ABSTRACT
Background
Anti-Müllerian hormone (AMH) has recently emerged as a promising biomarker for the detection of polycystic ovarian morphology. In polycystic ovary syndrome (PCOS), an elevated level of AMH has been suggested to add value to the Rotterdam criteria in cases of diagnostic uncertainty. In this study, we evaluated the correlation between AMH and PCOS, and the potential role of AMH in PCOS diagnosis.
Methods
A case-control study was performed on a total of 200 females, 100 of which were diagnosed with PCOS as per Rotterdam revised criteria (2003) and 100 as the control (non-PCOS group). Patient medical records were therefore retrieved for clinical, biochemical and ultrasound markers for PCOS diagnosis. Sensitivity, specificity, area under receiver operating characteristic (AUROC) curve, and multivariate linear regression models were applied to analyze our data.
Results
Mean serum levels of LH and AMH, and LH/FSH ratio were significantly different between compared groups. In the PCOS group, the mean serum AMH level was 6.78 ng/mL and LH/FSH ratio was 1.53 while those of controls were 2.73 ng/mL and 0.53, respectively (p < .001). The most suitable compromise between 81% specificity and 79% sensitivity was obtained with a cutoff value of 3.75 ng/mL (26.78 pmol/L) serum AMH concentration for PCOS prediction, with an AUROC curve of 0.9691.
Conclusion
Serum AMH cutoff level of 3.75 ng/mL was identified as a convenient gauge for the prediction of PCOS and an adjuvant to the Rotterdam criteria.
Introduction
Polycystic ovary syndrome (PCOS), the most common endocrine and gynecological disease in women of reproductive age, is a complex disorder distinctively identified by the union of hyperandrogenism (HA) and ovulatory dysfunction [Citation1]. PCOS is associated with a range of disordered reproductive features including menstrual irregularities and infertility [Citation2]. PCOS being the most common cause of oligo/amenorrhea (OA), makes it particularly correlated with primary infertility [Citation2,Citation3]. Additionally, patients with PCOS have been reported to express multiple metabolic manifestations including insulin resistance (in 35% of patients), obesity (35%), and dyslipidemia (70%), that still manifest even post-menopause [Citation2,Citation4].
Being a multifaceted disorder, several hormonal disturbances are associated with PCOS. For instance, previous studies have reported high levels of luteinizing hormone (LH) in PCOS, which stimulates hyper-secretion of androgens by theca cells [Citation5]. Likewise, numerous studies reported low levels of follicular stimulating hormone (FSH) in PCOS, resulting in incomplete growth of and failure in selecting a dominant follicle for ovulation, leading to chronic anovulation [Citation5,Citation6].
Anti-Mullerian Hormone (AMH), a glycoprotein that belongs to the transforming growth factor (TGF-β) family, is exclusively synthesized by granulosa cells in women [Citation7]. Besides its crucial embryonic role in sex determination by suppressing the Mullerian system to pave the way for male internal genitalia [Citation8], AMH has also been considered responsible for the ovarian dysfunction in PCOS [Citation9], and is thought to play a significant role in folliculogenesis [Citation10]. Evidence suggests that AMH may hinder follicular maturation and ovulation by inhibiting pre-antral follicles growth [Citation11], and FSH-stimulated aromatase enzyme expression [Citation12], which is essential for ovarian steroidogenesis. Furthermore, research suggests that AMH production is increased in women with PCOS [Citation13,Citation14], though the underlying cause for this elevation remains unclear. Elevated serum AMH levels in individuals with PCOS were also linked to more unfavorable clinical, endocrinological, and metabolic conditions [Citation15].
The physical and psychological impact of PCOS on the patient necessitates the existence of a multi-disciplinary team approach for its diagnosis, treatment, and follow-up [Citation5,Citation16]. Presently, the Rotterdam 2003 criteria has been the benchmark for PCOS diagnosis [Citation1]. One of its pitfalls, however, is the reliance on subjective input like ultrasound findings, patient’s recall of OA, and clinical hyperandrogenism [Citation17]. Therefore, scoring for AMH biomarker levels, either solely or along with the Rotterdam 2003 criteria, currently serves as a practical, quantitative, and effective approach for PCOS diagnosis [Citation18]. The advantage of relying on AMH levels over other reproductive hormones, such as inhibin B, estradiol (E2), LH, and FSH, is particularly attributed to its persistently high concentration throughout the menstrual cycle, pregnancy, and oral contraceptive usage. Moreover, unlike other hormones, it is an appropriate evocative of a female’s ovarian reserve [Citation17,Citation19].
In 2017, based on Rotterdam 2003 criteria, a meta-analysis by Ding et al. reported the prevalence of PCOS in Middle Eastern Women to be 16% [Citation20]. In Qatar, a study conducted on a cohort of 720 females reported 12.1% prevalence of PCOS [Citation21]. Owing to this remarkable prevalence of PCOS, national, regional, and international efforts are now directed toward the establishment of better predictive and diagnostic tools for PCOS. In this study, we recorded AMH levels in a sample of patients with and without PCOS to assess the correlation between AMH and PCOS, and to evaluate the sensitivity and specificity of using AMH as a potential biomarker to aid in PCOS diagnosis. We also studied the association between several other hormonal parameters including FSH, LH, E2, prolactin, thyroid stimulating hormone (TSH) and PCOS occurrence.
Methods
Participants and subject selection
The study population was selected from women who presented to the infertility clinic at Hamad Medical Corporation (HMC) in Qatar between January 2015 and December 2018. A total of 350 patient records were reviewed and after applying exclusion and inclusion criteria, 100 PCOS diagnosed and 100 non-PCOS female patients between the ages of 18 and 44 inclusive, were selected to constitute our case and control groups. We used convenient sampling – all subjects recruited from the infertility clinic – as this is the only clinic in HMC that measures AMH routinely for all patients. The case subjects must be clinically diagnosed with PCOS according to Rotterdam 2003 consensus, while the controls must not have a clinical diagnosis of PCOS and must not satisfy the Rotterdam criteria. Moreover, women with any other endocrine etiologies such as androgen-secreting tumors, congenital adrenal hyperplasia, hyperprolactinemia, and Cushing’s syndrome, were excluded. Rotterdam 2003 consensus defines PCOS by presence of at least two of the following three features: (1) oligo/amenorrhea (OA); (2) hyperandrogenism (HA), defined as clinical and/or biochemical evidence of Hyperandrogenism (Ferriman-Gallwey score >8; free androgen index >4, respectively); (3) polycystic ovarian morphology (PCOM) by transvaginal or pelvic ultrasound examination defined as, minimum of 12 follicles with 2–9 mm diameters in each ovary and/or increasing ovarian volume with a minimum size of 10 mm3 [Citation5].
Inclusion criteria:
All patients are females given the nature of the disease.
All patients are between the age of 18–44 years.
Exclusion criteria:
Patients with other endocrine diseases (e.g. 21-hydroxylase deficiency, hyperprolactinemia, Cushing’s disease and androgen-secreting tumors).
Presence of ovarian gynecological disorders.
Determining sample size
A sample of 66 from the positive group (people with the disease) and 66 from the negative group (people without the disease) would achieve 80% power to detect a difference of 0.08 between the area under receiver operating characteristic (AUROC) curve under the null hypothesis of 0.75 and an AUROC curve under the alternative hypothesis 0.83 using a two-sided z-test at a significance level of 0.05.
Data collection
For this study, information about the nationality, age, body mass index (BMI), levels of vitamin D, AMH, LH, FSH, E2, prolactin, TSH, presence of oligo/amenorrhea, ultrasound findings of PCOM, clinical evidence of hyperandrogenemia (hirsutism and acne), were extracted from the medical records at the infertility clinic of HMC.
Data analysis
AUROC curve was applied to determine the minimum cutoff with the best sensitivity and specificity of AMH in order to assess the diagnostic strength of AMH as a biomarker for PCOS. Multivariable logistic regression models were carried out to adjust for any confounding variables. All values are given as mean ± Standard error (SE) and 95% confidence interval (CI) unless otherwise specified. A p-value of <.05 was considered statistically significant. All Statistical analysis was performed in the College of Medicine at Qatar University using STATA 16.0 software.
Results
Demographic, clinical and endocrine characteristics of participants
We identified 200 subjects aged 18–44, 100 were PCOS cases and 100 were controls. The mean age of cases was slightly lower than that of controls. The majority of subjects (66% of cases and 57% of controls) were below 35 years of age. Additionally, participants were divided into four groups according to their BMI. One hundred fifty-eight participants were either overweight or obese and approximately half of them were PCOS cases. In terms of clinical features, 68 participants reported hirsutism, among which 82% were cases. Also, participants who reported having menstrual irregularities were mostly PCOS patients. Additionally, 14% of those who had PCOM on ultrasound did not satisfy the Rotterdam criteria for PCOS diagnosis, and hence, were classified as controls. Overall, there was no significant difference between cases and controls in all baseline characteristics, except PCOS clinical features. Demographic, clinical and endocrine characteristics of the PCOS group and non-PCOS group are summarized in .
Table 1. Demographic and clinical characteristics of PCOS group and non-PCOS group.
Comparison of hormone levels
The mean AMH level in cases was 6.80 ng/mL (CI: 5.99–7.60) compared to 2.72 ng/mL (CI: 2.19–3.25) in controls, which represents a 150% increase in the PCOS group. This difference was found to be statistically significant (p < .001). illustrates the distribution of AMH levels in cases and controls. Additionally, the mean LH level was significantly higher in cases compared to controls (6.17 mIU/mL, CI: 5.05–7.29 vs. 4.06 mIU/mL, CI: 3.62–4.50; p < .001). Mean levels of FSH, E2, and prolactin were lower in patients with PCOS compared to controls. However, the differences in the mean levels of these hormones were not statistically significant. The mean hormone levels in cases and controls are summarized in .
Figure 1. Two box plots representing the distribution of AMH levels in PCOS group and non-PCOS group. The minimum, median and maximum values of AMH are higher in the PCOS group compared to the non-PCOS group.
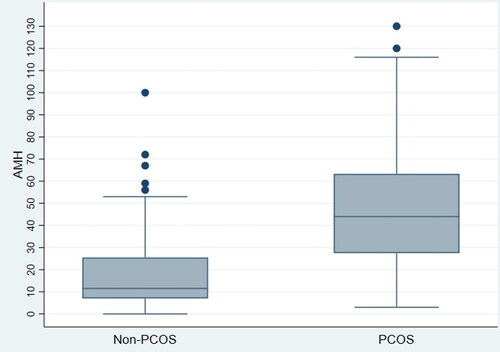
Table 2. Hormone levels in PCOS group and non-PCOS group.
AMH and other possible predictors in correlation with PCOS
A logistic regression model was utilized to investigate the correlation between PCOS and multiple possible predictors, including age, BMI, AMH, LH/FSH ratio, LH, FSH, E2, prolactin, TSH and vitamin D levels. Using a univariable logistic regression, we found that for every unit increase in AMH, a 5.7% increase in the odds of being a PCOS case is expected at any value of AMH (OR: 1.057, CI: 1.039–1.075; p < .001). This model also showed that LH level and LH/FSH ratio were significantly correlated with PCOS (). Further investigation with a forward multivariable logistic regression revealed that AMH and LH/FSH ratio were the only significant predictors associated with PCOS ().
Table 3. Possible predictors of PCOS (univariate model).
Table 4. Possible predictors of PCOS after adjustment.
Anti-Müllerian hormone cutoff value
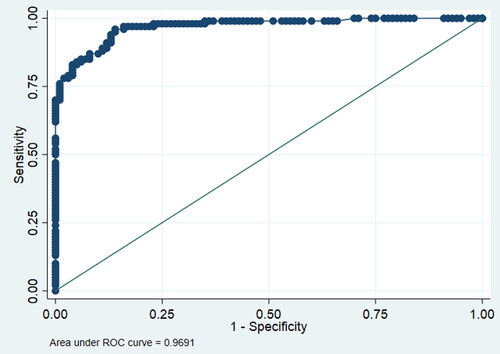
In an attempt to determine the value of AMH in predicting PCOS or as an additional criterion for PCOS diagnosis, a receiver operator curve was generated (). AUROC curve yielded a satisfactory result of 0.9691, with AMH cutoff value of 3.75 ng/mL (26.78 pmol/L) signifying a satisfactory specificity and sensitivity of 81% and 79%, respectively.
Figure 2. Receiver operating characteristic (ROC) curve for evaluating the diagnostic strength of anti-müllerian hormone (AMH) as a biomarker for polycystic ovary syndrome (PCOS). Area under the ROC curve = 0.9691. Cutoff value of AMH for predicting PCOS diagnosis = 26.8 pmol/L with specificity and sensitivity of 81% and 79% respectively.
Discussion
Characteristics of cases and controls
In the present study, there was no significant difference between the mean age of PCOS cases and controls, which is in congruence with the findings of other studies [Citation22,Citation23]. However, surprisingly, about 79% of the women in our study were obese or overweight as seen in . Moreover, our results, in consistency with others [Citation24–26], showed a significant linear association between women with PCOS with a BMI of 24 kg/m2 or more during their reproductive age, and the risk of anovulatory infertility. Thus, a healthy and controlled lifestyle with a maintained BMI of 18.5–24 kg/m2 can improve the ovulatory cycles of PCOS women or at the very least decrease the severity of the disease [Citation27,Citation28].
It has been well established that the clinical features of PCOS have been shown to be attributed to the excessive androgens in women [Citation6,Citation29]. One of the features, hirsutism, which is defined as excessive hair growth in a male pattern, is believed to be linked to the hair follicle receptors responding to hormonal changes, mainly the increasing levels of free testosterone in PCOS women [Citation30]. This would explain the high response seen in PCOS women to anti-hirsutism medications and creams [Citation31]. Similarly, acne, another androgen related feature of PCOS, is believed to be due to the hormonal imbalance expected in PCOS women, resulting in the excessive production and accumulation of sebum, thus, manifesting as acne. In one study [Citation32], acne was explained by the division of adipose gland cells and the synthesis of intracellular fat due to stimulation by increased androgen levels. In addition to hirsutism and acne, menstrual irregularities, another clinical feature in PCOS women, has been shown to be due to the high androgen levels which in turn stimulate dysfunctional ovulation by preventing the maturation of ovaries, thus manifesting as oligomenorrhea or amenorrhea, and ultimately leading to infertility [Citation33,Citation34]. Our findings are in congruence with Stein and Leventhal [Citation35], who, in 1935, were the first to describe the correlation of hirsutism and oligo/amenorrhea in PCOS and for this reason, PCOS is also known as Stein – Leventhal Syndrome.
AMH role in the detection of PCOM and PCOS
AMH has been emerging recently as a viable alternative to ultrasound. In addition to its low cost and independency of operator ultrasound skills, AMH has been well established to be a significant correlator and a biologic marker for the antral follicle counts (AFC), thus reflecting the ovarian reserve in the female population. Therefore, it can potentially replace the ultrasound in the detection of PCOM and hence it may play a role in the diagnosis of PCOS [Citation14,Citation36]. Moreover, these benefits may be particularly useful in the adolescent and reproductive age population and in certain cultures since evaluation of the ovaries by transvaginal ultrasonography may not be considered or even possible due to virginal status. In addition, the imaging quality of abdominal ultrasound is often impaired by obesity, which is prevalent in women with PCOS, as discussed before.
The strong involvement of AMH in the pathophysiology of PCOS has promoted the conduction of more research to assess the correlation between AMH and PCOS, and whether this biomarker could potentially be involved in facilitating the diagnosis of PCOS. According to the most recent PCOS guidelines, serum AMH levels should not yet be used as an alternative for PCOM detection or as a single test for PCOS diagnosis [Citation37]. Nonetheless, the authors left the door open for future contributions in this area. They stated that AMH tests are likely to become more accurate in the identification of PCOM with enhanced assay standardization, specified cutoff levels, and large-scale validation in populations of all ages and ethnicities [Citation37]. Therefore, our present study attempts to contribute to these efforts by showing that serum AMH level is strongly associated with PCOS (; ) and 150% higher in PCOS patients compared with the non-PCOS subjects (p < .001). Our findings also are consistent with several studies [Citation14,Citation38–40] which reported increased serum AMH levels in women with PCOS compared with controls, and proposed AMH as a diagnostic marker in PCOS. Moreover, AMH levels can also serve as a valuable tool to guide in understanding treatment response, enable personalized management, and predict both reproductive and long-term metabolic outcomes [Citation15].
Sensitivity and specificity
Our study showed that the best diagnostic potential of AMH was observed at a cutoff of 3.75 ng/mL (26.78 pmol/L), with a sensitivity and a specificity of 79% and 81%, respectively. A comparable AMH cutoff of 3.34 ng/mL was found in another study [Citation41] conducted in India, with a sensitivity and specificity of 98% and 93%, respectively. Our cutoff value was consistent to that of others in previous studies. In a study by Woweiko et al. [Citation42], which was conducted on a Taiwanese female population, a cutoff of 3.5 ng/mL yielded a respectable sensitivity and specificity of 74% and 79%, respectively. Similarly, another study by Dewailly et al. [Citation23], showed that the best compromise between sensitivity and specificity (76.1% and 74.6% respectively) was at a cutoff of 4.9 ng/mL. In addition, one study observed a higher sensitivity and specificity of 92% and 97%, respectively, at the same cutoff of 4.9 ng/mL. They concluded that AMH not only reflects AFC but also the degree of hyperandrogenism offering AMH as a better marker than follicle numbers per ovary [Citation43].
Other correlates in PCOS
LH/FSH ratio
Our study’s findings, in consistency with others [Citation44,Citation45], showed a statistically significant elevation in the LH/FSH ratio. However, in a study by Taylor et al. [Citation46], it was revealed that only 45.4% of the PCOS diagnosed women had an elevated LH/FSH ratio, showing that not all women with PCOS will have an elevated ratio. Subsequently, this study and others revealed that other factors, such as insulin, serve as the main drivers for the androgenesis witnessed in PCOS women [Citation46–48]. Additionally, the LH/FSH ratio’s variability during the menstrual cycle and with BMI also support the fact that an elevated ratio is not necessarily a sensitive diagnostic tool nor a predictor for PCOS [Citation45,Citation49].
Prolactin
Unlike the LH/FSH ratio, our study did not show a statistically significant correlation between the levels of prolactin and PCOS diagnosed women. This finding was not surprising as it was consistent with other studies [Citation45,Citation50]. In one study by Filho et al. [Citation51], where bromocriptine was given to a 100 PCOS patients, there was a significant decrease in prolactin levels but no effect on LH, FSH, ovulation and fertility. Moreover, another study by Li et al. [Citation52], revealed that the high levels of prolactin in women with PCOS, were explained by either exogenous medicinal intake or the presence of organic causes such as pituitary adenomas or macroprolactinomas. Similarly, another study by Parsanezhad et al. [Citation50], showed that, out of all the recruited women with PCOS, 16% had elevated prolactin levels, and out of the 16%, all were found to have an identifiable underlying cause for this elevated prolactin. The findings of these articles, in addition to our findings, suggest that a high prolactin level is neither a manifestation nor an association of PCOS.
Vitamin D
The findings of our study, in congruence with others [Citation53,Citation54], showed no statistically significant difference between Vitamin D levels and PCOS occurrence. However, although some studies did report an association, there was no clinical justification to be determined. The studies that did show an association, suggested that a possible explanation for the high prevalence of vitamin D deficiency in PCOS, is its association with insulin resistance, rather than with the disease itself [Citation55,Citation56]. Two articles [Citation57,Citation58] attempted to prove a correlation between vitamin D and PCOS by administering vitamin D supplements to PCOS patients and monitoring their hormonal parameters such as insulin levels. However, the attempts of these articles were also contradicted by other studies [Citation59,Citation60]. The discrepancies in results presented weaken the possible existence of a correlation between vitamin D and PCOS occurrence.
Study limitations
Potential perceived limitations of this study include the fact that all the analyzed data were from the infertility clinic at HMC, possibly contributing to selection bias. As explained previously, this is the only clinic in HMC that measures AMH routinely for all patients, which made it convenient for us to retrospectively recruit a sufficient number of cases and controls. Secondly, the metrics that we used to define PCOM relied on the Rotterdam 2003 consensus since the population of this study was selected from women who presented to the infertility clinic between 2015 and 2018. The definition of PCOM was revised in the latest PCOS guidelines (2018) and redefined as ‘a follicle number per ovary of ≥20 and/or an ovarian volume ≥10 mL on either ovary, ensuring no corpora lutea, cysts or dominant follicles are present’ [Citation37]. Changing the cutoff of the minimum number of follicles from 12 to 20 in the new guidelines is likely to make this criterion less sensitive and more specific. Thus, it is possible that some of the subjects that we classified as cases using the 2003 consensus (in whom we established that AMH is significantly higher) would be now classified as controls according to the new cutoff, given that they do not satisfy the other two criteria. Consequently, the observed associations may be dampened if the new cutoff was to be used. Thirdly, AMH interpretation is laboratory assay-dependent and there is no international standard to interpret its levels so far.
Conclusion
In conclusion, the findings of our study confirm the strong correlation between AMH and PCOS, and support the utilization of AMH as a screening test or an adjuvant to the Rotterdam criteria for PCOS diagnosis. Its linear relationship with PCOS and steadily elevated level throughout the course of the disease, makes it a valuable tool that deserves attention. Our findings, along with others, widen the possibilities for further research to be done using larger sample sizes for reliability, validation, and determination of a globally accepted AMH threshold for PCOS diagnosis. Consistency in results regarding this emerging biomarker will offer a feasible solution for challenges faced by the clinicians and researchers managing PCOS.
Authors’ contributions
All the authors contributed to the collection of data. MOM led the team, performed data analysis, contributed to the writing of the results and discussion, and reviewed and edited the final draft. SK wrote the methods and contributed to the writing of the introduction and discussion. ARI performed data analysis, contributed to the writing of the results and discussion. HS, SA, MA, performed data interpretation and comparison with other paper, in addition to writing the bulk of the discussion. AA contributed to the writing of the introduction and discussion. LA originated the research idea, formulated the research protocol, and took responsibility for study supervision and revision.
Ethical approval
This study has been approved by Institutional Review Board of Hamad Medical Corporation (MRC-01-19-144) and the Institutional Review Board of Qatar University (QU-IRB 1055-E/19). This study was conducted in full conformance with principles of the ‘Declaration of Helsinki’, Good Clinical Practice (GCP) and within the laws and regulations of Ministry of Public Health in Qatar. This research used medical records only (i.e. no follow up or interference in treatment). Also, patients’ identities were not disclosed. Therefore, there was no need for informed consent. This approach was approved by the Institutional Review Board of Hamad Medical Corporation (MRC-01-19-144) who stated the following in the Data Sharing Agreement ‘Except as otherwise specified herein, Institution grants Recipient the right to use and disclose the Data solely in fulfillment of its professional obligations in relation to the project described in Research Protocol No: MRC-01-19-144’.
Informed consent
Consent was not required as we relied on medical records solely.
Acknowledgements
The authors would like to thank Hamad Medical Corporation for approving this research and facilitating the process of data collection. Open Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from Hamad Medical Corporation. Restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of Hamad Medical Corporation.
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):1–7. doi:10.1016/j.fertnstert.2003.10.004.
- Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10(8):2107–2111. doi:10.1093/oxfordjournals.humrep.a136243.
- Siddiqui S, Mateen S, Ahmad R, et al. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J Assist Reprod Genet. 2022;39(11):2439–2473. doi:10.1007/s10815-022-02625-7.
- Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism. 2018;86:33–43. doi:10.1016/j.metabol.2017.09.016.
- Ibáñez L, Oberfield SE, Witchel S, et al. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr. 2017;88(6):371–395. doi:10.1159/000479371.
- Harrison TNH, Chang RJ. Ovarian response to follicle-stimulating hormone in women with polycystic ovary syndrome is diminished compared to ovulatory controls. Clin Endocrinol (Oxf). 2022;97(3):310–318. doi:10.1111/cen.14708.
- Vigier B, Picard JY, Tran D, et al. Production of anti-Müllerian hormone: another homology between sertoli and granulosa cells. Endocrinology. 1984;114(4):1315–1320. doi:10.1210/endo-114-4-1315.
- Visser JA, de Jong FH, Laven JS, et al. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131(1):1–9. doi:10.1530/rep.1.00529.
- di Clemente N, Josso N, Gouédard L, et al. Components of the anti-Müllerian hormone signaling pathway in gonads. Mol Cell Endocrinol. 2003;211(1–2):9–14. doi:10.1016/j.mce.2003.09.005.
- Carlsson IB, Scott JE, Visser JA, et al. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–2227. doi:10.1093/humrep/del165.
- McGee EA, Smith R, Spears N, et al. Müllerian inhibitory substance induces growth of rat preantral ovarian follicles. Biol Reprod. 2001;64(1):293–298. doi:10.1095/biolreprod64.1.293.
- Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891–4899. doi:10.1210/endo.142.11.8486.
- Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957–5962. doi:10.1210/jc.2003-030727.
- Rudnicka E, Kunicki M, Calik-Ksepka A, et al. Anti-müllerian hormone in pathogenesis, diagnostic and treatment of PCOS. Int J Mol Sci. 2021;22(22):12507. doi:10.3390/ijms222212507.
- Malhotra N, Mahey R, Cheluvaraju R, et al. Serum anti-mullerian hormone (AMH) levels among different PCOS phenotypes and its correlation with clinical, endocrine, and metabolic markers of PCOS. Reprod Sci. 2023;30(8):2554–2562. doi:10.1007/s43032-023-01195-y.
- Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update. 2009;15(4):477–488. doi:10.1093/humupd/dmp008.
- Saxena U, Ramani M, Singh P. Role of AMH as diagnostic tool for polycystic ovarian syndrome. J Obstet Gynaecol India. 2018;68(2):117–122. doi:10.1007/s13224-017-1066-4.
- Piltonen TT, Komsi E, Morin-Papunen LC, et al. AMH as part of the diagnostic PCOS workup in large epidemiological studies. Eur J Endocrinol. 2023;188(6):547–554. doi:10.1093/ejendo/lvad065.
- Sinha S, Sharan A, Sinha S. Anti-Mullerian hormone as a marker of ovarian reserve and function. Cureus. 2022;14(9):e29214. doi:10.7759/cureus.29214.
- Ding T, Hardiman PJ, Petersen I, et al. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96351–96358. doi:10.18632/oncotarget.19180.
- Dargham SR, Ahmed L, Kilpatrick ES, et al. The prevalence and metabolic characteristics of polycystic ovary syndrome in the Qatari population. PLoS One. 2017;12(7):e0181467. doi:10.1371/journal.pone.0181467.
- Sahmay S, Atakul N, Aydogan B, et al. Elevated serum levels of anti-Müllerian hormone can be introduced as a new diagnostic marker for polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2013;92(12):1369–1374. doi:10.1111/aogs.12247.
- Wiweko B, Maidarti M, Priangga MD, et al. Anti-mullerian hormone as a diagnostic and prognostic tool for PCOS patients. J Assist Reprod Genet. 2014;31(10):1311–1316. doi:10.1007/s10815-014-0300-6.
- Rich-Edwards JW, Spiegelman D, Garland M, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13(2):184–190. doi:10.1097/00001648-200203000-00013.
- Sam S. Obesity and polycystic ovary syndrome. Obes Manag. 2007;3(2):69–73. doi:10.1089/obe.2007.0019.
- Dokuzeylül Güngör N, Güngör K, Celik N, et al. Impact of body mass index and vitamin D on serum AMH levels and antral follicle count in PCOS. Eur Rev Med Pharmacol Sci. 2023;27(1):179–187. doi:10.26355/eurrev_202301_30870.
- Sharif E, Rahman S, Zia Y, et al. The frequency of polycystic ovary syndrome in young reproductive females in Qatar. Int J Womens Health. 2017;9:1–10. doi:10.2147/ijwh.S120027.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43(2):298–309. doi:10.1016/j.rbmo.2021.05.008.
- Lobo RA. Hirsutism in polycystic ovary syndrome: current concepts. Clin Obstet Gynecol. 1991;34(4):817–826. doi:10.1097/00003081-199112000-00019.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic ovary syndrome. N Engl J Med. 2016;375(1):54–64. doi:10.1056/NEJMcp1514916.
- Randall VA, Thornton MJ, Hamada K, et al. Mechanism of androgen action in cultured dermal papilla cells derived from human hair follicles with varying responses to androgens in vivo. J Invest Dermatol. 1992;98(6 Suppl):86s–91s. doi:10.1111/1523-1747.ep12462307.
- Witchel SF, Oberfield SE, Peña AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3(8):1545–1573. doi:10.1210/js.2019-00078.
- Wang HS, Chard T. IGFs and IGF-binding proteins in the regulation of human ovarian and endometrial function. J Endocrinol. 1999;161(1):1–13. doi:10.1677/joe.0.1610001.
- Mantzou D, Stamou MI, Armeni AK, et al. Impaired sexual function in young women with PCOS: the detrimental effect of anovulation. J Sex Med. 2021;18(11):1872–1879. doi:10.1016/j.jsxm.2021.09.004.
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstetr Gynecol. 1935;29(2):181–191. doi:10.1016/S0002-9378(15)30642-6.
- Palomaki GE, Kalra B, Kumar T, et al. Adjusting antimüllerian hormone levels for age and body mass index improves detection of polycystic ovary syndrome. Fertil Steril. 2020;113(4):876–884.e2. doi:10.1016/j.fertnstert.2019.12.012.
- Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–379. doi:10.1016/j.fertnstert.2018.05.004.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(3):941–945. doi:10.1210/jc.2005-2076.
- Dewailly D, Pigny P, Soudan B, et al. Reconciling the definitions of polycystic ovary syndrome: the ovarian follicle number and serum anti-Müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab. 2010;95(9):4399–4405. doi:10.1210/jc.2010-0334.
- Woo HY, Kim KH, Rhee EJ, et al. Differences of the association of anti-Müllerian hormone with clinical or biochemical characteristics between women with and without polycystic ovary syndrome. Endocr J. 2012;59(9):781–790. doi:10.1507/endocrj.ej12-0055.
- Saikumar P, Selvi VK, Prabhu K, et al. Anti mullerian hormone: a potential marker for recruited non growing follicle of ovarian pool in women with polycystic ovarian syndrome. J Clin Diagn Res. 2013;7(9):1866–1869. doi:10.7860/jcdr/2013/5530.3337.
- Chao KC, Ho CH, Shyong WY, et al. Anti-mullerian hormone serum level as a predictive marker of ovarian function in taiwanese women. J Chin Med Assoc. 2012;75(2):70–74. doi:10.1016/j.jcma.2011.12.007.
- Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–3129. Nov doi:10.1093/humrep/der297.
- Al-Hashimy D, Al-Rikaby H, Al-Khayaat E. Study of some hormonal disorders associated with polycystic ovarian syndrome in women in Thi Qar governorate. J Coll Educ Pure Sci. 2019;9(2):25–31. doi:10.32792/utq.jceps.09.02.03.
- Malini NA, Roy George K. Evaluation of different ranges of LH: FSH ratios in polycystic ovarian syndrome (PCOS) - clinical based case control study. Gen Comp Endocrinol. 2018;260:51–57. doi:10.1016/j.ygcen.2017.12.007.
- Berger MJ, Taymor ML, Patton WC. Gonadotropin levels and secretory patterns in patients with typical and atypical polycystic ovarian disease. Fertil Steril. 1975;26(7):619–626. doi:10.1016/S0015-0282(16)41228-8.
- Hernández-Jiménez JL, Barrera D, Espinoza-Simón E, et al. Polycystic ovarian syndrome: signs and feedback effects of hyperandrogenism and insulin resistance. Gynecol Endocrinol. 2022;38(1):2–9. doi:10.1080/09513590.2021.2003326.
- Ezeh U, Pisarska MD, Azziz R. Association of severity of menstrual dysfunction with hyperinsulinemia and dysglycemia in polycystic ovary syndrome. Hum Reprod. 2022;37(3):553–564. doi:10.1093/humrep/deac001.
- Taylor AE, McCourt B, Martin KA, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248–2256. doi:10.1210/jcem.82.7.4105.
- Hayashida SA, Marcondes JA, Soares JMJr, et al. Evaluation of macroprolactinemia in 259 women under investigation for polycystic ovary syndrome. Clin Endocrinol (Oxf). 2014;80(4):616–618. doi:10.1111/cen.12266.
- Parsanezhad ME, Alborzi S, Jahromi BN. A prospective, double-blind, randomized, placebo-controlled clinical trial of bromocriptine in clomiphene-resistant patients with polycystic ovary syndrome and normal prolactin level. Int J Fertil Womens Med. 2002;47(6):272–277.
- Filho RB, Domingues L, Naves L, et al. Polycystic ovary syndrome and hyperprolactinemia are distinct entities. Gynecol Endocrinol. 2007;23(5):267–272. doi:10.1080/09513590701297708.
- Krul-Poel YH, Snackey C, Louwers Y, et al. The role of vitamin D in metabolic disturbances in polycystic ovary syndrome: a systematic review. Eur J Endocrinol. 2013;169(6):853–865. Dec doi:10.1530/eje-13-0617.
- Davis EM, Peck JD, Hansen KR, et al. Associations between vitamin D levels and polycystic ovary syndrome phenotypes. Minerva Endocrinol. 2019;44(2):176–184. doi:10.23736/s0391-1977.18.02824-9.
- Hahn S, Haselhorst U, Tan S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114(10):577–583. doi:10.1055/s-2006-948308.
- Contreras-Bolívar V, García-Fontana B, García-Fontana C, et al. Mechanisms involved in the relationship between vitamin D and insulin resistance: impact on clinical practice. Nutrients. 2021;13(10):3491. doi:10.3390/nu13103491.
- Wehr E, Trummer O, Giuliani A, et al. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164(5):741–749. doi:10.1530/eje-11-0134.
- Kotsa K, Yavropoulou MP, Anastasiou O, et al. Role of vitamin D treatment in glucose metabolism in polycystic ovary syndrome. Fertil Steril. 2009;92(3):1053–1058. doi:10.1016/j.fertnstert.2008.07.1757.
- Pal L, Berry A, Coraluzzi L, et al. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28(12):965–968. doi:10.3109/09513590.2012.696753.
- Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutr Res. 2012;32(3):195–201. doi:10.1016/j.nutres.2012.02.001.
