Abstract
Objective
To investigate the physiological changes of serum homocysteine (Hcy) levels and to establish trimester-specific reference intervals of serum Hcy levels for Chinese pregnant women.
Method
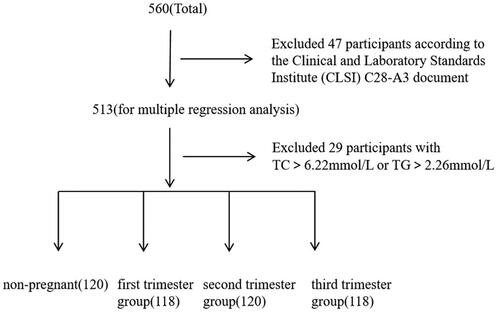
According to the guideline of the Clinical and Laboratory Standards Institute (CLSI) C28-A3 document, 476 healthy women were recruited in West China Second University Hospital, Sichuan University from January 2021 to October 2021. Among them, 120 were non-pregnant, 118 were in the first trimester, 120 were in the second and 118 were in the third trimester of gestation. The enzymatic cycling method was performed to detect serum Hcy levels. Non-parametric percentiles (2.5th percentile and 97.5th percentile) were calculated to establish the reference intervals for non-pregnant women and pregnant women in different trimester of gestation.
Results
There was a significant statistical difference for serum Hcy levels between non-pregnant women and pregnant women (p < 0.05), and serum Hcy levels in the first, second, and third trimesters of gestation were statistically different (p < 0.05). The trimester-specific reference intervals of serum Hcy levels were 4.35 ∼ 10.16 μmol/L, 3.38 ∼ 8.60 μmol/L, and 3.75 ∼ 11.17 μmol/L for pregnant women in the first, second, and third trimester of gestation, respectively.
Conclusions
Compared to non-pregnant women, serum Hcy levels physiologically decreased after pregnancy, and the physiological changes in serum Hcy levels during pregnancy were also found. Establishing trimester-specific reference intervals of serum Hcy levels for pregnant women was valuable for clinical practice.
Introduction
As a sulfur-containing amino acid, homocysteine (Hcy) is the primary product of the methionine cycle, which can convert back to methionine through 5-methyltetrahydrofolate and methylcobalamin [Citation1,Citation2]. The metabolism of Hcy is at the intersection of the following two pathways: the remethylation pathway generating methionine (requiring folic acid and vitamin B12, and methylene tetrahydrofolate and methionine synthase enzymes), and the transsulfurization pathway to irreversible production of cysteine and alpha-ketobutyrate (requiring vitamins B6-dependent catalysis of cystathionine beta synthase) [Citation3–5]. Defects in these two pathways can lead to the accumulation of Hcy, and congenital or acquired micronutrient deficiencies may play essential role in the pathological process [Citation6]. Elevated Hcy in peripheral blood releases nitric oxide (NO) and prostacyclin, which initiate the coagulation process and ultimately lead to endothelial damage [Citation7]. Elevated Hcy can also increase oxidative stress, reduce the utilization activity of NO, promote the proliferation of vascular smooth cells, and increase the releases of inflammatory cytokines [Citation8]. Therefore, elevated Hcy in blood may play roles in many vascular diseases, such as atherosclerotic changes, coronary dysfunction, and embolic diseases [Citation9,Citation10].
In recent years, some studies found that elevated Hcy was associated with adverse pregnancy outcomes and fetal malformations [Citation11]. HHcy induces hydrogen peroxide and superoxide to produce free radicals, which can cause oxidative damage to vascular endothelial cells, reduce the increase in the number of villous blood vessels, inhibit embryonic blood perfusion, and lead to adverse pregnancy outcomes, such as preeclampsia [Citation12], abortions [Citation13], low birth weight, and increased incidence of type 2 diabetes in the offspring [Citation14]. In addition, Hcy was also proved to be associated with oligohydramnios, fetal growth restriction, and impairment of placental transport [Citation11,Citation15,Citation16].
The determination of serum Hcy levels during pregnancy can reflect the mother’s metabolic status and help to predict the risk of adverse pregnancy outcomes. Although healthy Hcy differences between nonpregnant women and pregnant women have been demonstrated in Spain, Canada, and the United States [Citation15,Citation17,Citation18], due to differences in ethnicity, region, and culture, the applicability of this study to healthy Chinese women needs to be verified. However, Chinese clinical laboratory only established the Hcy reference interval of the general population. So, this study will explore the physiological changes of serum Hcy levels during pregnancy and develop valuable and reliable maternal pregnancy-specific reference intervals according to the clinical and Laboratory Standards Institute (CLSI) C28-A3 document.
Materials and methods
Participants
The healthy women who went to West China Second University Hospital, Sichuan University (Sichuan, Chengdu) for prenatal checkup or delivery were recruited from January 2021 to October 2021. A total of 476 healthy women were selected in this study, including 120 non-pregnant women, 118 pregnant women in the first trimester, 120 in the second trimester and 118 in the third trimester of gestation. All the pregnant women in our study were singletons. The selection of non-pregnant and pregnant women was based on the exclusion criteria drawn up according to CLSI C28-A3 guidelines: (1) having chronic diseases, liver diseases or endocrine diseases; (2) having a family history of genetic disease; (3) with body mass index (BMI) ≥28 kg/m2, or ≤18.5 kg/m2; (4) having a history of hypertension more than 3 years (systolic blood pressure [SBP] ≥ 140 mm Hg or diastolic blood pressure ≥90 mm Hg); (5) having abnormalities in the heart, liver, lungs, and kidneys; (6) received surgery within 4 months or blood transfusion or donation within 6 months; (7) took drugs within 2 weeks; (8) excessive smoking (more than 20 cigarettes per day) or drinking (more than 30 g per day); (9) excessive labor or exercise within two weeks. Participants’ age, blood pressure, and other biochemical indicators and the above information were collected by self-made questionaries, which participants completed before receiving Hcy detection. The ethics committee of West China Second University Hospital approved the study. All participants gave informed consent before the study commenced. The remaining number of participants included for the analysis of reference intervals of serum Hcy levels for pregnant women is shown in . This longitudinal study investigates the trimester-specific reference intervals of serum Hcy levels for pregnant women in China.
Laboratory analyses
All participants were asked to maintain a normal lifestyle and avoid excessive physical labor, alcohol, and smoking for 3 days before laboratory tests of Hcy. We told participants to fast overnight for at least 8 h and sit for at least 30 min before specimen collection. A separation gel vacutainer collected about 5 ml of blood (BD Biosciences, New Jersey, USA). Blood samples were left at room temperature for 30 min, and followed by centrifugation at 2500 to 3000 rpm. Serum Hcy levels were detected on Siemens ADVIA Chemistry XPT System (Siemens Healthcare, New York, USA) using Hcy reagent (MEIKANG Biotech, Ning Bo, China) by enzyme cycling method within 2 h after centrifugation. All experimental operations were carried out according to standard operating procedures calculated by referring to the operating manual. The limitation of Hcy measuring by enzyme cycling method was 3.5–50.0 μmol/l. In addition, two control concentrations of Hcy were also tested for quality control. The accuracy and precision were calculated referred to the document EP15-A described by CLSI, and were expressed as recovery and total coefficient of variation (CV), respectively. The recovery ranged from 95% to 105% and a total CV <5% were regarded as suitable for requirement of the protector.
Statistical analyses
Kolmogorov-smirnov test were performed to detect the distribution of continuous variables. Variables with a normal distribution were expressed as a mean ± standard deviation (SD) and were compared by the Student’s t-test or an analysis of variance (ANOVA). Variables with abnormal distribution were expressed as medians (interquartile range) and were compared by the Mann-Whitney U test or the Kruskal-Wallis H-test. Nonparametric methods were performed to establish trimester-specific reference intervals of serum Hcy levels. The 2.5th and 97.5th percentiles were calculated to express the minimum and maximum reference intervals. The p < 0.05 were considered statistically significant. All data analyses were performed by SPSS 22.0 software (SPSS, USA).
Results
Baseline characteristics of participants
The average age was 31.9 ± 4.7, 31.1 ± 4.3, 31.3 ± 4.0, and 30.4 ± 3.6 for included non-pregnant women and pregnant women in the first, second and third trimester of gestation, respectively. SBP, diastolic blood pressure (DBP), total cholesterol (TC) levels and triglyceride (TG) levels were also collected for these participants. The above results are shown in .
Table 1. Baseline Characteristic of the participants.
Serum Hcy levels and reference intervals in nonpregnant and pregnant women
Komlogorov-Smimov test showed that serum Hcy levels was abnormally distributed for pregnant women (p < 0.05).Still for non-pregnant women, serum Hcy levels showed normal distribution (p = 0.20). There was a statistically significant difference for serum Hcy levels between non-pregnant women and pregnant women (p < 0.05). So, reference intervals of serum Hcy for healthy non-pregnant and pregnant women should be established separately (as shown in ).
Table 2. The RIs of serum Hcy for healthy nonpregnant women and healthy pregnant women (μmol/L).
Serum Hcy levels and reference intervals in pregnant women during different trimesters of gestation
Komlogorov-Smimov test showed that serum Hcy levels were generally distributed in the first and second-trimester group (p > 0.05), and were not generally distributed in third-trimester group (p < 0.05). There were statistically significant differences for serum Hcy levels among pregnant women in the first, second, and the third trimesters of gestation (p < 0.05). According to CLSI C28-A3 guidelines, the RIs of serum Hcy and 90% CI for healthy non-pregnant women and healthy pregnant women were calculated. The reference intervals of serum Hcy levels for non-pregnant women were 5.59 ∼ 13.10 μmol/l. For pregnant women, reference intervals of serum Hcy levels were 4.35 ∼ 10.16 μmol/l in the first trimester, 3.38 ∼ 8.60 μmol/l in the second trimester and 3.75 ∼ 11.17 μmol/l in the third trimester (as shown in ).
Discussion
Healthy women were selected according to the exclusion criteria developed by the CLSI C28-A3 guidelines in this study to explore the physiological changes of serum Hcy during pregnancy. It is also worth mentioning that BMI using 28 kg/m2 is more suitable for Chinese people [Citation19].We found that (1) the reference interval of serum Hcy levels in non-pregnant women was 5.59 ∼ 13.10 μmol/l, (2) for pregnant women, reference intervals of serum Hcy levels were 4.35 ∼ 10.16 μmol/l in the first trimester, 3.38 ∼ 8.60 μmol/l in the second trimester and 3.75 ∼ 11.17 μmol/l in the third trimester. The results of our study will provide a practical reference value for clinical work and laboratory research.
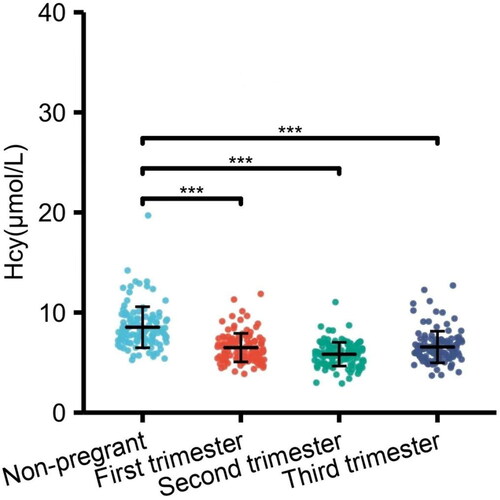
In our research, the serum Hcy levels of pregnant women was lower than that of non-pregnant women. Previous studies also showed the similar results, Yang et al. found that serum Hcy levels are 11.15 μmol/l in non-pregnant women compared with 8.66 μmol/l in pregnant women [Citation20]. Úbeda et al. demonstrated that serum Hcy levels were 30%–60% lower in pregnant women than in non-pregnant women [Citation21]. We also found that the serum Hcy levels decreased from the first trimester and reached the lowest value during the second trimester; although it steadily increased in the third trimester, it remained at a lower level than in non-pregnant women (). These findings are consistent with some previous work. Walker et al. reported that the normal values of Hcy during pregnancy in Canadian women are 5.6 ± 1.6 μmol/l in the first trimester, 4.3 ± 1.0 μmol/l in the second trimester and 5.6 ± 2.3 μmol/l in the third trimester [Citation17]. Murphy et al. demonstrated that the serum Hcy in Spanish women was 6.48 ± 1.30 μmol/l, 5.22 ± 1.29 μmol/l, and 5.16 ± 1.32 μmol/l in gestational week 8, 20, and 32, respectively [Citation15]. Velzing-Aarts et al. found that the serum Hcy during pregnancy in American were 9.42 μmol/l (5.50 ∼ 16.12 μmol/l) in gestational 8–16 weeks, 7.33 μmol/l (4.25 ∼ 12.64 μmol/l) in gestational 20–28 weeks and 7.60 μmol/l (4.46 ∼ 12.96 μmol/l) in gestational 36–42 weeks [Citation21].The reason of the slight discrepancy in values probably is the difference in race, region and sample type, and some studies use geometrical mean rather than median to analyze the variation trend [Citation20].
Figure 2. The change of serum Hcy levels (median) in healthy nonpregnant and pregnant woman in different trimesters.
Hcy is a promising biochemical marker, an early warning for common gestational diseases, such as preeclampisa, abortions, gestational diabetes, preterm birth. Lower Hcy levels in pregnancy may represent a physiologic adaptation to pregnancy mediated by the increase in estradiol concentrations during pregnancy [Citation22]. Holmes et al. proposed that a lower Hcy in pregnancy is associated with an altered maternal amino acid metabolism driven by fetal requirements [Citation23]. In addition, the lower Hcy level during pregnancy may be due to the hemodilution caused by the increased blood volume and elevated glomerular filtration rate .The fetus may also absorb a proportion of Hcy during pregnancy [Citation24].For pregnant women, partial RIs used in laboratory tests are still the same as that of the average population, which has not considered the unique physiological and biochemical changes during pregnancy [Citation20]. Establishing a medical serum Hcy RIs for commonly used testing items for pregnant women is conducive to improving the diagnostic level of clinicians for pregnant women, making subsequent examinations and treatments more rational, ensuring more significant utilization of medical resources, and so on.
There were some limitations for this study. At first, this study was single-central designed, only data from healthy pregnant women in Southwest China were included. When the results are expanded to other areas or other ethnic groups, they should be applicated with caution. Secondly, We known that continuous folic acid supplementation reduces HCY in pregnant women. Still, it is not related to the MTHFR gene polymorphism [Citation2], and we cannot determine whether pregnant women are taking folic acid and its dose. Therefore, when designing the experiment, we set the population as a random healthy population and did not collect folate and VB12 related data. As for VB6, we did not collect this data because the laboratory did not conduct this project. Last but not least, only blood pressure and two biochemical indicators were investigated in this study because of economic factors, which made this study incomplete in some degree.
In conclusion, pregnant women have lower serum Hcy levels than non-pregnant women, and the stories of serum Hcy in different trimester of gestation are different. We established trimester-specific reference intervals for pregnant women, which may provide a valuable reference for HHcy diagnosis, even predicting adverse pregnancy outcomes during pregnancy.
Authors contributions
Fan Yu designed the research study. Qing Li and Yuanting Tang performed data collection and data analysis. Hai Liu wrote the manuscript. Ting Liu made the tables and figures. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank all health workers for providing the data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Boyama BA, Cepni I, Imamoglu M, et al. Homocysteine in embryo culture media as a predictor of pregnancy outcome in assisted reproductive technology. Gynecol Endocrinol. 2016;32(3):1–195. doi:10.3109/09513590.2015.1102877.
- Mehrabani ZHA, Ghorbanihaghjo A, Melli MS, et al. Effects of folic acid supplementation on serum homocysteine and lipoprotein (a) levels during pregnancy. Bioimpacts. 2015;5(4):177–182. doi: 10.15171/bi.2015.26.
- Shuang L, Yuanpeng Z, Huijun W, et al. The effect of multiple single nucleotide polymorphisms in the folic acid pathway genes on homocysteine metabolism. Biomed Res Int. 2014;2014:560183. doi:10.1155/2014/560183.
- Ramin A, Ali M, Eric M, et al. Erratum: hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2014;10(4):281–288. doi: 10.3988/jcn.2014.10.4.281.
- Miner SE, Evrovski J, Cole DE. Clinical chemistry and molecular biology of homocysteine metabolism: an update. Clin Biochem. 1997;30(3):189–201. doi: 10.1016/s0009-9120(96)00172-5.
- Sukumar N, Adaikalakoteswari A, Venkataraman H, et al. Vitamin B12 status in women of childbearing age in the UK and its relationship with national nutrient intake guidelines: results from two national diet and nutrition surveys. BMJ Open. 2016;6(8):e11247. doi: 10.1136/bmjopen-2016-011247.
- Freyburger G, Labrouche S, Sassoust G, et al. Mild hyperhomocysteinemia and hemostatic factors in patients with arterial vascular diseases. Thromb Haemost. 1997;77(3):466–471. doi: 10.1055/s-0038-1655990.
- Van Guldener C, Stehouwer CDA. Hyperhomocysteinemia, vascular pathology, and endothelial dysfunction. Semin Thromb Hemost. 2000;26(3):281–289. doi: 10.1055/s-2000-8472.
- Ciaccio M, Bivona G, Bellia C. Therapeutical approach to plasma homocysteine and cardiovascular risk reduction. TCRM. 2008;4(1):219–224. doi: 10.2147/TCRM.S1807.
- Eskes TK. Clotting disorders and placental abruption: homocysteine–a new risk factor. Eur J Obstet Gynecol Reprod Biol. 2001;95(2):206–212. doi: 10.1016/s0301-2115(00)00492-9.
- Mascarenhas M, Habeebullah S, Sridhar MG. Revisiting the role of first trimester homocysteine as an index of maternal and fetal outcome. J Pregnancy. 2014;2014:123024. doi: 10.1155/2014/123024.
- Dodds L, Fell DB, Dooley KC, et al. Effect of homocysteine concentration in early pregnancy on gestational hypertensive disorders and other pregnancy outcomes. Clin Chem. 2008;54(2):326–334. doi: 10.1373/clinchem.2007.097469.
- Gaiday AN, Tussupkaliyev AB, Bermagambetova SK, et al. Gaiday AN, Tussupkaliyev AB, Bermagambetova SK, et al. Effect of homocysteine on pregnancy: a systematic review. Chem Biol Interact. 2018;293:70–76. doi:10.1016/j.cbi.2018.07.021.
- Yajnik CS, Deshpande SS, Jackson AA, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the pune maternal nutrition study. DIABETOLOGIA. 2008;51(1):29–38. doi: 10.1007/s00125-007-0793-y.
- Murphy MM, Scott JM, Arija V, et al. Maternal homocysteine before conception and throughout pregnancy predicts fetal homocysteine and birth weight. Clin Chem. 2004;50(8):1406–1412. doi: 10.1373/clinchem.2004.032904.
- Jansson T. Novel mechanism causing restricted fetal growth: does maternal homocysteine impair placental amino acid transport? J Physiol. 2009;587(Pt 17):4123. doi: 10.1113/jphysiol.2009.178327.
- Walker MC, Smith GN, Perkins SL, et al. Changes in homocysteine levels during normal pregnancy. Am J Obstet Gynecol. 1999;180(3 Pt 1):660–664. doi: 10.1016/s0002-9378(99)70269-3.
- Velzing-Aarts FV, Holm PI, Fokkema MR, et al. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am J Clin Nutr. 2005;81(6):1383–1389. doi: 10.1093/ajcn/81.6.1383.
- Gao M, Wei YX, Lyu J, et al. China kadoorie biobank collaborative group. [the cut-off points of body mass index and waist circumference for predicting metabolic risk factors in Chinese adults]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40(12):1533–1540.
- Yang Y, Jiang H, Tang A, et al. Changes of serum homocysteine levels during pregnancy and the establishment of reference intervals in pregnant Chinese women. Clin Chim Acta. 2019;489:1–4. doi: 10.1016/j.cca.2018.11.026.
- Úbeda N, Reyes L, González-Medina A, et al. Physiologic changes in homocysteine metabolism in pregnancy: a longitudinal study in Spain. Nutrition. 2011;27(9):925–930. doi: 10.1016/j.nut.2010.10.017.
- Mangoni AA, Jackson SH. Homocysteine and cardiovascular disease: current evidence and future prospects. Am J Med. 2002;112(7):556–565. doi: 10.1016/s0002-9343(02)01021-5.
- Holmes VA, Wallace JM, Alexander HD, et al. Homocysteine is lower in the third trimester of pregnancy in women with enhanced folate status from continued folic acid supplementation. Clin Chem. 2005;51(3):629–634. doi: 10.1373/clinchem.2004.032698.
- Cikot RJLM, Steegers-Theunissen RPM, Thomas CMG, et al. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br J Nutr. 2001;85(1):49–58. doi: 10.1079/bjn2000209.