed and treated with citrate buffer to repair the antigen, followed by 3% hydrogen peroxide to block the endogenous peroxidase activity. The sections were incubated with primary antibodies against Talin1 (1:200 14168-1-AP, Proteintech) and GLUT4 (1:100, # PA5-23052, Invitrogen), then with secondary antibodies. The nuclei were stained with hematoxylin after the sections were stained satisfactorily. Finally, dehydration, transparency, and sealing were performed. Five visual fields were randomly selected from each section under a microscope, and the average H-score of each visual field was used for comparison.
Cell transfection
siRNA was synthesized by RiboBio (Guangzhou, China), and the si-Talin1 (human-specific) sequence was 5′-GGAGAUGGUUA CCAAGUCAAA-3′. One day before transfection, Ishikawa cells were seeded in 6-well plates to ensure that the confluence of the cells reached 70%–90%. siRNA was transfected using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The plasmids were purchased from Tsingke (Beijing, China). The plasmid was transfected using Optimi Lipofectamine 3,000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Next, 48 h after transfection, cells were collected for RT-qPCR and western blot analysis.
Animal models
Animal experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 202207001). C57BL/6j mice aged six weeks were selected for modeling after one week of adaptation. PCOS-IR mice were administered letrozole (LTZ) dissolved in 1% sodium carboxymethyl cellulose (CMC) by intragastric administration at 1 mg/kg/d for 21 days. They were fed a high-fat diet, while the control group was fed the same amount of 1% sodium carboxymethyl cellulose and an ordinary diet. After 7 days of the administration, the estrous cycle was observed by daily vaginal discharge smear, and the estrous cycle’s disturbance indicated the PCOS model’s success. After 21 days of modeling, fasting blood samples were collected from the mice in the modeling and control groups, and fasting blood glucose, fasting insulin, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone were detected. After the mice were sacrificed, ovarian tissue was subjected to hematoxylin-eosin (HE) staining. One part of the uterus was used for RNA and protein extraction, and the other was fixed, dehydrated, embedded, and sliced.
Talin1 knockdown in vivo
Twelve female C57BL/6j mice and six males aged 8–12 weeks were selected. During the female estrous cycle, female and male mice were fed together in a ratio of 2:1 at 18 o’clock. The next day, at 8 o’clock, the occurrence of a vaginal plug was determined as 0.5 days of gestation. On day 2 of gestation, female mice were anesthetized. They injected into the uterine cavity with the animal transfection reagent and siRNA mixture, si-Talin1 on one side and si-NC on the other. Si-Talin1 (mouse-specific) was synthesized by RiboBio (Guangzhou, China), and the sequence was GGATGTAGCTGGTGGACTA. On the eighth day of gestation, six female mice were sacrificed, and the uteri of the mice were taken to observe the number of embryos in the uterus on both sides.
Twelve C57BL/6j female mice aged 8–12 weeks were selected. On the second day of gestation, female mice were anesthetized and injected with an animal transfection reagent and siRNA mixture into the uterine cavity. Six mice were injected with si-Talin1 on both sides of the uterine cavity, and the other six were injected with si-NC on both sides of the uterine cavity. The number of live births was recorded after a natural delivery.
Co‑immunoprecipitation (Co‑IP)
According to the Co-IP kit instructions (Thermo Scientific, Rockford, IL, USA), 10 μg Talin1 antibody and 20 μl Coupling resin reaction for two hours. Ishikawa cells were lysed using the IP Lysis/Wash Buffer, and 1 mg lysed cells were reacted with 80 μl control agarose resin for 1 h. Then, the bait is bound to the target protein, and finally, the resin is eluted to obtain the immune complex. Immune complexes were examined using western blotting.
Statistical analysis
SPSS 26.0 software and GraphPad Prism 8 software was used for statistical analysis. Data are expressed as the mean ± standard deviation. Two-tailed Student’s t-test was used to compare the two groups. Statistical significance was set at p < 0.05.
Results
Demographics
In this study, endometrial tissues were collected from 15 patients with PCOS-IR and 15 normal control patients. summarized the demographic characteristics of the two groups. Based on the results of RT-qPCR, pearson correlation analysis showed that the expression of Tlian1 in endometrial samples was negatively correlated with BMI (r=-0.165, p = 0.000), fasting blood glucose (FBG) (r=-0.662, p = 0.000), fasting insulin(r=-0.588, p = 0.001), LH(r=-0.482, p = 0.007), LH/FSH(r=-0.502, p = 0.005), and HOMA-IR(r=-0.612, p = 0.000). There was no correlation between Talin1 expression and age, FSH, or testosterone. PCOS is classified according to Rotterdam criteria as phenotype A (hyperandrogenism plus ovulatory dysfunction plus PCOM), phenotype B (hyperandrogenism plus ovulatory dysfunction), phenotype C (hyperandrogenism plus PCOM) and phenotype D (ovulatory dysfunction plus PCOM) [Citation18].Of the 15 patients with PCOS-IR in our study, seven patients had phenotype A, four had phenotype B, three had phenotype C, and one had phenotype D.
Table 1. Baseline characteristics of patients in two groups.
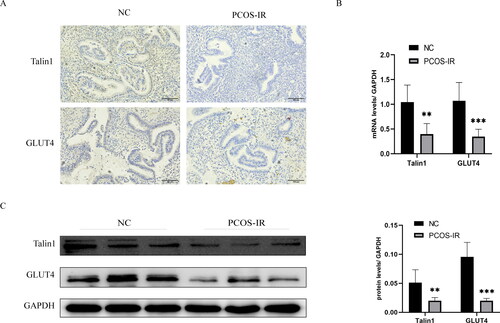
Low expression of Talin1 and GLUT4 in the endometrium of PCOS-IR patients
IHC results indicated that Talin1 and GLUT4 were expressed in both endometrial epithelium and stromal cells, mainly in the cytoplasm (). RT-qPCR results showed that the mRNA expression of Talin1 and GLUT4 in PCOS-IR patients were lower than in control patients (). Western blot analysis showed that the protein expression of Talin1 and GLUT4 in the endometrium of PCOS-IR patients were lower than in control patients ().
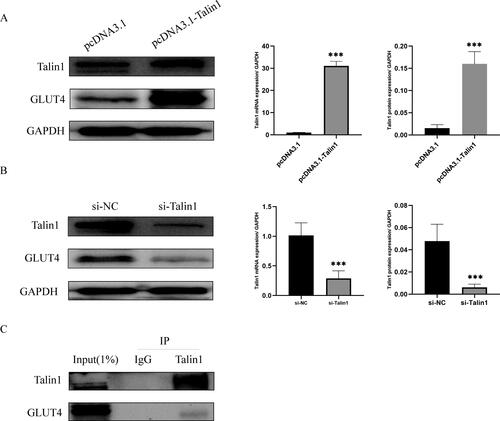
Talin1 affects GLUT4 expression
We silenced and overexpressed Talin1 in Ishikawa cells, and RT-qPCR and western blotting verified the silencing and overexpression efficiency. GLUT4 expression decreased after Talin1 silencing and increased after overexpression (). Co-IP assay showed that GLUT4 protein could be immunoprecipitated with Talin1 antibody as bait, indicating that Talin1 and GLUT4 proteins interacted ().
Establishment and verification of the PCOS-IR mouse model
After modeling, the estrous cycle of mice in the model group was disturbed and remained in the diestrus period, whereas the estrous cycle of mice in the control group was normal (). HE staining results showed that compared with the control group, cystic follicles were found in the ovarian tissue of mice in the model group, indicating that the PCOS model was successful (). Fasting blood glucose, fasting insulin, and HOMA-IR of mice in the model group were significantly higher than those in the control group. The mean value of HOMA-IR in the model group was greater than 2.69, indicating the success of the IR model (). Compared to the control group, the model mice had no significant difference in serum FSH levels. However, serum LH and T levels and LH/FSH levels were elevated ().
Figure 3. The role of Talin1 in endometrium of PCOS-IR was examined in vivo. (A) The estrus cycles in model and control mice; (B) ovarian changes in model and control mice; (C) Fasting blood glucose, fasting insulin, and HOMA-IR were measured in model and control mice; (D) FSH, LH, and T were measured in model and control mice; (E) Expression of Talin1 and GLUT4 in endometrium of PCOS-IR and control mice; (F) Effect of Talin1 on embryo implantation and live birth number in mice. *p < 0.05, **p < 0.01, ***p < 0.001.
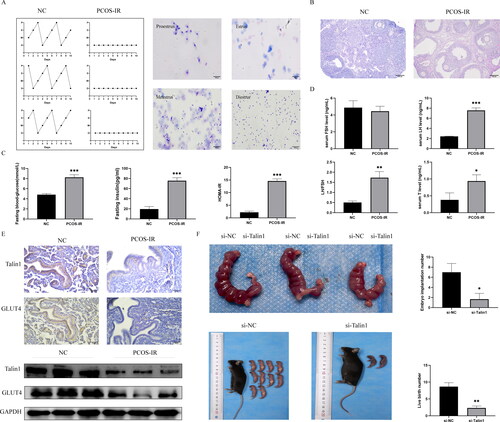
Low expression of Talin1 and GLUT4 in the endometrium of PCOS-IR mice
IHC results indicated that Talin1 and GLUT4 expression was lower in the endometrium of PCOS-IR mice than in the control mice. Western blotting results showed that the protein expression levels of Talin1 and GLUT4 were lower in PCOS-IR mice than in control mice ().
Effect of Talin1 on embryo implantation and live birth number in mice
After silencing Talin1 on one side of the uterus of pregnant mice, the number of embryos on one side of the uterus with Talin1 silencing was significantly lower than that on the other side of the uterus (). The number of live births after silencing Talin1 was significantly lower in the bilateral uterus of pregnant mice than in that of mice in the si-NC group ().
Discussion
PCOS is a common gynecological and endocrine disorder in women of reproductive age, with typical symptoms of hyperandrogenemia, ovarian dysfunction, sparse ovulation, and polycystic ovarian patterns. Increased risk of endometrial hyperplasia and endometrial cancer due to anovulation and decreased progesterone [Citation19]. However, the pathogenesis of PCOS remains unclear. IR is a common condition in polycystic ovary syndrome, aggravated by accumulation of adipose tissue associated with hyperandrogen [Citation20], and is involved in the pathogenesis and progression of polycystic ovary syndrome [Citation21]. IR and hyperinsulinemia affect the local secretion of insulin-like growth factor and insulin-like growth factor-binding proteins in the endometrium, resulting in abnormal endometrial proliferation and functional defects. Women with PCOS are at increased risk of pregnancy-induced hypertension, pre-eclampsia, gestational diabetes, and premature delivery, and offspring of women with PCOS are at increased risk of future metabolic and reproductive dysfunction [Citation22]. Impaired decidual trophoblast invasion and alterations in endovascular trophoblast invasion have been observed in women with PCOS, which may be associated with IR and hyperandrogenemia [Citation23, Citation24].We found that Talin1 expression was diminished in the endometrium of PCOS-IR patients, interacted with GLUT4, and affected GLUT4 expression and glucose metabolism in the endometrium. Furthermore, Talin1 can affect the receptivity of the endometrium in vivo and thus the number of embryos implanted and live births in mice.
The TLN gene encodes Talin, and Talin1, as its main component, is a key regulatory factor in activating integrin [Citation25]. Talin1 has been extensively studied in the field of reproductive system. Wang et al. [Citation26] found that Talin1 was significantly overexpressed in endometrial tissues and cells in adenomyosis, which may contribute to the development of adenomyosis by inducing epithelial-mesenchymal transition (EMT) to promote the migration and invasion of endometrial cells. Another study [Citation27] found that Talin1 overexpression may stimulate endometrial stromal cell proliferation and neovascularization in adenomyosis and promote the growth and survival of ectopic lesions. A recent study [Citation28] found that Talin1 expression was significantly increased in the fallopian tubes and villi of women with tubal pregnancies. Silencing Talin1 inhibited the invasion and migration of human chorionic trophoblast cells. It downregulated N-cadherin expression Focal adhesions (FAs) are protein mechanisms essential for cell adhesion, migration, and differentiation, and Talin1 regulates FAs to affect cell adhesion and signal transduction [Citation29]. Current studies suggest that talin1 is related to endometrial receptivity. Previous studies have found that the presence of embryos affects talin1 expression in the mouse endometrium, and a certain concentration of HCG can regulate its expression. Our previous study found that Talin1 can enhance endometrial cell adhesion and ultimately promote embryo implantation [Citation12, Citation13]. The typical clinical symptom of PCOS is hyperandrogen, and hyperandrogen can aggravate insulin resistance. However, this study f found no correlation between Talin1 and testosterone but correlated with FBG and fasting insulin, suggesting that Talin1 may not affect testosterone expression but affect insulin resistance in PCOS. Our study found low expression of Talin1 was found in the endometrium of PCOS-IR patients and mice. In vivo experiments showed that Talin1 could affect embryo implantation and the number of live births in mice, which confirmed the role of Talin1 in endometrium receptivity.
As a glucose transporter, GLUT-4 is involved in rapid glucose uptake by various cells to maintain glucose homeostasis. Previous studies have suggested that abnormal endometrial GLUT4 expression may be responsible for increased miscarriage rates in women with type 2 diabetes or PCOS [Citation30–32]. Several studies have found reduced mRNA and protein levels of GLUT-4 in the endometrium of PCOS patients compared to control patients[Citation33–35], whereas GLUT4 mRNA and protein levels in the endometrium of PCOS-IR patients were reduced to a greater extent [Citation36, Citation37]. Therefore, abnormal GLUT-4 expression may be an important mechanism of endometrial IR in patients with PCOS. GLUT4 plays a key role in endometrial receptivity. Long et al. [Citation38] showed that inhibiting GLUT4 expression in the mouse uterus affects embryo development and implantation. Our study found low GLUT4 expression in the endometrium of both PCOS-IR patients and PCOS-IR mice, similar to the results of previous studies. The Tlian1 expression in the endometrium was negatively correlated with FBG, while GLUT4 was involved in glucose uptake. In vitro experiments also revealed that silencing and overexpression of Talin1 affected GLUT4 expression and that Talin1 protein and GLUT4 protein interacted, suggesting that Talin1 may affect glucose metabolism and endometrial tolerance in patients with PCOS-IR by regulating GLUT4.
A strength of this study is that we have an experienced gynecologist to screen eligible cases and collect endometrium, avoiding bias due to lax case enrollment. Meanwhile, we revealed from tissue, in vitro, and in vivo experiments that Talin1 plays a role in PCOS-IR and may act through GLUT4. One of the limitations in this study was that we recruited endometrium from control patients, whereas women without any known pathological conditions may be more suitable. In addition, due to the limited number of cases included in this study, the correlation between PCOS phenotype and Talin1 expression could not be analyzed.
PCOS is often complicated by IR and endometrial receptivity insufficiency; however, the underlying mechanism remains unclear. This study investigated the causes of abnormal glucose metabolism and endometrial receptivity insufficiency in patients with PCOS-IR. In conclusion, we found that Tain1 and GLUT-4 expression was reduced in the endometrium of PCOS-IR patients and that Talin1 may affect glucose metabolism and endometrial receptivity in PCOS-IR patients through GLUT4.
Acknowledgments
We would like to thank Dr Xiaomou Wei and Baoyu He for their assistance with laboratory techniques.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data supporting the findings in this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kovacs G, Clarke S, Burger H, et al. Surgical or medical treatment of polycystic ovary syndrome: a cost-benefit analysis. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2002;16(1):1–7.
- Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update. 2021;27(3):584–618.
- Johnson JE, Daley D, Tarta C, et al. Risk of endometrial cancer in patients with polycystic ovarian syndrome: a meta‑analysis. Oncol Lett. 2023;25(4):168.
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034.
- Goodarzi M, Korenman S. The importance of insulin resistance in polycystic ovary syndrome. Fertil Steril. 2003;80(2):255–258. doi: 10.1016/s0015-0282(03)00734-9.
- Gough RE, Goult BA-O. The tale of two talins - two isoforms to fine-tune integrin signalling. FEBS Lett. 2018;592(12):2108–2125.
- Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int Rev Cell Mol Biol. 2011;289:117–147. doi: 10.1016/B978-0-12-386039-2.00004-3.
- Shen Y, Qin A. Regulation of embryonic signal on Talin1 in mouse endometrium. Reprod Sci. 2019;26(9):1277–1286. doi: 10.1177/1933719118815584.
- Liu J, He X, Qi Y, et al. Talin1 regulates integrin turnover to promote embryonic epithelial morphogenesis. Mol Cell Biol. 2011;31(16):3366–3377. doi: 10.1128/MCB.01403-10.
- von Wolff M, Ursel S, Hahn U, et al. Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2003;88(8):3885–3892. doi: 10.1210/jc.2002-021890.
- Medina R, Meneses A, Vera J, et al. Differential regulation of glucose transporter expression by estrogen and progesterone in Ishikawa endometrial cancer cells. J Endocrinol. 2004;182(3):467–478. doi: 10.1677/joe.0.1820467.
- Li J, Lin J, Yang Y, et al. Talin1 regulates the endometrial epithelial cell adhesive capacity by interacting with LASP1 and vitronectin. Reprod Biol. 2020;20(2):229–236. doi: 10.1016/j.repbio.2020.02.006.
- Chen S, Liu B, Li J, et al. Talin1 regulates endometrial adhesive capacity through the ras signaling pathway. Life Sci. 2021;274:119332. doi: 10.1016/j.lfs.2021.119332.
- Yang Y, Chen X, Saravelos SH, et al. HOXA-10 and E-cadherin expression in the endometrium of women with recurrent implantation failure and recurrent miscarriage. Fertil Steril. 2017;107(1):136–143.e2. doi: 10.1016/j.fertnstert.2016.09.016.
- Revised 2003. Consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
- Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78(8):782–5.
- Shafiee MN, Chapman C, Fau- Barrett D, et al. Reviewing the molecular mechanisms which increase endometrial cancer (EC) risk in women with polycystic ovarian syndrome (PCOS): time for paradigm shift? Gynecol Oncol. 2013;131(2):489–192.
- Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668–680. doi: 10.1016/S2213-8587(22)00163-2.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748–758.
- Dumesic DA, Abbott DH, Sanchita S, et al. Endocrine-Metabolic dysfunction in polycystic ovary syndrome: an evolutionary perspective. Curr Opin Endocr Metab Res. 2020;12:41–48.
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030.
- Palomba S, de Wilde MA, Falbo A, et al. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–592.
- Palomba S, Russo T, Fau- Falbo A, et al. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod. 2013;28(10):2838–2847.
- Palomba S, Russo T, Fau- Falbo A, et al. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. J Clin Endocrinol Metab. 2012;97(7):2441–2449.
- Haydari Z, Shams H, Jahed Z, et al. Kindlin assists talin to promote integrin activation. Biophys J. 2020;118(8):1977–1991. doi: 10.1016/j.bpj.2020.02.023.
- Wang YY, Duan H, Wang S, et al. Talin1 induces Epithelial-Mesenchymal transition to facilitate endometrial cell migration and invasion in adenomyosis under the regulation of microRNA-145-5p. Reprod Sci. 2021;28(5):1523–1539. doi: 10.1007/s43032-020-00444-8.
- Wang YY, Duan H, Wang S, et al. Upregulated Talin1 synergistically boosts β-estradiol-induced proliferation and pro-angiogenesis of eutopic and ectopic endometrial stromal cells in adenomyosis. Reprod Biol Endocrinol. 2021;19(1):70. doi: 10.1186/s12958-021-00756-7.
- Qiu P, Lin X, Deng G. [Talin1 is highly expressed in the fallopian tube and chorionic villi to promote trophoblast invasion in tubal pregnancy]. Nan Fang Yi Ke Da Xue Xue Bao. 2022;42(4):610–617.
- Dedden D, Schumacher S, Kelley CF, et al. The architecture of Talin1 reveals an autoinhibition mechanism. Cell. 2019;179(1):120–131.e13. doi: 10.1016/j.cell.2019.08.034.
- Zhai J, Liu CX, Tian ZR, et al. Effects of metformin on the expression of GLUT4 in endometrium of obese women with polycystic ovary syndrome. Biol Reprod. 2012;87(2):29.
- Schulte MM, Tsai JH, Moley KH. Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod Sci. 2015;22(1):6–14. doi: 10.1177/1933719114561552.
- Alam F, Islam MA, Khalil MI, et al. Metabolic control of type 2 diabetes by targeting the GLUT4 glucose transporter: intervention approaches. Curr Pharm Des. 2016;22(20):3034–3049. doi: 10.2174/1381612822666160307145801.
- Ujvari D, Hulchiy M, Calaby A, et al. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod. 2014;29(7):1526–1535. doi: 10.1093/humrep/deu114.
- Ormazabal P, Romero C, Quest A, et al. Testosterone modulates the expression of molecules linked to insulin action and glucose uptake in endometrial cells. Horm Metab Res. 2013;45(9):640–645. doi: 10.1055/s-0033-1345176.
- Fornes R, Ormazabal P, Rosas C, et al. Changes in the expression of insulin signaling pathway molecules in endometria from polycystic ovary syndrome women with or without hyperinsulinemia. Mol Med. 2010;16(3–4):129–136. doi: 10.2119/molmed.2009.00118.
- Mioni R, Chiarelli S, Xamin N, et al. Evidence for the presence of glucose transporter 4 in the endometrium and its regulation in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89(8):4089–4096. doi: 10.1210/jc.2003-032028.
- Mozzanega B, Mioni R, Granzotto M, et al. Obesity reduces the expression of GLUT4 in the endometrium of normoinsulinemic women affected by the polycystic ovary syndrome. Ann N Y Acad Sci. 2004;1034:364–374. doi: 10.1196/annals.1335.038.
- Long Y, Wang YC, Yuan DZ, et al. GLUT4 in mouse endometrial epithelium: roles in embryonic development and implantation. Front Physiol. 2021;12:674924. doi: 10.3389/fphys.2021.674924.