Abstract
Objective
GuizhiFulingWan (GFW) has been reported to be effective against polycystic ovary syndrome (PCOS) by possessing oxidative stress and inflammation which related to PI3K/AKT/NF-κB, Nrf2/HO-1 pathway. This study aims to probe the effects and mechanisms of GFW combined with rosiglitazone on PCOS via PI3K/AKT/NF-κB and Nrf2/HO-1 pathways.
Methods
A rat PCOS model established by dehydroepiandrosterone (DHEA) injection. The experiment was allocated to control, DHEA, GFW, rosiglitazone, GFW + rosiglitazone groups. Treatment for 30 days, we monitored weight and ovarian weight of rats. Fasting blood glucose (FBG), fasting insulin (FINS), homeostasis model assessment of insulin resistance (HOMA-IR), lipid metabolism indexes, estrous cycle and sex hormone-, inflammation-, oxidative stress-related factors were examined. Hematoxylin&eosin staining assessed ovarian tissue pathological changes. Western blot determined PI3K/AKT/NF-κB, Nrf2/HO-1 pathways-related markers.
Results
GFW and rosiglitazone treatment suppressed body weight and ovarian weight in PCOS rats. They also decreased FBG, FINS, HOMA-IR while inhibited total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) and enhanced high-density lipoprotein (HDL). They ameliorated estrous cycle, ovarian histological changes and follicular development. They restrained testosterone (T), luteinizing hormone (LH) and accelerated estradiol (E2), progesterone (P), follicle stimulating hormone (FSH). They inhibited glutathione peroxidase (GSH-Px), malondialdehyde (MDA), superoxide dismutase (SOD) in serum while increased GSH-Px, SOD and decrease MDA in ovarian tissues. They reduced C-reactive protein, interleukin-18 (IL-18), tumor necrosis factor-α (TNF-α), IL-6, IL-1β levels. GFW and rosiglitazone co-intervention regulated PI3K/AKT/NF-κB and Nrf2/HO-1 pathways in PCOS rats.
Conclusion
GFW alleviated ovarian dysfunction in PCOS rats, which may be related to the PI3K/AKT/NF-κB, Nrf2/HO-1 pathways.
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous, progressive, and refractory gynecological endocrine disease with unknown etiology [Citation1]. It was reported that PCOS is a hereditary disease that can affect women’s whole life, and the probability of daughters of PCOS patients suffering from PCOS is 60–70% [Citation2]. The clinical manifestations of PCOS are generally characterized by hyperandrogenemia, involving menstrual disorders, infertility, obesity, hirsutism, and acne [Citation3]. In addition, PCOS is often accompanied or induced by various complications, such as insulin resistance (IR), endometrial cancer, hypertension, metabolic syndrome, cardiovascular diseases, and low-grade chronic inflammation [Citation4,Citation5]. Due to the complex etiology of PCOS, it may be related to numerous factors such as genetics, diet, environment, and social psychology; coupled with its highly heterogeneous and diverse clinical symptoms, the treatment of PCOS is difficult [Citation6]. It has been reported that in PCOS, inflammation and oxidative stress are increased mainly through the PI3K/AKT/NF-κB signaling pathway [Citation7]. In addition, the activation of Nrf2/HO-1 pathway could inhibit oxidative stress and apoptosis of mouse ovarian granular cells, and reduce the phenotype of PCOS in mice [Citation8]. Therefore, it is feasible to study the relationship between these pathways and PCOS for the treatment of PCOS.
Western medicine treatment mainly focuses on adjusting the menstrual cycle, improving ovulation, surgery and lifestyle adjustment, etc., but none of them can permanently relieve the symptoms of patients, and may produce problems such as ovarian overstimulation, high ovulation rate, low pregnancy rate, and follicular luteinization [Citation9,Citation10]. As a result, an increasing number of PCOS patients seek complementary and alternative medical treatments, including traditional Chinese medicine (TCM).
The role of TCM and its active ingredients in the treatment of PCOS, diabetes, and improvement of IR has gradually attracted attention, providing a new idea for the development of PCOS-IR therapeutic drugs [Citation11]. GuizhiFulingWan (GFW), as a classic prescription for the treatment of gynecological diseases by Chinese physician Zhang Zhongjing in the Eastern Han Dynasty, is composed of Cinnamomi Ramulus, Poria, Paeoniae Alba Radix, Moutan Cortex, and Persicae Semen [Citation12]. According to the basic theory of TCM, GFW has the functions of dissipating phlegm and promoting blood circulation for removing blood stasis. GFW has been reported to be effective against PCOS in humans and animal models. For instance, GFW could effectively correct patients’ sex hormone disorders, ameliorate PCOS-IR, and play a positive role in ovulation and pregnancy [Citation13,Citation14]. Liu et al. clarified that GFW could effectively lessen autophagy of granulosa cells in PCOS rats by the activation of PI3K/AKT/mTOR pathway [Citation15]. In addition, GFW inhibited mice granulocyte autophagy of PCOS by H19/miR-29b-3p [Citation16]. GFW improved insulin sensitivity in PCOS insulin-resistant model rats by remodeling intestinal homeostasis [Citation17]. Furthermore, paeoniflorin, the active ingredient of Paeoniae Alba Radix in GFW, has been found to be effective against PCOS [Citation11]. Animal and in vitro experiments confirmed that paeoniflorin has the effect of improving IR [Citation18,Citation19]. However, the exact mechanism of GFW in the treatment of PCOS remains to be further probed.
Rosiglitazone belongs to thiazolidinediones, which can decrease plasma leptin levels in PCOS patients [Citation20] and ameliorate insulin sensitivity, thereby improving hyperandrogenism in PCOS patients, helping to restore menstrual cycle and advance ovarian ovulation [Citation21]. Currently, the efficacy of GFW combined with rosiglitazone on PCOS has not been reported. Therefore, this study took dehydroepiandrosterone (DHEA)-induced PCOS rat model as the research object to explore the specific mechanism of GFW combined with rosiglitazone in the treatment of PCOS, and to provide a reference for the treatment of PCOS with integrated traditional Chinese and Western medicine.
Materials and methods
Preparation of GFW extracting solution
Hangzhou Huadong Herbal Pieces Co., Ltd. offered and identified the Chinese Herbal Pieces of GFW. GFW extracting solution was prepared according to the process described in the Chinese Pharmacopeia (2010 Edition), as previously described [Citation22,Citation23]. In this study, the water-soluble components, fat-soluble components, and volatile oils of GFW were extracted, and the obtained extracts were uniformly mixed. After decoction and concentration according to a certain technological process, we obtained GFW extracting solution with crude drug content of 100 mg/mL.
Animals
SPF grade female SD rats (6-week old, 170–180 g) were acquired from Shanghai SLAC Laboratory Animal Co., Ltd. with the certificate number SCXK (Hu) 2017-0005. They were adaptively fed for 7 days in the Zhejiang Eyong Pharmaceutical Research and Development Center (SYXK (Zhe) 2021-0033) with a temperature- and humidity-controlled environment of 22 ± 2 °C and 50%-60% with a 12-h circadian rhythm. They had free access to water and normal chow.
Animal model of PCOS
In this study, 10 rats were randomly selected as the control group, and the remaining rats were used to prepare the PCOS model. The rats in the PCOS group were subcutaneously injected daily with DHEA (20200707, OKA Biotechnology Co., China) at a dose of 60 mg/kg body weight dissolved in 0.1 ml sesame oil, for 21 consecutive days [Citation24]. The control group received 0.1 ml sesame oil alone the same way once a day for 21 days. All rats underwent vaginal smear (Wright stain) examination every day after the molding to observe the morphological changes of epithelial cells and detect the estrous cycle of rats. Vaginal smears of rats that completely lost the changes of estrous cycle revealed that the model was successful.
Experimental design
In this study, 40 successfully modeled rats were selected and randomly divided into the DHEA group, GFW group, rosiglitazone group, and GFW + rosiglitazone group, with 10 rats in each group. The rats in the GFW group were given 200 mg/kg of GFW extracting solution by gavage. The group of rosiglitazone was administrated with 3 mg/kg rosiglitazone (R128083, Aladdin, China) by gavage. The group of GFW plus rosiglitazone received 200 mg/kg GFW extracting solution and 3 mg/kg rosiglitazone through gavage. The control and DHEA groups were given with saline alone the same way. All groups were continuously intervened for 30 days.
Vaginal smears (Wright stain)
All rats underwent vaginal smear examination every day to determine the stage of the estrous cycle. In brief, we collected vaginal cells by saline lavage every day at 9 am, followed by methanol fixation. After 5% Giemsa (G8220, Solarbio, China) staining, we assessed estrus under a light microscope (BX41, Olympus, Japan).
Examination of fasting blood glucose (FBG), fasting insulin (FINS), and homeostasis model assessment of IR (HOMA-IR)
After being anesthetized with isoflurane (induced at 4% and maintained at 3%), the blood samples were harvested from the orbital vein of rats. After centrifugation (2054×g, 10 min), we acquired serum samples. A blood glucose meter (580) offered by Yuwell (China) was selected to monitor FBG of each group. For determination of FINS, Rat Insulin ELISA Kit (PI606, Beyotime, China) was applied. HOMA-IR was calculated based on the formula: FBG (mmol/L) × FINS (mU/L)/22.5.
Determination of body weight and ovarian weight
The weight of the rats was monitored every two days throughout the experiment. Two hours after the last administration, we weighed the weight of each rat. After the last blood draw, all animals were euthanized by inhalation of excessive CO2. We immediately removed the ovarian tissue from each rat and weighed it. For fixation of partial ovarian tissues, 10% formalin (E672001, Sangon, China) was selected. The fixed tissues of rats were dehydrated, embedded, and then cut into 10 μm slices. The remaining ovarian tissue was used for Western blot analysis.
Determination of lipid metabolism levels
For determination of lipid metabolism levels, total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) levels were evaluated by applying an automatic biochemical analyzer (AU680, Beckman, USA).
Assessment of sex hormone levels
In this research, we determined serum sex hormone levels of rats. The contents of testosterone (T), estradiol (E2), progesterone (P), luteinizing hormone (LH), and follicle stimulating hormone (FSH) were analyzed utilizing rat T kit (MM-0577R1, MEIMAIN, China), rat E2 kit (MM-0575R1, MEIMAIN, China), rat P kit (MM-0551R1, MEIMAIN, China), rat LH kit (MM-0590R1, MEIMAIN, China), and rat FSH kit (MM-0566R1, MEIMAIN, China) respectively.
Hematoxylin and eosin (H&E) staining
H&E staining kit (BL700A) was introduced from Biosharp (China). After dewaxing and hydrating, hematoxylin was exploited to stain the slices of ovarian tissues (5 min) followed by differentiation with 1% hydrochloric acid (30 s). Next, 1% eosin solution was applied to counterstain the slices (120 s). Then, the slices underwent dehydration and transparency, which were then sealed by using neutral balsam (KGF032, KeyGEN, China). In the end, the pathological change of the ovarian tissues was analyzed by utilizing a microscopy (BX53M, Olympus, Japan) at the magnification of × 40, × 200, and × 400. Meanwhile, the follicles were classified and counted with the microscopy [Citation25].
Measurement of oxidative stress- and inflammation-related factors
Rat glutathione peroxidase (GSH-Px) kit (MM-20251R1), rat malondialdehyde (MDA) kit (MM-0385R1), rat superoxide dismutase (SOD) kit (MM-0386R1), rat C-reactive protein (CRP) kit (MM-0081R1), rat IL-1β kit (MM-0047R1), rat IL-6 kit (MM-0190R1), rat TNF-α kit (MM-0180R1), and rat IL-18 kit (MM-0194R1) were introduced from MEIMAIN (China). The contents of the above serum inflammation- and serum or ovarian tissue oxidative stress-related markers were measured with the manufacturer’s instructions.
Western blot
RIPA Buffer (KGP702, KeyGEN, China) was applied to extract total protein from the ovarian tissues. Next, BCA kit (KGP902, KeyGEN, China) was selected to quantify the lysed protein. After being denatured, the protein was electrophoresed for protein separation with 10% SDS-PAGE. The separated protein was transferred through loading onto nitrocellulose membrane, after which 5% bovine serum albumin (BSA, R00911, Leagene, China) was taken to block the membrane (37 °C, 60 min). Thereafter, the membrane underwent an overnight reaction with primary antibodies. The blots were visualized utilizing ECL reagent (GK10008, GlpBio, USA) after secondary antibody (1:1000, ab6721, Abcam, UK) treatment. Finally, the membrane was exposed under an Imaging System (Geliance 200, PerkinElmer, USA). The primary antibodies of Nrf2 (1:2000, AF0639), HO-1 (1:2000, AF5393), NF-κB p65 (1:2000, BF8005), PI3K P85 alpha (1:1000, AF6241), phospho-pan-AKT1/2/3 (Ser473, 1:1000, AF0016), pan-AKT1/2/3 (1:1000, AF6261), β-actin (1:20000, AF7018) were purchased from Affinity (USA).
Statistics
Data were analyzed and processed with SPSS software (16.0, IBM, USA). Data acquired from three independent experiments were manifested as mean ± standard deviation. One-way ANOVA with Tukey test was exploited for multiple comparisons. Kruskal-Wallis H test was employed to those with uneven variance. Statistical significance was set at p < .05.
Results
GFW and rosiglitazone alone or together suppressed body weight and ovarian weight in PCOS rats
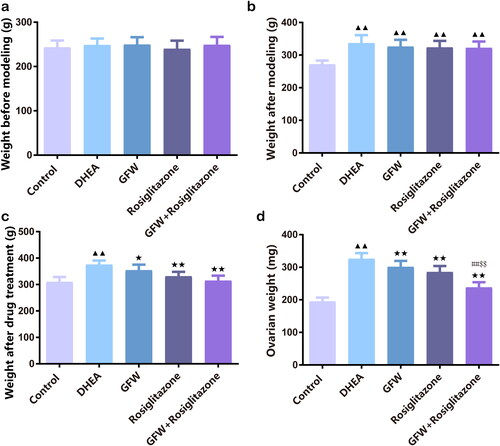
DHEA did not change the initial body weight of rats before modeling, but significantly increased the body weight after modeling and drug treatment, ovarian weight was also increased (, p < .01). We also found out that GFW and rosiglitazone alone or together greatly suppressed the body weight after drug treatment and ovarian weight (, p < .05). Furthermore, the addition of GFW plus rosiglitazone had significant impact on suppressing ovarian weight relative to GFW and rosiglitazone alone (, p < .01).
Figure 1. GFW and rosiglitazone alone or together suppressed body weight and ovarian weight in PCOS rats. (a) The initial weight of rats in each group before the experiment. (b) Weight of rats in each group after dehydroepiandrosterone (DHEA)-induced polycystic ovary syndrome (PCOS) modeling. (c) The final weight of rats in each group. (d) The ovarian weight of rats in each group. Data are expressed as mean ± SD, n = 10. ▲▲p < .01 vs. control group; ★p < .05, ★★p < .01 vs. DHEA group; ##p < .01 vs. Guizhi Fuling Wan (GFW) group; $$p < .01 vs. rosiglitazone group.
The FBG, FINS, and HOMA-IR levels in PCOS rats were decreased after GFW and rosiglitazone alone or co-treatment
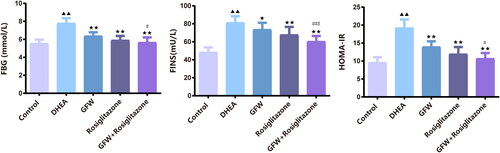
The group of DHEA was able to elevate the levels of FBG, FINS, and HOMA-IR than the control group (, p < .01). The group that received GFW and rosiglitazone alone or co-treatment seemed to alleviate the levels of FBG, FINS, and HOMA-IR than the DHEA group (, p < .05). Whilst the decrease of GFW on FBG, FINS, and HOMA-IR levels in PCOS rats was strengthened by rosiglitazone (, p < .05).
Figure 2. The FBG, FINS, and HOMA-IR levels in PCOS rats were decreased after GFW and rosiglitazone alone or co-treatment. (a) A blood glucose meter was selected to monitor fasting blood glucose (FBG) of each group. (b) For determination of fasting insulin (FINS), Rat Insulin ELISA Kit was applied. (c) The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated based on the formula: FBG (mmol/L) × FINS (mU/L)/22.5. Data are displayed as mean ± SD, n = 10. ▲▲p < .01 vs. control group; ★p < .05, ★★p < .01 vs. DHEA group; #p < .05, ##p < .01 vs. GFW group; $p < .05 vs. rosiglitazone group.
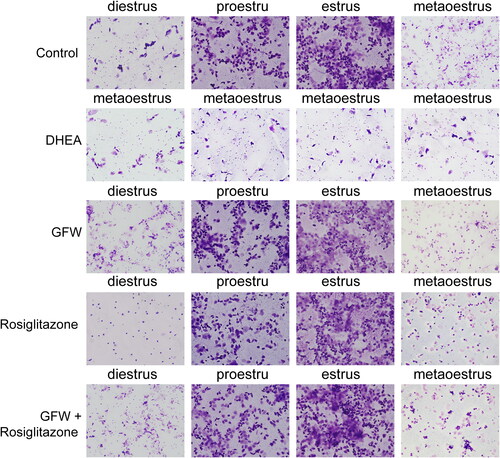
GFW and rosiglitazone alone or combination ameliorated estrous cycle in DHEA-induced PCOS rats
By conducting vaginal smears analysis, we discovered that the control group showed a full estrous cycle, most of the vaginal cells of the rats in proestru were nucleated epithelial cells; most of the vaginal cells of the rats in estrus were denucleated keratinocytes; the vaginal cells of the rats in metaoestrus were greatly reduced; most of the vaginal cells in diestrus were leukocytes (). In the DHEA group, the estrous cycle of the rats was disordered, which only showed metaoestrus; while other treatment groups recovered to a full estrous cycle ().
The effects of GFW and rosiglitazone on lipid metabolism levels in PCOS rats
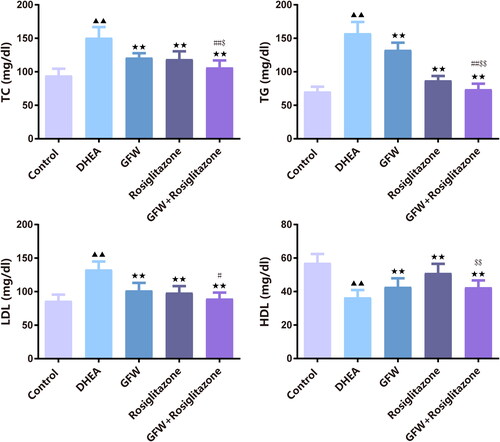
For determination of lipid metabolism levels, we found that there was increment of TC, TG, and LDL and decrease of HDL in the DHEA group compared to the control group (, p < .01). The addition of GFW and rosiglitazone alone or co-intervention attenuated the contents of TC, TG, and LDL and enhanced the content of HDL in PCOS rats (, p < .01). Significantly, the combined GFW and rosiglitazone had better effect on modulating lipid metabolism levels in PCOS rats than GFW and rosiglitazone alone (, p < .05).
Figure 4. The effects of GFW and rosiglitazone on lipid metabolism levels in PCOS rats. For determination of lipid metabolism levels, total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) levels were evaluated by applying an automatic biochemical analyzer. Data are displayed as mean ± SD, n = 10. ▲▲p < .01 vs. control group; ★★p < .01 vs. DHEA group; #p < .05, ##p < .01 vs. GFW group; $p < .05, $$p < .01 vs. rosiglitazone group.
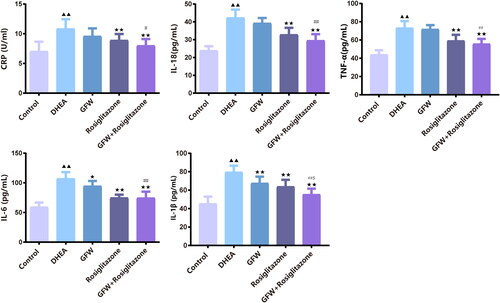
The effects of GFW and rosiglitazone on ovarian sex hormone levels in PCOS rats
As presented in , DHEA led to the up-regulation of T, LH, and the ratio of LH/FSH and the down-regulation of E2, P, and FSH in rats (p < .01). Nevertheless, introduction of GFW and rosiglitazone alone or combination restrained the elevation of T, LH, and the ratio of LH/FSH and accelerated the levels of E2, P, and FSH in rats in the presence of DHEA (, p < .05). Importantly, the combination of GFW and rosiglitazone had a stronger improvement on ovarian sex hormone levels than the single treatment group (, p < .05).
Figure 5. The effects of GFW and rosiglitazone on ovarian sex hormone levels in PCOS rats. The contents of testosterone (T), estradiol (E2), progesterone (P), luteinizing hormone (LH), and follicle stimulating hormone (FSH) were analyzed utilizing ELISA. Data are described as mean ± SD, n = 10. ▲▲p < .01 vs. control group; ★p < .05, ★★p < .01 vs. DHEA group; #p < .05, ##p < .01 vs. GFW group; $p < .05, $$p < .01 vs. rosiglitazone group.
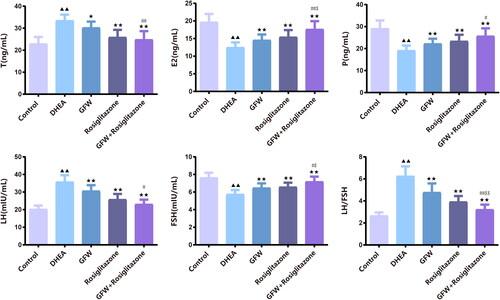
GFW and rosiglitazone co-intervention ameliorated ovarian histological change in PCOS rats
In , H&E staining results elucidated that the ovarian follicles at all levels of the rats in the control group were clearly visible. Relative to the control group, the rats in the DHEA group had obvious ovarian fibrosis, the number of preantral follicles and antral follicles lessened, and the number of atretic follicles raised. Compared with the DHEA group, the vacuoles and atretic follicles in the ovarian tissue of the GFW group and rosiglitazone group were notably reduced, the number of granulosa cells increased and arranged radially and neatly, the follicles of all levels began to appear, and the mature follicles enhanced notably; in the GFW + rosiglitazone group, the number of follicles in the ovarian tissues were abundant, the follicles at all levels were visible, and there were more corpus luteum and a small number of atresia follicles. In , relative to the control group, the number of primordial follicles, primary follicles, and secondary follicles in the ovarian tissues of the rats in the DHEA group lessened evidently, while the number of atretic follicles heightened strongly (p < .01); compared with the DHEA group, the number of primordial follicles in the ovarian tissues of the rats in the GFW + rosiglitazone group was extremely increased, the number of primary follicles in the ovarian tissues of the GFW group, rosiglitazone group, and GFW + rosiglitazone group was greatly increased, whereas the number of atretic follicles was extremely decreased (p < .05). The showed the overall structure of the ovary.
Figure 6. GFW and rosiglitazone co-intervention ameliorated ovarian histological change and follicular development in PCOS rats. (a) Hematoxylin and eosin (H&E) staining assessed the pathological change of the ovarian tissues (×40, ×200, ×400). (b) The number of follicles at all levels of the ovarian tissues was counted. (c) H&E staining of overall structure of ovary. Data are described as mean ± SD, n = 6. ▲▲p < .01 vs. control group; ★p < .05, ★★p < .01 vs. DHEA group; ##p < .01 vs. GFW group; $p < .05, $$p < .01 vs. rosiglitazone group.
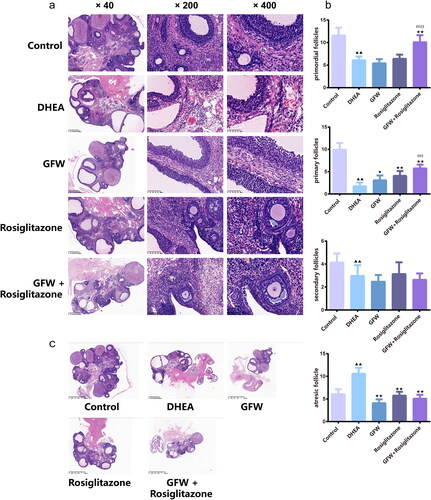
The combined GFW and rosiglitazone alleviated serum inflammation and oxidative stress response in PCOS rats
The levels of serum oxidative stress-related marker including GSH-Px, MDA, and SOD in the DHEA exposure group were largely higher than those in the control (, p < .01). GFW alone effectively alleviated the levels of serum GSH-Px, MDA and SOD in rats mediated by DHEA (, p < .05). More important, the suppression of GFW and rosiglitazone co-treatment on the expressions of oxidative stress-related marker in the serum of PCOS rats was stronger than that of each single treatment group (, p < .05). In , the levels of GSH-Px and SOD in ovarian tissue were decreased, the level of MDA was increased after DHEA exposure (, p < .01). GFW alone could increase the level of GSH-Px (, p < .01) and SOD, meanwhile, decrease the level of MDA (, p < .01). In addition, the effect of GFW + rosiglitazone on the expressions of oxidative stress-related marker in the ovarian tissue was more powerful than that of each single treatment group (, p < .01 or p < .05). Then, we examined serum inflammation-related factors. The DHEA exposure led to the inflammatory responses of rats, with an obvious increase of CRP, IL-18, TNF-α, IL-6, and IL-1β levels, which was reversed by rosiglitazone or GFW and rosiglitazone together (, p < .01). GFW alone prominently repressed the contents of serum CRP, IL-6 and IL-1β in PCOS rats (, p < .05). Meanwhile, the inhibitory function of GFW and rosiglitazone together on inflammation was stronger than that of GFW and rosiglitazone alone (, p < .05).
Figure 7. The combined GFW and rosiglitazone alleviated serum oxidative stress response in PCOS rats. (a) The serum glutathione peroxidase (GSH-Px), malondialdehyde (MDA), and superoxide dismutase (SOD) levels were examined by ELISA. (b) The ovarian tissue GSH-Px, MDA, SOD levels were examined by ELISA. Data are described as mean ± SD, n = 10. ▲▲p < .01 vs. control group; ★p < .05, ★★p < .01 vs. DHEA group; ##p < .01 vs. GFW group; $p < .05, $$p < .01 vs. rosiglitazone group.
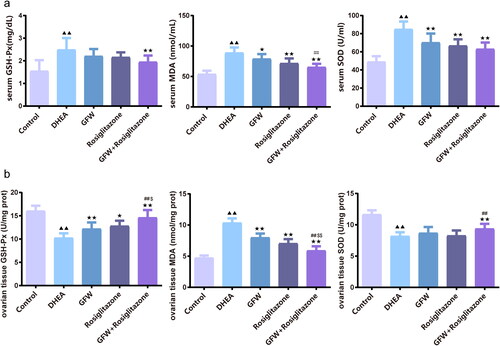
Figure 8. The combined GFW and rosiglitazone alleviated serum inflammatory response in PCOS rats. The serum C-reactive protein (CRP), interleukin-18 (IL-18), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) levels were assessed by ELISA. Data are described as mean ± SD, n = 10. ▲▲p < .01 vs. control group; ★p < .05, ★★p < .01 vs. DHEA group; ##p < .01 vs. GFW group; $p < .05 vs. rosiglitazone group.
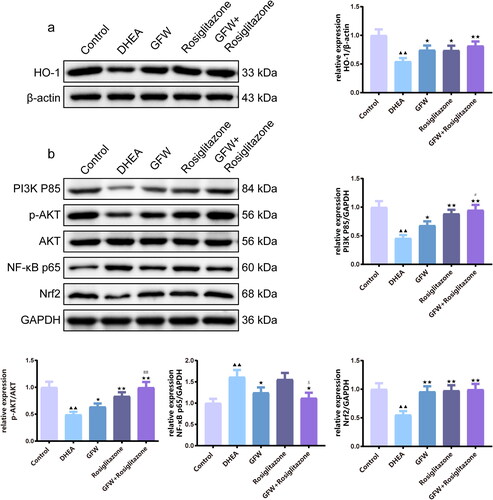
The effects of GFW and rosiglitazone on PI3K/AKT/NF-κB and Nrf2/HO-1 pathways of the ovarian tissues in PCOS rats
By conducting Western blot analysis, we discovered that DHEA caused the down-regulation of HO-1, PI3K P85, Nrf2 as well as the ratio of p-AKT/AKT, and the increase of NF-κB p65 in the ovarian tissues, while the above-mentioned proteins were reversed by GFW alone or GFW and rosiglitazone together (, p < .01). In addition to NF-κB p65 protein expression, rosiglitazone alone also evidently advanced the expressions of PI3K/AKT and Nrf2/HO-1 pathway-related marker in the ovarian tissues of PCOS rats (, p < .05). Overall, the combination of GFW and rosiglitazone regulated PI3K/AKT/NF-κB and Nrf2/HO-1 pathways more strongly in the ovarian tissues (, p < .05).
Figure 9. The effects of GFW and rosiglitazone on PI3K/AKT/NF-κB and Nrf2/HO-1 pathways of the ovarian tissues in PCOS rats. (a,b) The effects of GFW and rosiglitazone on phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/nuclear factor-kappaB (NF-κB) and nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathways of the ovarian tissues in PCOS rats were evaluated by Western blot. Data are manifested as mean ± SD, n = 3. ▲▲p < .01 vs. control group; ★p < .05, ★★p < .01 vs. DHEA group; #p < .05, ##p < .01 vs. GFW group; $p < .05 vs. rosiglitazone group.
Discussion
Hyperandrogenism plays a crucial role in the occurrence and development of PCOS. The ovary contains enzymes that mediate insulin production and metabolism, while it is reported that hyperinsulinemia and IR can interfere with the synthesis of sex hormone-binding globulin in the liver, resulting in increased androgen synthesis and causing hyperandrogenism in PCOS patients [Citation26]. In addition, hyperandrogenism can also aggravate hyperinsulinemia [Citation27] via affecting altered glucose tolerance and impaired IR in women [Citation28]. From the clinical data of women diagnosed with PCOS, the hyperandrogenic environment can inhibit the follicle development, leading to follicular atresia, resulting in decreased E2 level, excessive LH secretion, and insufficient FSH secretion [Citation29]. And low level of FSH and high level of androgen can promote the rapid luteinization of granulosa cells and stop the development of small antral follicles, thus causing abnormal follicle maturation and ovulation function in PCOS patients, resulting in polycystic changes in the ovary [Citation30]. Therefore, an increasing number of studies believe that IR and hyperandrogenism are the pathological basis of PCOS. Previous studies have found that DHEA can elevate the level of T in women, thereby creating a high androgen environment, and DHEA replication of animal PCOS is an internationally recognized ideal modeling method [Citation31]. In this study, after establishing a rat PCOS model with the above method, it was found that the levels of FBG, FINS, HOMA-IR, LH, T, and blood lipids (TC and TG) increased, while the levels of E2 and FSH decreased; under the light microscope, the follicles showed cystic expansion, increased atretic follicles, decreased corpus luteum and granulosa cells. These indicated that the IR was induced, sex hormone and lipid metabolism were disordered, and ovarian ovulation function was affected, showing the success of PCOS modeling.
Scientific research elucidated that GFW ameliorated IR in PCOS with the potential mechanism of modulating intestinal flora to control inflammation [Citation17]. Furthermore, GFW could obviously decrease FBG, FINS, and T levels and augmented the level of adiponectin in PCOS patients to ameliorate IR and lipid metabolism, lessen LH/FSH, E2, and P levels to advance mature follicle development and ovulation [Citation32,Citation33]. Our study found that GFW can improve the weight and ovary quality of PCOS rats. At the same time, GFW regulated the estrus cycle of PCOS rats which controlled by mature follicle development and ovulation. The consistency between this study and previous studies lies in the improvement of sex hormone levels, lipid metabolism levels, inflammation, and changes in polycystic ovary by GFW alone. More importantly, our study confirmed that co-treatment of GFW and rosiglitazone was better than rosiglitazone or GFW alone to balance the secretion of sex hormones, promote follicle development and improve ovulation in rats. These findings suggested that integrated traditional Chinese and Western medicine exhibits a significant role in improving PCOS.
At present, although the mechanism of IR in PCOS patients is still not fully understood, the relationship between chronic inflammation, oxidative stress, and IR has been confirmed by many studies [Citation34–36]. Studies have shown that oxidative stress is involved in the physiological and pathological processes of PCOS. Many clinical manifestations of PCOS, such as hyperandrogenemia, obesity and IR, may lead to local and systemic oxidative stress, further aggravating the disorder of glucose and lipid metabolism and sex hormone metabolism in PCOS patients [Citation37]. Research suggested that the level of TNF-α in the follicular fluid of PCOS patients is notably correlated with the concentration of oxidative stress state, because TNF-α presents a considerable role in the initiation and development of inflammation and oxidative stress, and induces the production of IL-6 and IL-8 cytokines, therefore, PCOS patients are more susceptible to oxidative stress and inflammation [Citation38]. PI3K/AKT has been reported to mediate the increase of TNF-α, IL-1β, IL-6, and IL-8 gene expression in the uterus of PCOS rats [Citation39]. In granulosa cells of PCOS patients, WNT5a overexpression augmented inflammation and oxidative stress mainly through the PI3K/AKT/NF-κB pathway [Citation7]. Autophagy was activated in the ovarian tissue of the DHEA-induced PCOS rat model via PI3K/AKT pathway [Citation40]. Researches showed that the phosphorylation of NF-κB p65 was increased by high glucose which induce inflammation, and the phosphorylation of AKT was increased which induce autophagy [Citation41]. Zhang et al. demonstrated by network pharmacological analysis that the pharmacological mechanism of GFW in the treatment of primary dysmenorrhea is related to inflammation [Citation42]. The main compound of GFW could inhibit inflammation and oxidative stress [Citation43]. In addition, as the main active ingredient of GFW, Paeoniflorin regulates oxidative stress by regulating the PI3K/AKT pathway and improving endometrial hyperplasia induced by PCOS in mice [Citation44]. Another scientific study reported that GFW can effectively ameliorate kidney damage, and its mechanism is associated with the activation of Nrf2/HO-1 pathway, inhibition of NLRP3 inflammasome, and improvement of intestinal flora dysregulation [Citation45]. Our report clarified that co-treatment of GFW and rosiglitazone could more effectively suppress inflammation and oxidative stress in the serum and ovarian tissue of PCOS rats via regulating PI3K/AKT/NF-κB and Nrf2/HO-1 pathway than GFW or rosiglitazone monotherapy, and suggested that its mechanism was related to increase the expression of HO-1, PI3K P85, Nrf2 as well as the ratio of p-AKT/AKT and decrease the expression of NF-κB p65.
Although our studies have verified the effect of GFW combined with rosiglitazone on PCOS via PI3K/AKT/NF-κB pathway and Nrf2/HO-1 pathway, our choice of experimental model is simple and the studies of molecular mechanism are not profound enough. In the following experiments, different animal models of PCOS will serve as effective models to clarify the precise mechanism deeply between drugs and PCOS.
In this study, based on the analysis of insulin signal transduction and related inflammatory responses and oxidative stress responses, it can be seen that GFW combined with rosiglitazone can more effectively augment insulin sensitivity, lessen inflammation and oxidative stress responses in PCOS rats than rosiglitazone alone. The mechanism is related to the regulation of PI3K/AKT/NF-κB pathway and Nrf2/HO-1 pathway. The results of this study provide a scientific basis for the therapy of PCOS ovulation disorder with the combination of traditional Chinese and Western medicine and broaden the application scope of classical prescriptions.
Ethical approval
All experimental animals were approved by the Animal Experimentation Ethics Committee of Zhejiang Eyong Pharmaceutical Research and Development Center (Approval NO.: SYXK-2021-0033).
Author’s contribution
Study designed by Feilan Xuan; manuscript drafted by Yongju Ye; data collected by Weimei Zhou; data analyzed and interpretated by Yuefang Ren and Jiali Lu; statistical analyzed by Aixue Chen and Ruiying Jin. All authors read the final manuscript and approved to publish.
Supplemental Material
Download MS Word (17.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be provided upon request.
Additional information
Funding
References
- Li X, Fang Z, Yang X, et al. The effect of metformin on homocysteine levels in patients with polycystic ovary syndrome: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2021;47(5):1–12. doi: 10.1111/jog.14725.
- Stener-Victorin E, Padmanabhan V, Walters KA, et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):bnaa010.
- Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321–336. doi: 10.1097/AOG.0000000000002698.
- Calderon-Margalit R, Siscovick D, Merkin SS, et al. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid-intima-media thickness: the coronary artery risk development in young adults women’s study. Arterioscler Thromb Vasc Biol. 2014;34(12):2688–2694. doi: 10.1161/ATVBAHA.114.304136.
- Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. doi: 10.1016/j.jsbmb.2018.04.008.
- Rothenberg SS, Beverley R, Barnard E, et al. Polycystic ovary syndrome in adolescents. Best Pract Res Clin Obstet Gynaecol. 2018;48:103–114. doi: 10.1016/j.bpobgyn.2017.08.008.
- Zhao Y, Zhang C, Huang Y, et al. Up-regulated expression of WNT5a increases inflammation and oxidative stress via PI3K/AKT/NF-κB signaling in the granulosa cells of PCOS patients. J Clin Endocrinol Metab. 2015;100(1):201–211. doi: 10.1210/jc.2014-2419.
- Ji R, Jia F, Chen X, et al. Carnosol inhibits KGN cells oxidative stress and apoptosis and attenuates polycystic ovary syndrome phenotypes in mice through Keap1-mediated Nrf2/HO-1 activation. Phytother Res. 2023;37(4):1405–1421. doi: 10.1002/ptr.7749.
- Wang R, Li W, Bordewijk EM, et al. First-line ovulation induction for polycystic ovary syndrome: an individual participant data meta-analysis. Hum Reprod Update. 2019;25(6):717–732. doi: 10.1093/humupd/dmz029.
- Bordewijk EM, et al. Laparoscopic ovarian drilling for ovulation induction in women with anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;2(2):Cd001122.
- Ong M, Peng J, Jin X, et al. Chinese herbal medicine for the optimal management of polycystic ovary syndrome. Am J Chin Med. 2017;45(3):405–422. doi: 10.1142/S0192415X17500252.
- Meng W, Ta N, Wang F. Add-on effect of guizhi fuling formula to mifepristone for endometriosis: a meta-analysis of randomized controlled trials. Medicine. 2019;98(33):e16878. doi: 10.1097/MD.0000000000016878.
- Tao LTA, Liu M. Effect of guizhifu ling pill combined with berberine on patients with polycystic ovary syndrome with insulin resistance. Chin J Exp Tradit Med Formulae. 2013;19:320–323.
- Wu JDH, Liu Y . Clinical research progress of guizhi fuling wan in the treatment of infertility. J Guangzhou Univ Tradit Chin Med. 2020;37:586–590.
- Liu M, Zhu H, Zhu Y, et al. Guizhi fuling wan reduces autophagy of granulosa cell in rats with polycystic ovary syndrome via restoring the PI3K/AKT/mTOR signaling pathway. J Ethnopharmacol. 2021;270:113821. doi: 10.1016/j.jep.2021.113821.
- Wu P, Zhu Y, Li J, et al. Guizhi fuling wan inhibits autophagy of granulosa cells in polycystic ovary syndrome mice via H19/miR-29b-3p. Gynecol Endocrinol. 2023;39(1):2210232.
- Zhu Y, Li Y, Liu M, et al. Guizhi fuling wan, Chinese herbal medicine, ameliorates insulin sensitivity in PCOS model rats with insulin resistance via remodeling intestinal homeostasis. Front Endocrinol. 2020;11:575. doi: 10.3389/fendo.2020.00575.
- Li Y-C, Qiao J-Y, Wang B-Y, et al. Paeoniflorin ameliorates fructose-induced insulin resistance and hepatic steatosis by activating LKB1/AMPK and AKT pathways. Nutrients. 2018;10(8):1024. doi: 10.3390/nu10081024.
- Ma Z, Liu H, Wang W, et al. Paeoniflorin suppresses lipid accumulation and alleviates insulin resistance by regulating the rho kinase/IRS-1 pathway in palmitate-induced HepG2Cells. Biomed Pharmacother. 2017;90:361–367. doi: 10.1016/j.biopha.2017.03.087.
- Stout D, Fugate S. Thiazolidinediones for treatment of polycystic ovary syndrome. Pharmacotherapy. 2005;25(2):244–252. doi: 10.1592/phco.25.2.244.56943.
- Li Y, Tan J, Wang Q, et al. Comparing the individual effects of metformin and rosiglitazone and their combination in obese women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril. 2020;113(1):197–204. doi: 10.1016/j.fertnstert.2019.09.011.
- Han L, Guo X, Bian H, et al. Guizhi fuling wan, a traditional Chinese herbal formula, sensitizes cisplatin-resistant human ovarian cancer cells through inactivation of the PI3K/AKT/mTOR pathway. Evid Based Complement Alternat Med. 2016. 2016;2016:4651949.
- National Commission of Chinese Pharmacopoeia. Pharmacopoeia of peoples republic of China. Vol. 1. Beijing: China Medical Science and Technology Press; 2010.
- Yu Y, Cao Y, Huang W, et al. β-Sitosterol ameliorates endometrium receptivity in PCOS-Like mice: the mediation of gut microbiota. Front Nutr. 2021;8:667130. doi: 10.3389/fnut.2021.667130.
- Xu Y, Pan C-S, Li Q, et al. The ameliorating effects of bushen huatan granules and kunling wan on polycystic ovary syndrome induced by dehydroepiandrosterone in rats. Front Physiol. 2021;12:525145. doi: 10.3389/fphys.2021.525145.
- Singh P, Agress A, Madrigal VK, et al. Massive ovarian growth in a woman with severe Insulin-Resistant polycystic ovary syndrome receiving GnRH analogue. J Clin Endocrinol Metab. 2019;104(7):2796–2800. doi: 10.1210/jc.2018-02464.
- Wang J, Wu D, Guo H, et al. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940. doi: 10.1016/j.lfs.2019.116940.
- Zhang B, Wang J, Shen S, et al. Association of androgen excess with glucose intolerance in women with polycystic ovary syndrome. Biomed Res Int. 2018;2018:6869705. doi: 10.1155/2018/6869705.
- Zhao H, Song X, Zhang L, et al. Comparison of androgen levels, endocrine and metabolic indices, and clinical findings in women with polycystic ovary syndrome in Uygur and Han ethnic groups from Xinjiang province in China. Med Sci Monit. 2018;24:6774–6780. doi: 10.12659/MSM.909715.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18(1):104. doi: 10.1186/s12967-020-02277-0.
- Rababa’h AM, Matani BR, Ababneh MA. The ameliorative effects of marjoram in dehydroepiandrosterone induced polycystic ovary syndrome in rats. Life Sci. 2020;261:118353. doi: 10.1016/j.lfs.2020.118353.
- Lin J, Xu Y, Zhang AH, et al. Effect of guizhi fuling wan combined with Western medicine in the treatment of polycystic ovary syndrome. China’s Health Standards Management. 2019;10:92–94.
- Lou XL, Chen Y. Clinical study on treating infertility caused by ovulatory disorder in polycystic ovary syndrome with yulin zhu and guizhi fuling wan. Journal of New Chinese Medicine. 2019;51:43–47.
- Kalyan S, Patel MS, Kingwell E, et al. Competing factors link to bone health in polycystic ovary syndrome: chronic low-grade inflammation takes a toll. Sci Rep. 2017;7(1):3432. doi: 10.1038/s41598-017-03685-x.
- Milutinović DV, Nikolić M, Veličković N, et al. Enhanced inflammation without impairment of insulin signaling in the visceral adipose tissue of 5α-dihydrotestosterone-induced animal model of polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2017;125(8):522–529. doi: 10.1055/s-0043-104531.
- Saglam E, Canakci CF, Sebin SO, et al. Evaluation of oxidative status in patients with chronic periodontitis and polycystic ovary syndrome: a cross-sectional study. J Periodontol. 2018;89(1):76–84. doi: 10.1902/jop.2017.170129.
- Murri M, Luque-Ramírez M, Insenser M, et al. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–288. doi: 10.1093/humupd/dms059.
- Artimani T, Karimi J, Mehdizadeh M, et al. Evaluation of pro-oxidant-antioxidant balance (PAB) and its association with inflammatory cytokines in polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2018;34(2):148–152. doi: 10.1080/09513590.2017.1371691.
- Zhang Y, Hu M, Meng F, et al. Metformin ameliorates uterine defects in a rat model of polycystic ovary syndrome. EBioMedicine. 2017;18:157–170. doi: 10.1016/j.ebiom.2017.03.023.
- Xie F, Zhang J, Zhai M, et al. Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-Akt pathway. Reproduction. 2021;162(1):73–82. doi: 10.1530/REP-20-0643.
- Lei L, Zhao J, Liu X-Q, et al. Wogonin alleviates kidney tubular epithelial injury in diabetic nephropathy by inhibiting PI3K/akt/NF-κB signaling pathways. Drug Des Devel Ther. 2021;15:3131–3150. doi: 10.2147/DDDT.S310882.
- Zhang S, Lai X, Wang X, et al. Deciphering the pharmacological mechanisms of guizhi-fuling capsule on primary dysmenorrhea through network pharmacology. Front Pharmacol. 2021;12:613104. doi: 10.3389/fphar.2021.613104.
- Li X, Gu LW, Ran QS, et al. Protective effects of three phenylallyl compounds from guizhi decoction ox-LDL-induced oxidative stress injury of human brain microvascular endothelial cells. Zhongguo Zhong Yao Za Zhi. 2016;41(12):2315–2320.
- Liu S, Cao X, Zhang T, et al. Paeonol ameliorates endometrial hyperplasia in mice via inhibiting PI3K/AKT pathway-related ferroptosis. Phytomedicine. 2023;109:154593. doi: 10.1016/j.phymed.2022.154593.
- Xu B, Zheng J, Tian X, et al. Protective mechanism of traditional Chinese medicine guizhi fuling pills against carbon tetrachloride-induced kidney damage is through inhibiting oxidative stress, inflammation and regulating the intestinal flora. Phytomedicine. 2022;101:154129. doi: 10.1016/j.phymed.2022.154129.