 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
This study elucidated the efficacy of Relugolix (REL) on the reduction of uterine volume and clinical symptoms for the treatment of adenomyosis.
Methods
We conducted a retrospective cohort study of patients who received REL (40 mg for about 20 weeks) and who underwent a hysterectomy for adenomyosis or fibroids. We divided patients into two groups: adenomyosis coexisting with fibroids (Group A) and fibroids only (Group B); the groups were determined by a postoperative pathological examination. The primary end points were the percent reduction in uterine volume, adenomyotic lesion, and the largest fibroid volume at week 16. The secondary end points were the rate of amenorrhea, pelvic pain, and anemia at week 12.
Results
A total of 56 patients participated in the current study: 20 in Group A and 36 in Group B. Regarding the largest fibroid volume, there was no significant difference between the two groups. Uterine volume after REL treatment was significantly decreased in Group A (43%), as compared to Group B (27%) (p = .00972), In Group A, adenomyotic lesion was decreased by 61%. Irrespective of the group, adenomyosis showed a significant reduction compared to uterine fibroids (p < .001). There was no statistically significant difference in the mitigation of symptoms (amenorrhea, pelvic pain, and anemia) between the two groups.
Conclusions
REL is more effective in reducing adenomyotic lesion than uterine fibroids and in relieving symptoms (amenorrhea, pelvic pain, and anemia). It can be expected that REL will also be used as a preoperative treatment for adenomyosis.
Introduction
Uterine adenomyosis is estimated to affect approximately 20% of women and is often complicated by fibroids [Citation1]. The presence of adenomyosis was observed in 15% to 57% of patients who underwent a hysterectomy for fibroids [Citation2], while fibroids were detected in 22% of patients diagnosed with adenomyosis [Citation3]. Manifestations of adenomyosis usually include heavy menstrual bleeding, chronic pelvic pain, and infertility [Citation4]. The main objective of treating adenomyosis is symptom management, but the choice of how to do so depends on the woman’s age, clinical symptoms, and reproductive status.
Adenomyosis is essentially characterized by its estrogen dependency and progesterone resistance [Citation5]. Treatments for adenomyosis include dienogest (DNG) and the levonorgestrel-releasing intrauterine system (LNG-IUS), both of which are effective for pain and bleeding but have not been proven effective for severe adenomyosis due to their progesterone resistance [Citation6,Citation7]. On the other hand, GnRH agonists (GnRH-a) are known to be effective in the treatment of adenomyosis, as these hormone therapies reduce mitigating estrogens [Citation8]. Moreover, a comparison between DNG and GnRH-a showed no difference in terms of pelvic pain reduction. In addition, GnRH-a induced a greater reduction of bleeding and uterine volume [Citation9]. However, GnRH-a has a flare-up effect, which is known to delay its efficacy and worsen the symptoms of low estrogen levels [Citation10]. GnRH antagonists (GnRH-ant) have the advantage over GnRH-a that they do not have a flare-up effect and thus avoid the initial worsening of symptoms due to elevated estrogen levels [Citation11]. As a result, a few new oral nonpeptide GnRH antagonists have been approved, and some are under development [Citation12]. Relugolix (REL), a GnRH-ant, was approved in Japan in March 2019 to treat uterine fibroids and in December 2021 to treat endometriosis-associated pain [Citation13]. Although another GnRH antagonist, Linzagolix, has been reported to reduce uterine adenomyosis and improve symptoms in a case report [Citation14], the effects of REL on uterine and adenomyotic lesion volume reduction and the mitigation of symptoms in adenomyosis have not yet been clarified. Thus, we aimed to confirm the efficacy of REL for treating adenomyosis.
Methods
We conducted a retrospective cohort study of patients who received REL (40 mg) and who underwent a hysterectomy for adenomyosis or fibroids at Kobe University Hospital between March 2019 and December 2020. This study was approved by the institute’s ethical committee (B220108). The patients were divided into a group of cases with adenomyosis coexisting with fibroids (Group A) and fibroids only (Group B); the groups were determined by a postoperative pathological examination. Both groups were administered REL (40 mg for about 20 weeks) and underwent a subsequent hysterectomy.
The primary end points at week 16 were the percent reduction of uterine volume, adenomyotic lesion, and the percent change in the volume of the largest fibroid. They were assessed by means of magnetic resonance imaging (MRI) before and at 16 weeks after treatment. The secondary end points at week 12 included the following: the percentage of women who reported amenorrhea, the percentage of women with pelvic pain scoring zero on the numerical rating scale (NRS) score, and the percentage of women who had an increase of more than 2 g/dl from their baseline hemoglobin level.
On the assumption that both the shape of the uterus, adenomyotic lesion and the largest fibroid are spheroid, uterine, adenomyotic lesion and fibroid volumes were calculated using the following formula:
Where:
D1 = the longest diameter of the uterus, adenomyotic lesion, or fibroid (unit of length: cm)
D2 = the longest diameter of the uterus, adenomyotic lesion, or fibroid that is perpendicular to D1 (unit of length: cm)
D3 = the diameter of the uterus, adenomyotic lesion, or fibroid that crosses the intersection of D1 and D2
(intersection ‘Z‘) and is perpendicular to the D1/D2 plane (unit of length: cm).
These diameters, D1, D2, and D3, were measured by MRI.
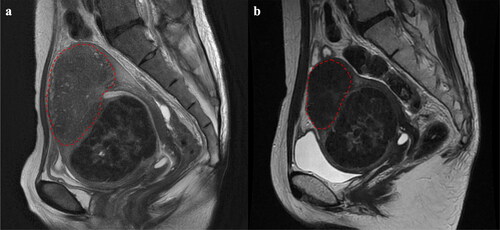
Currently, adenomyosis is broadly classified into three groups: (1) internal adenomyosis, (2) adenomyoma, and (3) external adenomyosis. These groups are further subdivided based on specific characteristics and features [Citation15,Citation16]. We classified adenomyosis into three categories using these classifications, estimated the volume of the affected areas, and then compared them. As demonstrated in , we calculated the volume under the assumption that the region encapsulated by the red dotted line represents an adenomyotic lesion using the following formula: D1 x D2 x D3 x π/6 (π = 3.14).
Figure 1. Determination of the adenomyotic lesion. (a) The extent of the adenomyotic lesion prior to treatment is indicated by the red dotted line. (b) the extent of the adenomyotic lesion 16 weeks after treatment is indicated by the red dotted line. We quantified the uterine, fibroid, and adenomyotic lesion volumes both prior to and 16 weeks post-treatment utilizing the formula for the volume of an ellipsoid, as described in the Material and Methods section.
Analyses were performed with the use of R version 2.7-2. Intergroup comparisons were assessed using the t-test, the Mann-Whitney U test, and Fisher’s exact test. A p value of <.05 was statistically significant.
Results
A total of 56 patients were administered REL (40 mg for about 20 weeks) and underwent a subsequent hysterectomy. Twenty patients with adenomyosis coexisting with fibroids were classified in Group A, and 36 patients with fibroids only were classified in Group B. These groups were divided by the results of a postoperative pathological examination. There were no significant differences among the two groups regarding age, body mass index, parity, incidence of menorrhagia, pelvic pain, and minimum hemoglobin levels (). A total laparoscopic hysterectomy (TLH) was the most common method for the hysterectomy performed. Moreover, there was no significant difference among the two groups regarding uterine volume. However, in Group B, the volume of the primary uterine fibroid was larger than that in Group A (). Adenomyosis is classified into three groups, with internal adenomyosis (IA) accounting for 25%, adenomyoma (AM) for 55%, and external adenomyosis (EA) for 20%.
Table 1. Patient characteristics of a total of 56 women with adenomyosis and fibroids in Group A, and fibroids only in Group B.
Uterine volume, which was calculated from the diameter of the XYZ axis described in the Materials and Methods section, was decreased after REL treatment by 43% in Group A and 27% in Group B (), and there was significant different between the two groups in this regard (p = .00972). Changes in the volume of the largest fibroid after REL treatment in Group A did not differ significantly from those in Group B (). In addition, in Group A, the adenomyotic lesion was decreased by 61% after REL treatment (). Regardless of the group, when focusing only on the lesions, the shrinking effect of adenomyosis by REL was significantly greater than that of the largest fibroid (p < .001) (). The reduction rates of the classified subtype of adenomyosis showed no significant differences. (IA −65 ± 11.1% vs AM −51 ± 15.3% vs EA −59 ± 20.4% p = .1993) As well, there was no statistically significant difference in the mitigation of symptoms (amenorrhea, no pelvic pain, and baseline hemoglobin level) between the two groups.
Figure 2. Efficacy end points. (a) Percent change in uterine volume from baseline to week 16 (GroupA -43 ± 19.1% vs -27.7 ± 21.2%, p = .00972), (b) Percent change in the volume of the primary uterine fibroid from baseline to week 16 (GroupA -33 ± 29.0% vs -24.7 ± 27.4%, p = .297). Group a = adenomyosis and fibroids; GroupB = fibroids only.
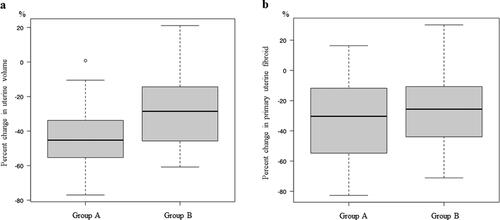
Figure 3. Percent change in adenomyosis and Myoma from baseline to week 16 when focusing on lesions regardless of group (adenomyosis -61 ± 14.4% vs fibroid -33 ± 29.0%, p < .001).
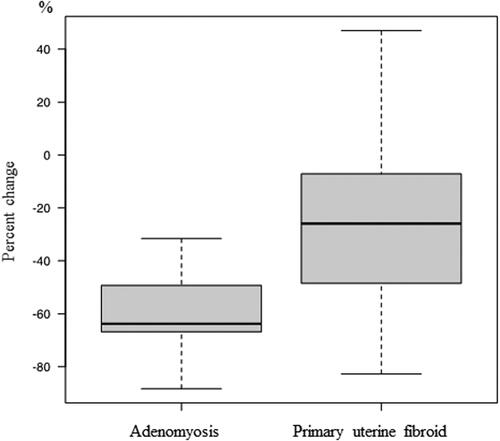
Table 2. Efficacy of treatment.
Discussion
Our study is the first report of a reduction in uterine volume and adenomyotic lesion for adenomyosis with REL, which was also effective in mitigating symptoms (menorrhagia and pelvic pain). Especially, adenomyotic lesion volume showed the most marked reduction compared to uterine volume and fibroid volume. In our study, uterine volume was reduced more in patients with adenomyosis compared with fibroids only, even though the reduction of fibroids with REL didn’t differ between the two groups. This result can be attributed to the fact that adenomyosis shrinks earlier than the normal myometrium. In fact, adenomyosis is marked by elevated levels of estrogen caused by the enhanced expression of estrogen receptors [Citation4,Citation6]. The condition causes a reduction in the effectiveness of progesterone receptors, resulting in the development of progesterone resistance [Citation17]. Some studies have also reported that uterine adenomyosis has estrogen dependence and progesterone resistance [Citation5,Citation18–20]. Hyperestrogenism is thought to be involved in neoangiogenesis and growth factors and inflammation in adenomyosis [Citation5]. It has already been reported that GnRH agonist therapy for adenomyosis affects inflammation, angiogenesis, and apoptosis [Citation21]. From the above, the overexpression of estrogen receptors in adenomyosis may have made the effect of GnRH antagonists more pronounced, thus resulting in a significantly reduced uterine volume in adenomyosis coexisting with fibroids.
In this study, we have reported for the first time that REL has a greater shrinking effect on adenomyosis than on fibroids. In uterine fibroid growth, estrogen induces the expression of the progesterone receptor, and progesterone and its receptor are essential and sufficient for tumor growth, as indicated by the stimulation of cell proliferation, the accumulation of the extracellular matrix, and cellular hypertrophy [Citation22]. On the other hand, uterine adenomyosis has estrogen dependence and progesterone resistance. These differences in hormonal effects between fibroid and adenomyosis may be related to differences in the reduction effects of REL. Furthermore, in uterine fibroids, fibrous tissue such as the extracellular matrix may make it physically more difficult for the fibroid to shrink. However, as reported by Matsushima et al. GnRH-a for uterine adenomyosis rapidly returns to reversibility after treatment [Citation23], and the same may occur with GnRH-ant. These finding suggest that REL is therefore expected to be used as a preoperative treatment of adenomyosis, and it is recommended that GnRH-ant be continued until immediately before surgery.
Due to hypoestrogenic symptoms such as menopausal symptoms or osteoporosis, REL can only be administered for six months. Therefore, the duration of treatment is limited in non-operative cases. AI-Hendy et al. have proposed on a REL combination therapy (40 mg of REL, 1 mg of estradiol, and 0.5 mg of norethindrone acetate, 24 weeks) as a treatment for uterine fibroids [Citation24]. REL combination therapy can also reduce uterine volume and relieve hypoestrogenic symptoms. Similar effects can be expected for adenomyosis, and further studies of a long-term administration of REL combination therapy are ongoing.
There are some limitations to the present study. First, since hysterectomy is not typically the first-line treatment for adenomyosis and medical therapy is often prioritized, it was not possible to simply investigate the shrinking effect of REL on adenomyosis compared to a normal uterus. Therefore, we retrospectively examined the treatment effects on adenomyosis by comparing cases of uterine fibroids that underwent hysterectomy with or without a pathological diagnosis of adenomyosis. Second, as for the volume of adenomyosis, the adenomyoma was more accurately measured, but the volume of internal adenomyosis and external adenomyosis may be somewhat inaccurate because the boundaries were not clear in some cases. Third, it was somewhat difficult to accurately assess bleeding and pain in a self-reported survey. To evaluate the effects of REL on shrinking the uterus or reducing adenomyotic lesions, as well as symptom management, larger studies are needed.
In this study, it was shown for the first time that REL can reduce uterine volume not only in uterine fibroids but also in adenomyosis. It can be expected that REL will also be used as a preoperative treatment and the mitigation of symptoms for these two conditions.
Ethical approval
Ethics Committee of the Institution approved the study protocol on 23 August 2022(No. B220108).
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent for publication
The consent to publish the study results was obtained from all participants.
Acknowledgments
The authors thank Mr. Brian Nolan, for the assistance in editing this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
All data were obtained from Kobe University medical records.
Additional information
Funding
References
- Naftalin J, Hoo W, Pateman K, et al. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. 2012;27(12):1–5. doi: 10.1093/humrep/des332.
- Taran FA, Weaver AL, Coddington CC, et al. Understanding adenomyosis: a case control study. Fertil Steril. 2010;94(4):1223–1228. doi: 10.1016/j.fertnstert.2009.06.049.
- Naftalin J, Hoo W, Pateman K, et al. Is adenomyosis associated with menorrhagia? Hum Reprod. 2014;29(3):473–479. doi: 10.1093/humrep/det451.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109(3):371–379. doi: 10.1016/j.fertnstert.2017.12.030.
- Donnez J, Stratopoulou CA, Dolmans M-M. Uterine adenomyosis: from disease pathogenesis to a new medical approach using GnRH antagonists. Int J Environ Res Public Health. 2021;18:9941.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109(3):398–405. doi: 10.1016/j.fertnstert.2018.01.013.
- Osuga Y, Hayashi K, Kanda S. Long-term use of dienogest for the treatment of primary and secondary dysmenorrhea. J Obstet Gynaecol Res. 2020;46(4):606–617. doi: 10.1111/jog.14209.
- Khan KN, Kitajima M, Hiraki K, et al. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod. 2010;25(11):2878–2890. doi: 10.1093/humrep/deq240.
- Fawzy M, Mesbah Y. Comparison of dienogest versus triptorelin acetate in premenopausal women with adenomyosis: a prospective clinical trial. Arch Gynecol Obstet. 2015;292(6):1267–1271. doi: 10.1007/s00404-015-3755-5.
- Bedaiwy MA, Mousa NA, Casper RF. Aromatase inhibitors prevent the estrogen rise associated with the flare effect of gonadotropins in patients treated with GnRH agonists. Fertil Steril. 2009;91(4 Suppl):1574–1577. doi: 10.1016/j.fertnstert.2008.09.077.
- Osuga Y, Enya K, Kudou K, et al. Oral Gonadotropin-Releasing hormone antagonist relugolix compared with leuprorelin injections for uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2019;133(3):423–433. doi: 10.1097/AOG.0000000000003141.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of Endometriosis-Associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40. doi: 10.1056/NEJMoa1700089.
- Harada T, Osuga Y, Suzuki Y, et al. Relugolix, an oral gonadotropin-releasing hormone receptor antagonist, reduces endometriosis-associated pain compared with leuprorelin in japanese women: a phase 3, randomized, double-blind, noninferiority study. Fertil Steril. 2022;117(3):583–592. doi: 10.1016/j.fertnstert.2021.11.013.
- Donnez O, Donnez J. Gonadotropin-releasing hormone antagonist (linzagolix): a new therapy for uterine adenomyosis. Fertil Steril. 2020;114(3):640–645. doi: 10.1016/j.fertnstert.2020.04.017.
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109(3):389–397. doi: 10.1016/j.fertnstert.2018.01.024.
- Munro MG. Classification and reporting systems for adenomyosis. J Minim Invasive Gynecol. 2020;27(2):296–308. doi: 10.1016/j.jmig.2019.11.013.
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35(5):592–601. doi: 10.1016/j.rbmo.2017.06.016.
- Bulun SE, Cheng Y-H, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103. doi: 10.1016/j.mce.2005.11.041.
- Yilmaz BD, Bulun SE. Endometriosis and nuclear receptors. Hum Reprod Update. 2019;25(4):473–485. doi: 10.1093/humupd/dmz005.
- Kitawaki J, Kado N, Ishihara H, et al. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002;83(1-5):149–155. doi: 10.1016/s0960-0760(02)00260-1.
- Khan KN, Kitajima M, Hiraki K, et al. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod. 2010;25(3):642–653. doi: 10.1093/humrep/dep437.
- Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–1355. doi: 10.1056/NEJMra1209993.
- Matsushima T, Akira S, Yoneyama K, et al. Recurrence of uterine adenomyosis after administration of gonadotropin-releasing hormone agonist and the efficacy of dienogest. Gynecol Endocrinol. 2020;36(6):521–524. doi: 10.1080/09513590.2019.1683818.
- Al-Hendy A, Lukes AS, Poindexter AN, 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384(7):630–642. doi: 10.1056/NEJMoa2008283.
