Abstract
Objective
To analyze pregnancy outcomes of women with one abnormal value (OAV) during oral glucose tolerance test (OGTT) or OGTT-intolerance, compared with gestational diabetes mellitus (GDM) and normal glucose tolerance (NGT) pregnant women, according to whether they received any health intervention or not.
Methods
An observational retrospective study was designed including pregnant women who gave birth at Hospital del Mar, Barcelona (Spain) during December/2014–July/2018. Baseline characteristics, pregnancy outcomes and health interventions were obtained from a database collected previously for other study. Inclusion criteria were singleton pregnancies with OAV or OGTT-intolerants who gave birth at the Hospital. GDM screening followed a two-step approach: 50 g O’Sullivan test and 100 g 3-hour OGTT if the former was abnormal.
Results
From a total of 2,662 pregnancies, 326 (12.2%) had GDM, 87 OAV (3.3%), 65 OGTT intolerance (2.4%) and 2,184 were NGT women. First trimester HbA1c in both OAV and OGTT-intolerant women was significantly higher than in NGT group, and significantly lower than in GDM pregnants. No differences in obstetric outcomes were found between OGTT-intolerants and NGT/GDM groups. Treated OGTT-intolerants had greater gestational age at delivery than non-treated ones (weeks, 39.6 ± 1.2 vs 38.0 ± 4.0, respectively). In OAV women, significant differences were observed in newborns’ birthweight (g, 3227.3 ± 500.8 vs 3351.1 ± 436.7, vs GDM) and gestational age at birth (weeks, 38.7 ± 1.8 vs 39.3 ± 1.9, vs NGT), but not in macrosomia/pre-eclampsia. No differences were found according to treatment in OAV.
Conclusions
OAV and OGTT-intolerants account for a third of pregnant women referred to Diabetes Unit. Their rates of preterm birth, pre-eclampsia and macrosomia were not different from NGT or GDM women.
Introduction
Gestational diabetes mellitus (GDM), defined as hyperglycemia detected beyond the second trimester of pregnancy, raises the risk of adverse obstetric outcomes [Citation1]. Since its treatment lowers this risk [Citation2], an accurate identification of these women is needed. GDM is detected with an oral glucose tolerance test (OGTT), with two different strategies accepted depending on population characteristics. A one-step approach consists of performing a 2-h, 75 g OGTT for all pregnant women after gestational week 24 using the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria. The two-step approach involves a 50 g glucose challenge test (O’Sullivan test) followed by a 100 g 3-h oral glucose tolerance test (OGTT) only if the former is abnormal. In this case, GDM diagnosis requires at least 2 abnormal glucose values during OGTT.
In Spain, two-step approach is the advised strategy [Citation3], since GDM can be ruled out in most patients with O’Sullivan test which is better tolerated.
Nevertheless, two scenarios related to carbohydrate intolerance may arise, where pregnancy management and obstetric risks are unclear. The first scenario is vomiting during OGTT, thus impeding GDM diagnosis/exclusion. This situation is termed ‘OGTT-intolerance’ and it has been poorly analyzed. Its prevalence is 4%–8.2% [Citation4,Citation5], and only one study [Citation4] described its possible consequences on maternal-fetal outcomes. All these women have an abnormal 50 g OGTT, and therefore an impaired glucose tolerance can be assumed, entailing a higher obstetric risk. Nevertheless, there is no general agreement on the medical management of these women.
The second scenario is the presence of only one abnormal glucose value (OAV) in the OGTT, which is not diagnostic of GDM but points to a certain degree of glucose imbalance. Literature shows conflicting results on this condition: some described a similar risk to GDM women, recommending a similar management [Citation6–8], while others found no differences in outcomes between normoglycemic (Normal Glucose Tolerance, NGT) patients and those with OAV regardless of specific treatment [Citation9].
Based on the above, the aim of this study was to analyze the obstetric outcomes of OGTT-intolerants and OAV pregnant women in a 100 g 3-h OGTT compared with GDM and NGT women. The secondary objectives were to assess the prevalence of these conditions in our population and the impact of an educational intervention on maternal-fetal outcomes.
Material and methods
A retrospective study of a prospective observational cohort was conducted out at the Hospital del Mar, Barcelona, Spain, between December/2014 and July/2018. The study aimed to analyze the impact of first-trimester glycosylated hemoglobin (HbA1c) levels in multi-ethnic pregnant women. The cohort’s characteristics and study methods have been published elsewhere [Citation10]. Women aged ≥18 years with a singleton pregnancy were included. Exclusion criteria were preexisting diabetes (type 1 or 2), meeting the American Diabetes Association criteria for diabetes mellitus [fasting plasma glucose ≥126mg/dL and/or an HbA1c ≥6.5% (48 mmol/mol)] in first trimester without a previous diagnosis of diabetes, or multiple pregnancies. Women with a miscarriage or voluntary pregnancy termination and those lost to follow-up were also excluded.
Since 2013, our protocol for diabetes in pregnancy included HbA1c and fasting plasma glucose concentrations at the first antenatal blood testing. Pregnant women with HbA1c <6.5% (48 mmol/mol) and fasting glucose <126mg/dL did not receive any intervention until 24–28 weeks of gestation, when they underwent routine two-step GDM screening. The two-step approach involves a 50 g 1-h glucose challenge test (O’Sullivan test) in every pregnant woman, which is followed by a 100 g 3-h oral glucose tolerance test (OGTT) only if the former is abnormal. The OGTT protocol requires women to follow a balanced diet starting three days before the test with a minimum carbohydrate intake of 150 g/day. Patients fast overnight and have blood drawn at baseline and hourly until 3 h after the glucose load. OGTT solution is chill, and no antiemetic drug is routinely administered. GDM diagnosis implies having two or more glucose values above National Diabetes Data Group cutoff points: plasma glucose over 105, 190, 165, and 145 mg/dL for fasting, 1-, 2-, and 3-h post-glucose load, respectively [Citation11].
Women with OAV or vomits repeat the OGTT after two weeks. For this study, ‘OAV’ women had presented OAV in both OGTT, and only those vomiting in both OGTT were considered ‘OGTT-intolerants’.
Women with GDM, OAV or ‘OGTT-intolerance’ were referred to the Diabetes Unit. GDM patients were treated following GDM guidelines [Citation1]. Women with OAV and OGTT-intolerants were asked to self-monitor blood glucose, both fasting and one-hour post-prandial for 7 days, if more than 60% of values are higher than glucose goals (>90mg/dl fasting, >140mg/dl 1 h post-prandial) treatment was started according to GDM guidelines. Patients who presented <60% abnormal values received basic dietary counseling and were discharged from the Diabetes Unit. Consequently, every woman who attended the visit was considered as treated, given that a dietary education was always provided. Throughout the study period, all women received standard care from a midwife/obstetrician.
All data were collected from electronic medical records and transferred to a central database. The study was conducted following the Declaration of Helsinki principles and with local Ethics Committee of Clinical Research approval.
Macrosomia was defined as a newborns’ birthweight >4000 g. Pre-eclampsia was defined as new-onset or worsening hypertension after 20 weeks’ gestation with the co-existence of at least one more condition: proteinuria (protein/creatinine ratio >30mg/mmol), other maternal organ dysfunction or fetal growth restriction (fetal growth < p10) [Citation12,Citation13]. Indications for elective Cesarean section were: suspected fetal macrosomia (ultrasound estimated fetal weight >4000 g), 2 or more previous Cesarean sections, or placenta previa.
Statistical analysis was performed using the statistical software package IBM SPSS Statistic version 25.0. Data were expressed as mean ± standard deviation (SD) for continuous variables and as frequencies and percentages for qualitative variables. Chi-square or Fisher’s test were applied to determine the association between qualitative variables and Student’s t-test to compare quantitative variables. The comparison of obstetrical outcomes between groups was adjusted for initial BMI and age using multivariate linear regression analysis for continuous variables and logistic regression analysis for categorical variables. Statistical significance was set at p < 0.05.
Results
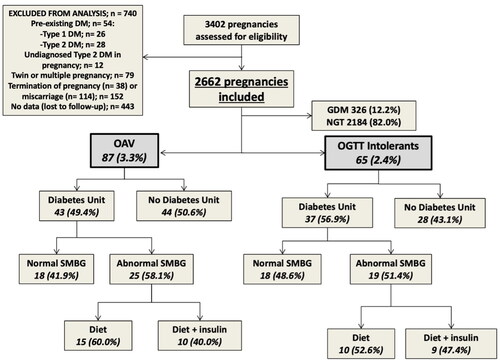
During the study, 3,402 deliveries were registered at the hospital, 740 of which were excluded (). Finally, 2,662 pregnancies were included for analysis. In the first OGTT, 160 women had OAV (6.0%), but after repeating the test 37 women had normal OGTT, 36 were diagnosed with GDM and 87 had OAV. Overall, 326 women (12.2%) had GDM, 87 presented OAV (3.3%) and 65 were OGTT-intolerants (2.4%). The percentages of these conditions from the total of pregnant women referred to Diabetes Unit were 18.2% for OAV and 13.5% for OGTT-intolerants. Women who attended Diabetes Unit and needed treatment are shown in .
Figure 1. Flow diagram of the study and analyzed groups.
GDM, gestational diabetes mellitus; DM, diabetes mellitus; NGT, normal glucose tolerance; OAV, one abnormal value; OGTT, oral glucose tolerance test; SMBG, self-measurement blood glucose.
OGTT-intolerant women
Regarding baseline characteristics, first-trimester HbA1c in OGTT-intolerant pregnant women was significantly higher than in NGT but lower than in GDM women (). Moreover, OGTT-intolerants had significantly less history of GDM compared with GDM women, and a different ethnic distribution. No differences in obstetric outcomes were found between OGTT-intolerants and the other groups ().
Table 1. Baseline characteristics and maternal-fetal outcomes in OGTT-intolerant pregnant women.
Comparing treated vs non-treated OGTT-intolerants, the former had more family history of diabetes and greater gestational age at delivery, with no differences in other outcomes.
OAV
Baseline characteristics and maternal-fetal outcomes of OAV pregnant women are shown in . First-trimester HbA1c of OAV women was significantly higher than in NGT and lower than in GDM group. OAV also had higher pre-pregnancy body mass index (BMI) than NGT, but similar to GDM women. Nevertheless, OAV women gained more weight during pregnancy than GDM patients. Moreover, OAV had more history of previous GDM than NGT group, but lower than GDM patients, who presented more diabetes family history than OAV ones. Regarding maternal-fetal outcomes, although mean newborns’ birthweight in OAV women was higher than in GDM mothers, macrosomia rate did not differ between groups. Gestational age at birth was lower in OAV mothers than in NGT women; however, no differences were observed in preterm birth rates. No other outcome differences were found.
Table 2. Baseline characteristics and maternal-fetal outcomes for one abnormal value (OAV) pregnant women.
Treated vs non-treated OAV women had similar characteristics except for history of GDM, and no significant differences were found in outcomes, although a trend toward more preterm births in non-treated women was observed (p = 0.09).
Discussion
This study provides new evidence on two frequent situations during pregnancy: OGTT-intolerance and OAV in 3-h 100 g OGTT. Both conditions comprise a 6% of all pregnancies and represented approximately 30% of pregnant women referred to Diabetes Clinics. Nevertheless, these women had no differences in major obstetric outcomes, regardless of the therapeutic strategy.
In our multi-ethnic population, 5.7% women presented one of these conditions: 3.3% OAV and 2.4% OGTT-intolerance. The reported prevalence of OAV varies between 3.7 and 19% [Citation6–9,Citation14–18], which can be explained by the different diagnostic cutoff points used and maternal factors related to glucose intolerance risk, such as ethnicity. In these studies, OAV prevalence was similar to GDM; however, in our population, their prevalence was lower than GDM. These differences could be explained, at least partially, since in our protocol women with OAV repeated the OGTT, allowing its reclassification as NGT or GDM.
Regarding pregnant women with OGTT-intolerance, the scant data available showed a 2-4 times higher prevalence than in this study [Citation4,Citation5]. Gastric intolerance has been related to the amount of glucose administered, since it raises solution osmolarity and delays gastric emptying [Citation19]. Some recommendations suggest chilling OGTT solution overnight or giving antiemetic drugs to improve tolerance [Citation20]. In our population, OGTT was administered cold and, in case of vomiting, the test was rescheduled with the same glucose amount; this could explain the lower rate of OGTT-intolerants compared with other studies that did not systematically repeat the test [Citation4,Citation5].
In case of vomit recurrence, some studies recommend performing a new test with less glucose (75 g or 50 g), which has disclosed the same area under the curve as 100 g OGTT and better tolerance [Citation5,Citation19]. Other investigators proposed replacing OGTT by self-monitoring of capillary blood glucose [Citation21,Citation22], or by HbA1c measurement [Citation5]. Indeed, Sermer [Citation23] demonstrated that second-trimester fasting plasma glucose levels are an independent predictor of macrosomia, but without a threshold at which risk increased. However, using fasting glycemia instead of OGTT would probably underdiagnose a significant number of women with GDM.
Given the frequency of these clinical situations, it seems necessary to evaluate their impact on pregnancy outcomes and ascertain the need for specific management. We can hypothesize that women with OAV or OGTT-intolerance have a glucose metabolism impairment since they all had a previous abnormal 50 g OGTT. Moreover, as found in this study, family and personal history of diabetes and/or GDM were also more frequent in OAV and OGTT-intolerant women than in NGT. Furthermore, HbA1c values in first trimester were higher in these women than in those with NGT, but lower than in GDM. Therefore, these women could be at higher risk of adverse pregnancy outcomes.
Regarding women with OAV, no differences were found in rates of macrosomia, preterm birth or the rest of the pregnancy outcomes assessed, although their newborns had a slightly higher mean birthweight than those of GDM women and lower gestational age at birth than NGT women. Similarly, Forest [Citation16] found no differences when comparing perinatal outcomes among four groups: normal, OAV treated women, OAV untreated women, and GDM group. Nevertheless, most of the available evidence shows contradictory results. In this respect, a 2016 meta-analysis by Roeckner [Citation8], including 23 studies and 4466 pregnant women with OAV, observed a higher risk of macrosomia, Cesarean delivery, higher birthweight, large for gestational age, neonatal hypoglycemia and 5 min’ Apgar <7, compared with normal OGTT women. Further, other recent studies have also found higher obstetrical risk in OAV women [Citation9,Citation24,Citation25]. The study of Kang et al. [Citation9] included 2120 pregnant NGT women and 137 women with OAV in the first OGTT that did not receive a specific intervention. After the second test, 28.5% of women with OAV were reclassified as GDM, and another 29.2% maintained OAV results, while the last 42.3% of patients showed a normal glucose tolerance at retesting. Regarding outcomes, they described a 12.8% higher macrosomia rates in OAV group vs NGT women, while there were no differences in macrosomia between OAV and GDM pregnants. Finally, Kim et al. [Citation17] analyzed Korean women with 1-h OAV in a 100 g OGTT and reported increased adverse pregnancy outcomes compared with women with normal OGTT, but also higher obstetrical risk than 2-h or 3-h OAV groups. In the present study, we did not separate women with 1-h, 2-h, or 3-h OAV, and it may be hypothesized that grouping all women into a single category could have masked possible differences.
Regarding OGTT-intolerant women, no differences in obstetric results were observed comparing with NGT or GDM women. An observational study [Citation4] in Arab and Hindu population of 100 g OGTT-intolerant women described rates of 9.1% of macrosomia and 21.5% of cesarean section, but no comparison was made with GDM women.
Another relevant aspect concerning these situations is whether an intervention may have an impact on pregnancy outcomes. Our protocol for OGTT-intolerant women includes a visit with a Diabetes Nurse, but surprisingly only 50% of women attended the appointment. These women had a significantly greater gestational age at delivery compared with non-compliant ones, and a trend toward more preterm births was observed. Interestingly, they had more family history of diabetes compared to non-treated women, and probably more disease awareness. Further trials are required to confirm the real benefits of this intervention.
This study found no differences in obstetrical outcomes between treated and non-treated OAV women, and previous studies on this issue revealed contradictory results [Citation7,Citation8,Citation16]. In line with these results, the 2018–2019 ACOG guidelines state that strong evidence to support the benefits of treating OAV women as GDM is not yet available [Citation26].
Our study has its limitations. Firstly, all data were retrospective and gathered from a database created for another purpose; however, OAV and OGTT-intolerant women were rechecked individually to confirm correct data. Secondly, the high nonattendance rate at Diabetes Clinic’s visit may have biased the results. Moreover, the study was not designed to compare pregnancy outcomes according to its attendance at Diabetes Clinic, and may be underpowered to detect treatment effects. Finally, as mentioned above in the text, we did not separate OAV women according to whether the abnormal glucose value in OGTT was at fasting, at 1, 2, or 3-h measurement, therefore hypothetical differences between groups could have been masked.
In conclusion, in our population OAV and OGTT-intolerance occurred in almost a third of all pregnant women referred to Diabetes Clinic. The rates of preterm birth, pre-eclampsia, and macrosomia of OAV and OGTT-intolerant women were not different from NGT or GDM ones. Although this study found no differences in obstetric results when comparing women who underwent a specific intervention and those who did not, possible benefits of an intervention should be explored in further trials designed for the purpose.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- American Diabetes Association. 15. Management of diabetes in pregnancy: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):1–6.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38.
- Grupo Español de Diabetes y Embarazo (GEDE). Asistencia a la gestante con diabetes. Guía de práctica clínica actualizada en 2014 [assistance to pregnant woman with diabetes. 2014 updated clinical practice guideline]. Av Diabetol. 2015;31(2):45–59.
- Agarwal MM, Punnose J, Dhatt GS. Gestational diabetes: problems associated with the oral glucose tolerance test. Diabetes Res Clin Pract. 2004;63(1):73–74. doi: 10.1016/j.diabres.2003.08.005.
- Fachnie JD, Whitehouse FW, McGrath Z. Vomiting during OGTT in third trimester of pregnancy. Diabetes Care. 1988;11(10):818. doi: 10.2337/diacare.11.10.818.
- Greenberg VR, Lundsberg LS, Reddy UM, et al. Perinatal outcomes in obese women with one abnormal value on 3-hour oral glucose tolerance test. Am J Perinatol. 2022;39(5):464–472. doi: 10.1055/s-0041-1740005.
- Langer O, Brustman L, Anyaegbunam A, et al. The significance of one abnormal glucose tolerance test value on adverse outcome in pregnancy. Am J Obstet Gynecol. 1987;157(3):758–763. doi: 10.1016/s0002-9378(87)80045-5.
- Roeckner JT, Sanchez-Ramos L, Jijon-Knupp R, et al. Single abnormal value on 3-hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;215(3):287–297. doi: 10.1016/j.ajog.2016.04.040.
- Kang S, Kim MH, Kim MY, et al. Progression to gestational diabetes mellitus in pregnant women with one abnormal value in repeated oral glucose tolerance tests. Diabetes Metab J. 2019;43(5):607–614. doi: 10.4093/dmj.2018.0159.
- Mañé L, Flores-Le Roux JA, Benaiges D, et al. Role of First-Trimester HbA1c as a predictor of adverse obstetric outcomes in a multiethnic cohort. J Clin Endocrinol Metab. 2017;102(2):390–397.
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National diabetes data group. Diabetes. 1979;28(12):1039–1057.
- Tranquilli AL, Brown MA, Zeeman GG, et al. The definition of severe and early-onset preeclampsia. Statements from the international society for the study of hypertension in pregnancy (ISSHP). Pregnancy Hypertens. 2013;3(1):44–47. doi: 10.1016/j.preghy.2012.11.001.
- Martins JG, Biggio JR, Abuhamad A, Society for Maternal-Fetal Medicine (SMFM). Society for maternal-fetal medicine consult series #52: diagnosis and management of fetal growth restriction: (replaces clinical guideline number 3, april 2012). Am J Obstet Gynecol. 2020;223(4):B2–B17. doi: 10.1016/j.ajog.2020.05.010.
- Di Cianni G, Seghieri G, Lencioni C, et al. Normal glucose tolerance and gestational diabetes mellitus: What is in between? Diabetes Care. 2007;30(7):1783–1788. doi: 10.2337/dc07-0119.
- Corrado F, Benedetto AD, Cannata ML, et al. A single abnormal value of the glucose tolerance test is related to increased adverse perinatal outcome. J Matern Fetal Neonatal Med. 2009;22(7):597–601. doi: 10.1080/14767050902801801.
- Forest JC, Massé J, Garrido-Russo M. Glucose tolerance test during pregnancy: the significance of one abnormal value. Clin Biochem. 1994;27(4):299–304. doi: 10.1016/0009-9120(94)00029-8.
- Kim HS, Chang KH, Yang JI, et al. Clinical outcomes of pregnancy with one elevated glucose tolerance test value. Int J Gynaecol Obstet. 2002;78(2):131–138. doi: 10.1016/s0020-7292(02)00129-7.
- Bevier WC, Fischer R, Jovanovic L. Treatment of women with an abnormal glucose challenge test (but a normal oral glucose tolerance test) decreases the prevalence of macrosomia. Am J Perinatol. 1999;16(6):269–275. doi: 10.1055/s-2007-993871.
- Schwartz JG, Phillips WT, Blumhardt MR, et al. Use of a more physiologic oral glucose solution during screening for gestational diabetes mellitus. Am J Obstet Gynecol. 1994;171(3):685–691. doi: 10.1016/0002-9378(94)90082-5.
- Moore TR, Hauguel-De Mouzon S, Catalano P. Diabetes in pregnancy. In: Resnik R, Lockwood CJ, Moore TR, Greene MF, Copel JA, Silver RM, editors. Creasy and Resnik’s maternal-fetal medicine: principles and practice. 8th ed. Philadelphia (PA): Elsevier; 2019.
- Rudge MV, Calderon IM, Ramos MD, et al. Perinatal outcome of pregnancies complicated by diabetes and by maternal daily hyperglycemia not related to diabetes. A retrospective 10-year analysis. Gynecol Obstet Invest. 2000;50(2):108–112. doi: 10.1159/000010293.
- Negrato CA, Jovanovic L, Tambascia MA, et al. Mild gestational hyperglycaemia as a risk factor for metabolic syndrome in pregnancy and adverse perinatal outcomes. Diabetes Metab Res Rev. 2008;24(4):324–330. doi: 10.1002/dmrr.815.
- Sermer M, Naylor CD, Gare DJ, et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. The Toronto Tri-Hospital gestational diabetes project. Am J Obstet Gynecol. 1995;173(1):146–156. doi: 10.1016/0002-9378(95)90183-3.
- Simsek D, Akselim B, Altekin Y. Do patients with a single abnormal OGTT value need a globally admitted definition such as "borderline GDM"? Pregnancy outcomes of these women and the evaluation of new inflammatory markers. J Matern Fetal Neonatal Med. 2021;34(22):3782–3789. doi: 10.1080/14767058.2021.1946779.
- Kung WJ, Kuo HY, Lee CH, et al. Association between gestational abnormal glucose tolerance and maternal-fetal outcomes. J Obstet Gynaecol Res. 2022;48(10):2505–2513. doi: 10.1111/jog.15350.
- ACOG. Practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–64.
