Abstract
Unilateral non-hemorrhagic adrenal infarction (NHAI) is a very uncommon cause of acute abdomen in pregnancy. Diagnosis is highly challenging due to its rarity, heterogeneity of clinical presentation, and inconclusiveness of the initial workup. Timely recognition is pivotal to ensuring optimal outcomes. Here we describe a case of spontaneous unilateral NHAI diagnosed in a singleton pregnant woman at 32 weeks’ gestation at our centre and provide the findings of an extensive literature review on the topic. We identified 22 articles describing 31 NHAI cases in 30 obstetric patients: NHAI occurs more frequently on the right side and in the third trimester, and diagnosis is formulated more than 24 h after clinical presentation in 50% of cases; second-level imaging is always necessary to reach a definitive diagnosis and start appropriate treatment. A high degree of clinical suspicion is needed to promptly recognize NHAI in pregnancy, thus allowing appropriate multidisciplinary management and timely treatment initiation. Promotion of knowledge and awareness of NHAI as a potential cause of acute abdomen in pregnancy is mandatory to improve clinical practice and, ultimately, perinatal outcomes.
Introduction
Adrenal infarction (AI) in pregnancy is a very rare, although potentially life-threatening, clinical entity [Citation1]. AI can be hemorrhagic or non-hemorrhagic [Citation2], with the latter being much less frequent. Although antiphospholipid-antibody syndrome has been identified as one of the main risk factors for AI [Citation3], pregnancy, with its hypercoagulability state [Citation4], represents a condition at risk, particularly for non-hemorrhagic AI (NHAI) [Citation1].
Diagnosis of NHAI is challenging due to the rarity of the condition as well as the variability of clinical manifestation, especially in unilateral cases with no adrenal insufficiency [Citation5]. The inconclusiveness of the initial workup further increases the diagnostic challenge, leading clinicians toward more common non-obstetric causes of acute pain in pregnancy, such as biliary or renal colic. Yet, prompt diagnosis is pivotal to implementing appropriate therapeutic management and avoiding clinical deterioration [Citation6].
So far, only case reports or small case series have been reported on the topic, and an extensive review of the literature is lacking [Citation1,Citation2,Citation5,Citation7–11]. Here we describe a case of spontaneous unilateral NHAI in a pregnant woman and perform a literature review to provide an in-depth update on this rare clinical entity.
Methods
Case report
We retrospectively collected the medical records of the woman diagnosed with acute spontaneous unilateral NHAI at our university center. This was the first case of AI identified at our center since its establishment in 1981. A written informed consent was obtained from the patient to use her anonymized clinical information for the purpose of this reporting. Since only anonymized data were employed, approval of the Ethical Committee of Fondazione IRCCS San Gerardo dei Tintori was waived.
Information sources and search strategy
We obtained all articles related to our topic from the international electronic bibliographic databases PubMed/MEDLINE, ISI Web of Knowledge, and Cochrane. The articles were identified using a combination of MeSH terms including the keywords “pregnancy”, “adrenal”, “infarction”. The search was limited to studies reported in English, French, and Italian. We did not employ temporal or publication status limits to restrict our search. Once an article was considered relevant, the full text was retrieved. The references included in the selected articles were also reviewed for related citations. Data collection and analysis were performed between 1 February 2022 and 31 May 2022.
Study selection and data retrieval
Three independent researchers (S.O., F.F., E.M.) screened the titles and abstracts obtained to select the most relevant articles. The three researchers selected the final studies to be included in the review after applying the eligibility criteria independently. Any conflict between researchers was resolved by senior consultants’ assessment (I.C., P.V.). Studies were considered eligible for review if they investigated clinical and radiological presentation in obstetric patients with a diagnosis of unilateral or bilateral NHAI. The following data were collected: authors, year of publication, maternal age at diagnosis, gestational age (GA; weeks, days) at diagnosis, obstetric history, side of NHAI, presenting symptoms and vitals, results of blood workup, type and findings of imaging, treatment, clinical course, GA at and mode of delivery, and follow-up.
Results
Clinical case
A 21-year-old woman, gravida 2 para 1, with a spontaneously conceived pregnancy presented at 322/7 weeks’ gestation to the Emergency Department (ED) of a nearby mother and child hospital for uterine contractions. Her obstetric history included an uncomplicated pregnancy two years before with a term spontaneous vaginal delivery of a healthy male neonate. The patient reported no previous use of hormonal contraception and family history was unremarkable. Her pregestational Body Mass Index (BMI) was 29 Kg/m2. A two-days hospitalization had occurred two weeks before for intravenous iron administration due to severe iron deficient anemia (Hb 8.9 g/dL).
On hospital presentation, vital signs were normal; irregular uterine contractions were identified, and a transvaginal ultrasound scan showed a shortened cervix of 20 mm. The patient was admitted and given intravenous tocolysis and intramuscular steroids for fetal lung maturation. Nasopharyngeal swab for SARS-CoV-2 detection performed upon admission was negative.
Ten hours after admission, the woman started complaining of a sudden-onset right upper quadrant and flank pain, associated with nausea and two bouts of non-bloody emesis. She was afebrile and had moderate right upper quadrant and flank tenderness. There were no contractions and cervical length was stable. She was administered intravenous acetaminophen, with transient resolution of the pain, which subsequently recurred and worsened. An abdominal ultrasound scan performed 5 h after pain’s onset was negative.
Considering the progressive worsening of the pain and its unresponsiveness to intravenous analgesics, the woman was transferred to our second-level hospital. Vital signs at admission showed mild hypertension (130/80 mmHg) and tachycardia (95 bpm), with a slightly elevated respiratory rate (RR 24) and normal oxygen saturation (SpO2 100%); she was afebrile. Clinical examination revealed a severe right upper quadrant and flank tenderness with rebound pain. The absence of uterine contractions and a 20 mm-long cervix were confirmed. The obstetric ultrasound was regular. Electrocardiogram and chest X-ray had negative results. Also, arterial gas analysis was unremarkable. Blood laboratory analyses were notable only for mild leukocytosis and anemia (). D-dimer was 1516 ng/mL, normal for the patient’s gestational age[Citation12]. Urine analysis was unrevealing. An abdominal ultrasound scan was performed (12 h after pain’s onset), which showed a 3-mm right perirenal fluid flap (). The right kidney’s appearance was normal, and there was no ureteral dilation or urolithiasis; Doppler signal in the right renal vein was regular. The woman was administered analgesics, which allowed for temporary pain control until a new episode of acute right flank pain associated with emesis occurred 4 h later. The patient underwent another scan (17 h after pain’s onset): the right perirenal fluid flap was stable and a new, minimal periduodenal fluid component was recognized ().
Figure 1. (A, B). Abdominal ultrasound scans. Minimal perirenal (1A) and periduodenal (1B) fluid (yellow arrows).
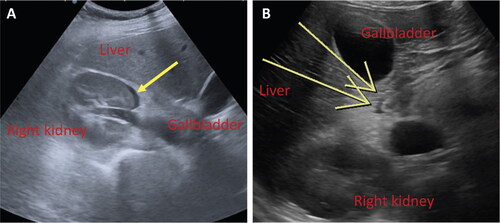
Table 1. Blood tests at admission.
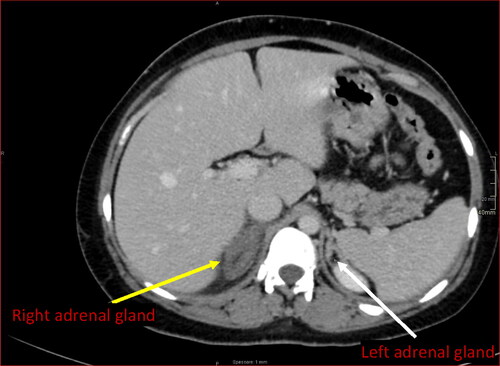
Being the sonography inconclusive, the woman’s pain was severe and only transiently responsive to analgesics, and a definitive diagnosis was absent, a computed tomography (CT) scan with contrast was performed (21 h after pain’s onset). The scan revealed an enlarged and edematous right adrenal gland with preserved morphology and no enhancement following intravenous contrast; stranding at ipsilateral peri-adrenal and peri-para-renal fat was also identified (), with no evidence of bleeding. The left adrenal gland was normal. Altogether, the findings suggested a diagnosis of right unilateral NHAI.
Figure 2. CT scan with contrast. CT scan displays an enlarged and edematous right adrenal gland with preserved morphology, no enhancement following intravenous contrast and inflammatory peri-adrenal fat stranding (yellow arrow). Note usual enhancement of the contralateral adrenal gland (white arrow).
Endocrinologist and haematologist consultations were requested. Doppler ultrasound of lower extremities was performed and turned out negative. Analgesics and prophylactic anticoagulation with enoxaparin 4000 IU once a day were established, with complete pain remission in 24 h (48 h after pain onset). displays the findings of endocrinological and thrombophilia screening. Clinical and biochemical follow-up showed the absence of adrenal insufficiency.
Table 2. Endocrinological and thrombophilia and screening.
The patient was discharged after 8 days of hospitalization. Spontaneous onset of labor occurred at 374/7 weeks’ gestation and the woman gave birth vaginally to a healthy female neonate of 2805 grams. She was discharged on day 3 postpartum with 4000 IU enoxaparin to be continued for 6 weeks. An endocrinological follow-up 6 weeks after discharge showed regular adrenal function.
Review of the literature
We found 22 articles describing a total of 31 NHAI cases that occurred in 30 obstetric patients (). The median maternal age was 25 years (IQR, 24–29.8), with two (6.7%) women >35. Three (10%) patients had a BMI ≥30 kg/m2. One (3.3%) case smoked during pregnancy, and another had a history of ulcerative colitis. There were two twin pregnancies.
Table 3. Review of cases of NHAI in obstetric patients.
In 23(74.2%) cases, the NHAI occurred after 28 weeks gestation. There was one postpartum event (day 1, n. 14). NHAI was unilateral in 28 (90.3%) cases, with involvement of the right gland in 21 (75%). The remaining three cases were bilateral: two simultaneous (n. 11 and 21) and one sequential (n. 26, 27).
Pain was identified as presenting symptom in all cases, with upper quadrant and/or flank localization in 29/31 events. Accompanying nausea and vomiting was described in 14 (45.2%) cases. Prodromal symptoms were recognized in 4 women (n. 1, 3, 5, and 17) and preempted the diagnosis of NHAI by a time interval ranging from a few hours to 2 weeks. Pyelonephritis was suspected in one case (n. 3), whereas in the remaining three pain was thought to be of colic origin. In one case (n. 26), a condition of painful myoma syndrome was initially suspected [Citation1].
Vitals at presentation were reported in 54.8% of events: 8 (47.1%) women showed abnormal vitals, with mild tachycardia and tachypnea being the most common (n = 5 each). Fever was present only in one case (n. 15). Blood tests were normal in 15/29 cases; mild-to-moderate leukocytosis was described in 13 (44.8%) and slightly-to-modestly increased C-reactive protein (CRP, median 5.1 mg/dL, IQR 3.1-7.5 mg/dL) in 6 (20.7%).
Abdominal ultrasound was employed as first-line imaging in 27 (87.1%) events, with an inconclusive report in 19 (70.4%). Abnormal findings included swollen adrenal gland (n. 10 and n. 6), peri-adrenal fluid (n. 10), trace fluid in the Morrison’s pouch (n. 26), gallbladder sludge (n. 5 and n. 17), and pyelocaliceal dilation with evidence of kidney stones (n. 8). Twenty-two women underwent a CT scan, with the use of contrast in 20/22. In all cases, an enlarged hypodense adrenal gland was recognized. MRI was performed in 19 women; it was an adjunction to the CT scan in 10 of them.
Analgesics were the first-line approach in 26/30 cases, and anticoagulant therapy was employed after diagnosis of NHAI in 27 (90%). The three patients with no anticoagulation had been retrospectively diagnosed by radiological imaging review (n. 18, 19, and 26).
Four cases needed cortisone supplementation due to adrenal insufficiency (n. 1–3, 11); only one of them presented a bilateral event (n. 11). The second bilateral NHAI case (n. 21) showed normal adrenal function, likely due to just a minor ischemic involvement of the contralateral gland. Similarly, the woman with metachronous bilateral involvement did not require cortisone supplementation due to the resolution of the first ischemic event (n. 26) by the time the second occurred (n. 27).
Complete pain remission allowing pregnancy’s continuation was obtained in 19/30 pregnant women, whereas 7 (23.3%) required immediate delivery (n = 5 urgent cesarean sections, CS, and n = 2 induction of labor) for persistent pain unresponsive to analgesics.
The median gestational age at delivery was 37 weeks (IQR 36–385/7 weeks, min-max 32–41;), with 7 (36.8%) preterm births <37 weeks. Results of thrombophilia screening were positive in 12/26 cases, and the most common abnormal finding was factor VIII elevation (n = 4 cases), which, in one case (n. 20), was also confirmed 3 months postpartum. Atrophy of the affected adrenal gland was identified in 5/9 patients with an available follow-up radiological investigation.
Data regarding anticoagulation management after hospital discharge were available in 18 (60%) women. Either low molecular weight heparin or warfarin were employed, for a duration of time ranging from 2 weeks to 11 months. Only the patient with simultaneous bilateral NHAI (n. 11) showed persistent adrenal insufficiency requiring cortisol supplementation at 4 months postpartum.
Discussion
Here we describe a rare case of spontaneous acute unilateral NHAI in a pregnant woman in the third trimester with a favorable outcome. We also provide an extensive review of this condition to promote knowledge and increase awareness among obstetricians and frontline healthcare workers managing pregnant women with an acute abdomen. AI is a very rare cause of non-uterine abdominal or flank pain in pregnancy. It can be hemorrhagic or non-hemorrhagic [Citation2], with NHAI being far less common than the hemorrhagic form and, thus, more rarely suspected.
The first case of AI in pregnancy has been reported in 1936 [Citation13]; since then, only thirty-one cases of NHAI in pregnancy have been described. The actual prevalence of NHAI during gestation is still not known, although a recent retrospective review of MRI examinations in pregnant women with acute abdominal pain has reported a figure of 1.3% [Citation1].
Adrenal vein thrombosis is thought to be the primary event in both HAI and NHAI, with gland hemorrhage occurring during the reperfusion phase of necrotic or damaged vessels after thrombosis [Citation13–15]. A recently published retrospective review of adrenal vein thrombosis in pregnancy has identified a prevalence of 1.5 per 10,000 births [Citation16].
The anatomy of the adrenal gland makes it prone to infarction in hypercoagulable states. The arterial supply is rich, but the venous drainage is limited to a single vein [Citation17]. Thus, conditions characterized by a pro-thrombotic status, such as antiphospholipid-antibody syndrome [Citation3], inflammatory bowel diseases [Citation18,Citation19], and pregnancy are at increased risk for AI [Citation4,Citation20]. Additional pregnancy-related risk factors include heightened gland stimulation and local blood flow [Citation21] and reduced venous drainage due to the compression from the gravid uterus, which all become more marked during the third trimester. Of note, we identified 23 out of 31 (74.2%) cases occurring >28 weeks’ gestation. In our patient, the event happened at 32 weeks.
AI can be unilateral or bilateral, with bilateral cases mostly identified among individuals with antiphospholipid-antibody syndrome [Citation3].
When unilateral, AI more commonly occurs in the right gland, due to a short, direct venous drainage into the inferior vein cava, which favors venous stasis and thrombosis. This is particularly true during pregnancy for the mechanical compression caused by the dextroposition of the uterus [Citation5,Citation16]. Among 31 NHAI cases identified in our review, 22 (71%) saw involvement of the right gland, as it was in our patient. Diagnosis of AI is challenging due to the rarity of the condition as well as the variability of clinical manifestation, especially in unilateral cases with no biological signs of adrenal insufficiency [Citation5,Citation9].
Unilateral AI can present with acute-onset, severe, upper right or left abdominal quadrant and/or flank pain non-responsive to analgesics and usually associated with emesis. This is what we observed in our case. However, the clinical presentation of unilateral AI may vary from patient to patient. This further increases the difficulty in correctly diagnosing unilateral AI by pointing to other more common non-obstetric causes of acute pain, including biliary or renal colic, cholecystitis, pyelonephritis, appendicitis, pneumonia, or pleuritis. Placental abruption, uterine rupture, pulmonary embolism, acute pancreatitis, gonadic vein thrombosis, and ovarian torsion should also be included in the differential diagnosis. In some cases, uterine contractions can be present, thus deceitfully suggesting threatened preterm labor [Citation5]. It is unclear whether the threatened preterm labor diagnosed in our patient was a prodromal sign of the unilateral NHAI or a separate event.
A challenge in diagnosing unilateral AI is additionally increased by the inconclusiveness of the initial workup. Blood laboratory analyses can identify a mild reactive leukocytosis in some cases, as we observed in our patient.
Ultrasonography may be limited because of the challenging location of the gland, the patient’s habitus, and the gravid uterus. Rarely, a small amount of perirenal fluid can be identified [Citation2,Citation10], as occurred in our patient but only at the second scan performed 12 h after pain’s onset al. so, in a few cases a swollen adrenal gland has been recognized [Citation10]. In turn, both CT scans and MRI can provide diagnostic information [Citation22–24]. However, potential fetal risks related to ionizing radiation and iodinated contrast material exposure contra indicate CT use in favor of MRI before 25 weeks’ gestation. Yet, MRI is usually not available in an emergency context and the use of gadolinium-based contrast enhancement during pregnancy is still controversial [Citation25,Citation26], thus making CT scan the most frequently performed imaging in these cases [Citation10]. Considering the advanced gestational age at the presentation of our case and the unavailability of MRI in the ED, we decided to perform a contrast-enhanced CT scan, which showed an enlarged hypodense adrenal gland without hyperenhancement and inflammatory peri-adrenal fat stranding.
The challenge in correctly identifying unilateral AI explains the delay usually observed between clinical presentation and diagnosis, which has been reported to be greater than 24 h in 50% of cases[Citation16]. We needed 21 h to reach a definitive diagnosis. The later the diagnosis is formulated, the later the appropriate therapeutic management is initiated, thus possibly increasing the risk of contralateral gland involvement and clinical status deterioration [Citation6].
Since the underlying cause of AI in pregnancy is believed to be adrenal vein thrombosis [Citation13–15], evaluation for thrombophilia is pivotal. A condition of thrombotic microangiopathy should also be excluded. Abnormal congenital or acquired thrombophilia screening has been identified in several cases of NHAI in pregnant women. Yet, NHAI can also occur in pregnancies with no evidence of thrombophilia disorders, likely due to the increased thrombogenic risk posed by the gestation itself [Citation20]. Nonetheless, additional risk factors for thrombosis can be identified in most of the published cases, including age >35 years, overweight or obese BMI, active smoking, and chronic inflammatory diseases. This suggests that NHAI should be even more suspected if risk factors for thrombosis, alongside gestation, are identified in women presenting with an acute abdomen and inconclusive initial workup.
Our case showed overweight BMI, substantially increased factor VIII activity (223%), homozygosity for MTHFR A1298C mutation, and heterozygosity for factor V Leiden G1691A mutation. This is the first case with a combination of three different thrombophilia disorders. Of note, both high factor VIII levels and factor V Leiden mutation constitute clinically relevant risk factors for venous thromboembolism [Citation27,Citation28].
Alongside thrombophilia and thrombotic status assessment, an endocrinological evaluation is necessary to identify women requiring cortisone supplementation [Citation8]. Interpretation of blood cortisol levels in pregnancy can be challenging, due to a physiological increase in both total and free cortisol levels [Citation6]. The use of trimester-specific cutoffs is mandatory to correctly identify adrenal insufficiency [Citation29]. In our case, a basal cortisol value of 32.7 mcg/dL was widely above the specific cutoff for the third trimester (21 mcg/dL), thus ruling out the need for cortisone supplementation. We identified four cases of NHAI in pregnancy with adrenal insufficiency (3 unilateral and 1 bilateral), and only one of them showed clinical signs (hypotension). Pain medications are the cornerstones of the therapeutic management of NHAI [Citation5,Citation30].
In addition, anticoagulation should always be considered to avoid ischemic events in the contralateral gland [Citation2]. However, awareness of an increased risk for adrenal hemorrhage as well as hemorrhage at childbirth for pregnant women has to be maintained, especially with therapeutic doses of anticoagulants. Since no standard anticoagulation protocol is currently available for NHAI, hematology consultation is mandatory. Our review identified two pregnant patients not receiving anticoagulation who experienced complete remission and an uncomplicated perinatal outcome[Citation1]; however, in a third case, a contralateral NHAI occurred 18 weeks after the first event [Citation2]. Also, 27 of the 31 published cases were treated with therapeutic doses of anticoagulant, with only one woman receiving prophylactic dosage. The hematologist consulted for our case suggested a prophylactic dosage of enoxaparin, to be continued till delivery and for 6 weeks afterwards. Of note, there is also a lack of consensus on the appropriate duration of anticoagulation after delivery, with published data ranging from two weeks to 11 months.
Mode of delivery is usually not influenced by the occurrence of NHAI unless pain persists notwithstanding analgesic therapy and anticoagulation [Citation11]. Five out of 31 NHAI cases required an urgent cesarean section for inadequate pain control. Our patient had a complete resolution of symptoms, thus allowing for pregnancy continuation.
Follow-up of patients with NHAI is pivotal to identifying those at higher risk for adrenal insufficiency [Citation5,Citation16], although no consensus on the type of investigations as well as on follow-up duration exists [Citation7]. NHAI can either completely resolve or evolve into gland atrophy, with the latter scenario increasing the risk of adrenal insufficiency in case of a contralateral event. This is particularly relevant if another pregnancy is planned, which may then require prophylactic anticoagulation. In our case, an endocrinological follow-up was performed 6 weeks postpartum and was regular.
In conclusion NHAI is a very rare cause of non-uterine abdominal pain in pregnant women. However, it can be life-threatening for both the mother and the fetus if associated with adrenal insufficiency. Early recognition and prompt multidisciplinary management are pivotal to improve outcomes. Thus, knowledge of all the potential clinical and radiological patterns of presentation is mandatory. Our work provides an extensive update on the topic and promotes awareness of this condition as a differential diagnosis among frontline healthcare professionals managing pregnant women with acute onset of abdominal pain.
Ethical approval
Since only anonymized data were employed, approval of the Ethical Committee of Fondazione IRCCS San Gerardo dei Tintori was waived.
Consent form
A written informed consent was obtained from the patient to use her anonymized clinical information for the purpose of this reporting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Glomski SA, Guenette JP, Landman W, et al. Acute nonhemorrhagic adrenal infarction in pregnancy: 10-year MRI incidence and patient outcomes at a single institution. AJR Am J Roentgenol. 2018;210(4):1–12. doi: 10.2214/AJR.17.18739.
- Guenette JP, Tatli S. Nonhemorrhagic adrenal infarction with magnetic resonance imaging features during pregnancy. Obstet Gynecol. 2015;126(4):775–778. doi: 10.1097/AOG.0000000000000884.
- Riddell AM, Khalili K. Sequential adrenal infarction without MRI-detectable hemorrhage in primary antiphospholipid-antibody syndrome. AJR Am J Roentgenol. 2004;183(1):220–222. doi: 10.2214/ajr.183.1.1830220.
- Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114(5-6):409–414. doi: 10.1016/j.thromres.2004.08.004.
- Chasseloup F, Bourcigaux N, Christin-Maitre S. Unilateral nonhaemorrhagic adrenal infarction as a cause of abdominal pain during pregnancy. Gynecol Endocrinol. 2019;35(11):941–944. doi: 10.1080/09513590.2019.1622088.
- Yuen KC, Chong LE, Koch CA. Adrenal insufficiency in pregnancy: challenging issues in diagnosis and management. Endocrine. 2013;44(2):283–292. doi: 10.1007/s12020-013-9893-2.
- Shah N, Deshmukh H, Akbar MJ, et al. Unilateral adrenal infarction in pregnancy with associated acute hypoadrenalism and subsequent spontaneous biochemical and radiological resolution. Clin Case Rep. 2022;10(2):e05442. doi: 10.1002/ccr3.5442.
- Warda F, Soule E, Gopireddy D, et al. Acute unilateral nonhemorrhagic adrenal infarction in pregnancy. AACE Clin Case Rep. 2021;7(3):228–229. doi: 10.1016/j.aace.2020.12.015.
- Green PA, Ngai IM, Lee TT, et al. Unilateral adrenal infarction in pregnancy. BMJ Case Reports. 2013;2013(aug23 1):bcr2013009997–bcr2013009997. doi: 10.1136/bcr-2013-009997.
- Chagué P, Marchi A, Fechner A, et al. Non-hemorrhagic adrenal infarction during pregnancy: the diagnostic imaging keys. Tomography. 2021;7(4):533–544. doi: 10.3390/tomography7040046.
- Reichman O, Keinan A, Weiss Y, et al. Non-hemorrhagic adrenal infarct in pregnancy - a rare clinical condition diagnosed by non-contrast magnetic resonance image. Eur J Obstet Gynecol Reprod Biol. 2016;198:173–174. doi: 10.1016/j.ejogrb.2015.12.021.
- Gutiérrez García I, Pérez Cañadas P, Martínez Uriarte J, et al. D-dimer during pregnancy: establishing trimester-specific reference intervals. Scand J Clin Lab Invest. 2018;78(6):439–442. doi: 10.1080/00365513.2018.1488177.
- Hall EM, Hemken L. The adrenal glands. A clinical and pathologic study. Arch Intern Med (Chic). 1936;58(3):448–468. doi: 10.1001/archinte.1936.00170130077005.
- Fox B. Venous infarction of the adrenal glands. J Pathol. 1976;119(2):65–89. doi: 10.1002/path.1711190202.
- Keele DV, Keele KD. Haemorrhagic suprarenal infarction. Br Med J. 1942;2(4275):687–691. doi: 10.1136/bmj.2.4275.687.
- Descargues P, Battie C, Huissoud C, et al. Pregnancy and thrombosis: adrenal vein thrombosis. A retrospective descriptive study of 14 cases. Eur J Obstet Gynecol Reprod Biol. 2019;233:38–42. doi: 10.1016/j.ejogrb.2018.10.055.
- Dobbie JW, Symington T. The human adrenal gland with special reference to the vasculature. J Endocrinol. 1966;34(4):479–489. doi: 10.1677/joe.0.0340479.
- Khandelwal A, Krishna JS, Khandelwal K, et al. Bilateral adrenal infarction in Crohn’s disease. Indian J Endocrinol Metab. 2013;17(5):933–935. doi: 10.4103/2230-8210.117227.
- Ornaghi S, Barnhart KT, Frieling J, et al. Clinical syndromes associated with acquired antithrombin deficiency via microvascular leakage and the related risk of thrombosis. Thromb Res. 2014;133(6):972–984. doi: 10.1016/j.thromres.2014.02.014.
- James AH. Pregnancy-associated thrombosis. Hematology Am Soc Hematol Educ Program. 2009;2009(1):277–285. doi: 10.1182/asheducation-2009.1.277.
- Carr BR, Parker CR, Jr., Madden JD, et al. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 1981;139(4):416–422. doi: 10.1016/0002-9378(81)90318-5.
- Ames DE, Asherson RA, Ayres B, et al. Bilateral adrenal infarction, hypoadrenalism and splinter haemorrhages in the ‘primary’ antiphospholipid syndrome. Br J Rheumatol. 1992;31(2):117–120. doi: 10.1093/rheumatology/31.2.117.
- Thuerl C, Altehoefer C, Spyridonidis A, et al. Imaging findings in the rare catastrophic variant of the primary antiphospholipid syndrome. Eur Radiol. 2002;12(3):545–548. doi: 10.1007/s003300101019.
- Wheatley T, Gallagher S, Dixon AK. Adrenal insufficiency and bilateral adrenal enlargement: demonstration by computed tomography. Postgrad Med J. 1985;61(715):435–438. doi: 10.1136/pgmj.61.715.435.
- Aco G. Committee opinion no. 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstetrics and Gynecology. 2017;130(4):e210–e216.
- De Santis M, Straface G, Cavaliere AF, et al. Gadolinium periconceptional exposure: pregnancy and neonatal outcome. Acta Obstet Gynecol Scand. 2007;86(1):99–101. doi: 10.1080/00016340600804639.
- Jenkins PV, Rawley O, Smith OP, et al. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol. 2012;157(6):653–663. doi: 10.1111/j.1365-2141.2012.09134.x.
- Martinelli I, De Stefano V, Taioli E, et al. Inherited thrombophilia and first venous thromboembolism during pregnancy and puerperium. Thromb Haemost. 2002;87(5):791–795. doi: 10.1055/s-0037-1613085.
- Lebbe M, Arlt W. What is the best diagnostic and therapeutic management strategy for an addison patient during pregnancy? Clin Endocrinol. 2013;78(4):497–502. doi: 10.1111/cen.12097.
- Bockorny B, Posteraro A, Bilgrami S. Bilateral spontaneous adrenal hemorrhage during pregnancy. Obstet Gynecol. 2012;120(2 Pt 1):377–381. doi: 10.1097/AOG.0b013e31825f20a7.
- Mathew R, Ali A, Sanders K, et al. Adrenal infarction in pregnancy secondary to elevated plasma factor VIII activity. Cureus. 2021;13(11):e19491. doi: 10.7759/cureus.19491.
- Padilla RM, Way AR, Soule E, et al. Diffusion weighted imaging in unilateral adrenal infarction: a case of colicky right upper quadrant pain in a pregnant female. Cureus. 2021;13(2):e13289. doi: 10.7759/cureus.13289.
- Jerbaka M, Slaiby T, Farhat Z, et al. Left flank pain during pregnancy with an unpredictable etiology: think of nonhemorrhagic adrenal infarction. Future Sci OA. 2021;7(8):FSO718. doi: 10.2144/fsoa-2021-0022.
- Sidibe S, Perazzini C, Cassagnes L, et al. The role of computed tomography inadrenal gland infarction diagnosis duringpregnancy: two case reports. J Med Vasc. 2021;46(1):28–31. doi: 10.1016/j.jdmv.2020.11.004.
- Cunningham TK, Maydanovych S, Draper H, et al. Adrenal infarction in the immediate postnatal period. J Obstet Gynaecol. 2019;39(3):410–411. doi: 10.1080/01443615.2018.1472557.
- Fei YF, Gonzalez-Brown V, Rood K, et al. Non-hemorrhagic unilateral adrenal infarct in pregnancy. IJCRIOG. 2019;5:1. doi: 10.5348/100044Z08YF2019CR.
- Hynes D, Jabiev A, Catanzano T. Nonhemorrhagic adrenal infarction in pregnancy: magnetic resonance imaging and computed tomography evaluation. J Comput Assist Tomogr. 2019;43(6):884–886. doi: 10.1097/RCT.0000000000000887.
- Agarwal KA, Soe MH. Cryptogenic adrenal infarction: a rare case of unilateral adrenal infarction in a pregnant woman. BMJ Case Rep. 2019;12(3):e228795. doi: 10.1136/bcr-2018-228795.
- Aljenaee KY, Ali SA, Cheah SK, et al. Unilateral adrenal infarction in pregnancy secondary to elevated factor VIII. Saudi Med J. 2017;38(6):654–656. doi: 10.15537/smj.2017.6.18520.
- Moliere S, Gaudineau A, Koch A, et al. Usefulness of diffusion-weighted imaging for diagnosis of adrenal ischemia during pregnancy: a preliminary report. Emerg Radiol. 2017;24(6):705–708. doi: 10.1007/s10140-017-1530-6.
- Sormunen-Harju H, Sarvas K, Matikainen N, et al. Adrenal infarction in a healthy pregnant woman. Obstet Med. 2016;9(2):90–92. doi: 10.1177/1753495X15627959.
- Lamba A. And you thought hormonewere the problem in pregnancy. J Lousiana State Med Soc. 2015;167(3):151.
- Hoen N, Ziane G, Grange C, et al. [Unilateral adrenal ischemia during third trimester of pregnancy: about two cases]. Gynecol Obstet Fertil. 2011;39(5):e73-6–e76. doi: 10.1016/j.gyobfe.2011.03.005.
- Schmitt C, Debord M-P, Grange C[, et al. Adrenal vein thrombosis during pregnancy. J Gynecol Obstet Biol Reprod. 2010;39(1):68–71. doi: 10.1016/j.jgyn.2009.09.012.
