Abstract
Sexual hormone binding globulin (SHBG) is associated with the endocrine and reproductive systems. We aimed to investigate the role of SHBG in the reproductive process. Therefore, we conducted a secondary analysis of the PCOSAct (Polycystic Ovary Syndrome and Acupuncture Clinical Trial) study, which involved 21 sites in China and a total of 1000 women with PCOS. Out of these, 954 women with SHBG were included in the analysis. Through multivariate analysis of ovulation predictors, we found that age, BMI, estradiol, testosterone, and SHBG all showed a positive predictive value for ovulation (p = 0.0211, 0.0011, 0.0211, 0.0029, 0.0434, respectively). However, the LH to FSH ratio had a negative predictive value (p = 0.0539). Higher quartiles of SHBG were associated with a higher rate of ovulation, and per quartile increased was statistically significant (HR = 1.138, 95%CI [1.054,1.229]). The association remained significant even after adjusting for testosterone (HR = 1.263, 95%CI [1.059, 1.507]). On the other hand, quartiles of testosterone and estradiol did not exhibit any significant tendency toward ovulation. SHBG demonstrated predictive ability for ovulation, conception, pregnancy, and live birth (p < 0.05), and this correlation remained significant after adjusting intervention. Kaplan-Meier curves illustrated that increased levels of SHBG were a factor in high rates of ovulation, conception, and pregnancy. In comparison to other sexual hormones, a higher baseline level of SHBG was related to increased ovulation.
Introduction
Ovulatory dysfunction, hyperandrogenism, and polycystic ovaries are all characteristics of polycystic ovarian syndrome (PCOS), which has a global prevalence of between 5 and 20% [Citation1]. It is considered to be a leading cause of female infertility in women of reproductive age [Citation2]. Additionally, 43% of adult women and nearly one-third of adolescent teenagers with PCOS have metabolic syndrome [Citation3].
Sex hormone-binding globulin (SHBG) is a glycoprotein produced in the liver that binds to sexual hormones and circulates these bound-state hormones in the bloodstream as biologically inactive forms. Previous studies have confirmed that lower levels of SHBG are associated with PCOS patients, indicating hyperandrogenemia [Citation4]. A recent meta-analysis found that the SHBG polymorphism rs35785886, which had eight or more (TAAAA)n-pentanucleotide repeats, was linked to PCOS risk and low levels of serum SHBG concentrations in PCOS patients [Citation5]. Genome-wide association studies (GWAS) have confirmed that the level of SHBG is determined by a group of genes involved in de novo lipogenesis. Inhibitors of these genes or thyroid hormone receptor β agonists, along with other therapies, could be a promising direction for the prevention and treatment of PCOS by increasing SHBG levels [Citation6].
Several studies have shown that low levels of prepregnant SHBG might result in metabolic diseases during pregnancy, such as gestational diabetes mellitus [Citation7], adverse cardio-metabolic diseases [Citation8], which may increase the risk of negative reproductive outcomes. Another observational study of two randomized clinical trials (RCTs) revealed that among PCOS women, those with lower SHBG levels tended to have poorer rates of conception, pregnancy and live birth [Citation9]. Although some studies have investigated the connection between SHBG levels and reproductive outcomes, there have been few clinical studies investigating the connection between SHBG levels and ovulation. Therefore, we sought to determine if elevated SHBG levels are indicative of higher ovulation and other reproductive outcomes among women with PCOS based on prior findings and the fact that both PCOS and SHBG contribute to reproductive problems.
Materials and methods
Design and target population
This is a secondary analysis of metabolic parameters and reproductive outcomes data from the Acupuncture and Clomiphene in Polycystic Ovary Syndrome Trial (PCOSAct), a large-sample, multicenter, two-by-two factorial randomized controlled clinical trial conducted from 2012 to 2015 in mainland China. All 1,000 subjects were diagnosed with PCOS according to the modified Rotterdam criteria [Citation2,Citation10]. Women aged from 20 to 40 years with oligomenorrhea (defined as a menstrual interval ≥35 days and/or ≤8 menses in the past year) or amenorrhea combined with clinical hyperandrogenism (modified Ferriman-Gallwey score ≥5) [Citation11,Citation12] and/or polycystic ovaries (defined as the presence of >12 antral follicles (≤9mm) and ovarian volume >10 ml by ultrasound) were considered to have PCOS. Documentation of patency of at least one tube and a normal uterine cavity was required by hysterosalpingogram, HyCosy, or diagnostic laparoscopy. Male partners were required to have a sperm concentration ≥ 15x106/ml and total motility ≥40% or viable sperm count ≥ 9 million [Citation13,Citation14]. The study design, methods, and inclusion and exclusion criteria described in details [Citation15] and the main results [Citation16] has been published.
In brief, 954 women with PCOS who had baseline measurements of SHBG were enrolled in the present study, and reproductive outcomes, including ovulation, conception, pregnancy, pregnancy loss, and live birth were recorded at the end of study. Progesterone was measured every week to determine whether the subjects ovulated during the trial. Ovulation was defined as ovulation if she had an elevated progesterone level (>5 ng/mL); the ovulation rate was calculated as the proportion of subjects who had ovulated at least once during all tested cycles to all subjects. Serum hCG was measured per week and conception was defined as a positive serum hCG. Pregnancy was defined as fetal sacs in the uterus with primitive heart beat under ultrasonography.
The trial was registered with ClinicalTrials.gov (No. NCT01573858). The study was approved by the regional ethics committee at The First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin, China. All participants and their partners provided written informed consent before participation in the study.
Data collection
Baseline laboratory, anthropometric, degree of hirsutism, acne, and ultrasonography measurements were performed after an overnight fast, and all biochemical assays were performed in a core laboratory. Fasting blood collected at baseline was used for all hormone assays [Citation17]. Total T was measured by radioimmunoassay (Siemens Diagnostics, Deerfield, Illinois), with intra-assay and inter-assay coefficients of variation less than 7.1%. SHBG levels were available for 954 of the 1,000 women enrolled in the trial, and these were measured in samples stored at −80 °C using an ELISA assay with no pre-dilution. BMI was calculated as weight in kilograms divided by height in meters squared.
Statistical analysis
Descriptive statistics were used to compare characteristics between different groups. Continuous variables were reported as means with standard deviations, and categorical variables were summarized in frequency and percentages. Multiple logistic regression analysis was used to obtain odds ratios (ORs) to assess the predictive ability of variables for ovulation. Cox proportional hazards regression was used to analyze the associations between SHBG, testosterone, and estradiol and ovulation. In predefined analyses, SHBG, testosterone, and estradiol were examined as quartiles based on distribution in the entire study population. Based on the observed distribution, SHBG and testosterone were further examined as a dichotomous variable comparing quartile 4 with quartiles 1 to 3. Univariate logistic regression analysis was used to assess the predictive ability of SHBG for fertile outcomes. Kaplan-Meier survival curves illustrated the association between SHBG levels and several reproductive outcomes over time, including ovulation, conception, pregnancy, miscarriage, and live birth, and the log-rank test assessed statistical significance. The time was defined as from randomization to the week women ovulated, the day women got conception, pregnancy, miscarriage and live birth. All analyses were performed in SAS version 9.3 software (SAS Institute Inc), and p-values <0.05 were considered statistically significant.
Results
The biometric features and fasting serum levels of participants at baseline were described in . To investigate the prognostic value of ovulation-related factors, we performed a multivariate analysis (). The results showed that age (OR = 1.063, 95%CI [1.009-1.119]), BMI (OR = 0.922, 95%CI [0.884–0.962]), LH/FSH (OR = 0.791, 95%CI [0.654-0.956]), E2 (OR = 1.002, 95%CI [1.000-1.003]), T (OR = 0.653, 95%CI [0.494-0.863]), and SHBG (OR = 1.008, 95%CI [1.000-1.015]) were independent predictors of ovulation (p < 0.05). After adjusting treatment, all variables except for LH/FSH remained significant (p < 0.05).
Table 1. Characteristics of the subjects.
Table 2. Multivariate analysis of predictors of ovulation according to the multiple logistic regression analysis.
We calculated the hazard ratios (HRs) for ovulation across quartiles of baseline serum levels of SHBG, T, free T, and E2 to further explore the predictive ability of each baseline sexual hormone (). Women in the highest quartile of SHBG had a higher risk of ovulation compared to those in the lower quartile of SHBG, and the per-quartile increase of SHBG had statistical significance (p < 0.001). Because of the biological interplay between testosterone and SHBG, we adjusted the testosterone, and per-quartile increase of SHBG (HR = 1.139; 95%CI [1.055,1.229]; p = 0.0009) and Q4 vs. Q1-3 of SHBG (HR = 1.263; 95%CI [1.059,1.507]; p = 0.0093) still had statistical significance. The p values for Q2, Q3, and Q4 of free T were all negative. E2 showed no obvious correlation with ovulation.
Table 3. HR for Ovulation across quartiles of serum SHBG, testosterone, and estradiol.
In the univariate analysis (), SHBG showed predictive value for ovulation, conception, pregnancy and other fertile outcomes. SHBG positively predicted ovulation, conception, clinical pregnancy and live birth (p < 0.05), and this correlation remained significant after adjusting treatment.
Table 4. Univariate analysis of relationship of SHBG level with various pathological factors.
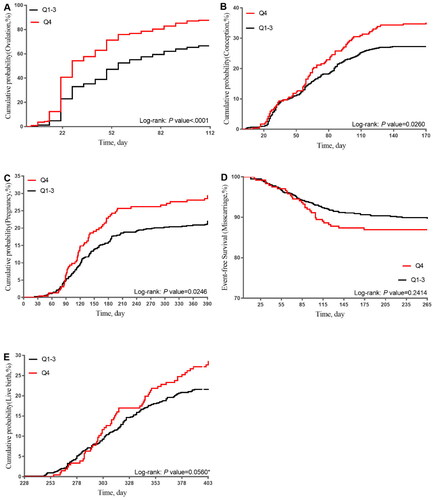
The Kaplan-Meier curves () presented the survival data by SHBG for reproductive outcomes. The highest quartile (Q4) of SHBG had significantly higher rates of ovulation, conception, and pregnancy compared to the first three quartiles (Q1-Q3) of SHBG over time (p < 0.0001; p = 0.0260; p = 0.0246). High SHBG showed no significant association with miscarriage (p = 0.2414). Furthermore, Q4 of SHBG had a relatively higher live birth rate, although the p value (p = 0.056) was borderline significant.
Discussion
In this study, SHBG had the strongest prediction for ovulation and women with high SHBG, independent of testosterone, had a higher ovulation rate. Additionally, women in the high SHBG quartile had a significantly higher conception rate and pregnancy rate. These two findings indicated that among women with PCOS, the baseline SHBG level was associated with higher ovulation, which might have further effects on conception, pregnancy, and live birth.
High SHBG levels may contribute to high ovulation, conception, and pregnancy rates, and probably a high live birth rate may be achieved when more subjects were enrolled. SHBG with higher quartiles had higher ovulation rate after adjustment for testosterone, and testosterone as well as estradiol showed no similar tendency. Therefore, we considered that the association between SHBG and ovulation was independent of testosterone and estradiol. Most previous studies focused on the relationships between other sexual hormones and ovulation. FSH/LH ratio [Citation18], preovulatory E2/−1E2 ratio, premature LH surge [Citation19], surge in serum and urinary LH [Citation20] or AMH [Citation21] might link to follicular development. However, our research indicated that SHBG was also lined with ovulation rather than other parameters. In addition, follicles produce progesterone during their development; baseline levels of progesterone do not have strong predictive power for ovulation. Besides, we typically use a threshold of progesterone >5 ng/ml to confirm ovulation in their cycles, rather than baseline levels of progesterone. The levels of SHBG in the menstrual cycle may exhibit some variation, but overall, it can be considered relatively stable. This study investigated the relationship between baseline hormone levels and the occurrence of ovulation, and we found that baseline SHBG was associated with ovulation instead of baseline progesterone.
Additionally, several studies have shown contradictory results regarding ovulation. Compared with PCOS women with anovulation, women with regular ovulation had statistically higher serum SHBG [Citation22]. However, LH, and Androstenedione could be considered as reliable predictors for depend on for recruiting ideal candidates with clomiphene citrate resistant polycystic ovarian disease, but not SHBG [Citation23]. Our study found that SHBG was more associated with ovulation. According to a recent study, the number of follicles measuring 15-20 mm were predicted by SHBG mRNA levels on day 1, regardless of age. The number of follicles measuring 15-20mm increased by 2.688 units for every unit rise in SHBG mRNA expression [Citation24]. A polymorphism study conducted in PCOS patients undergoing IVF-ET (in vitro fertilization-embryo transfer) found that SHBG rs6259 GA + AA genotype carriers had a decreased number of retrieved oocytes and embryos as well as fertility rate [Citation25]. However, due to the limited number of clinical research on the relationship between SHBG and reproductive outcomes in women with PCOS, more research is needed.
We further calculated the quartiles of SHBG, T, and E2, and conducted adjusted analyses due to the interaction between SHBG and T, as well as E2. Higher levels of SHBG were associated with a higher ovulation rate, and this correlation remained statistically significant after adjustment for T. However, there was no similar trend observed for T or E2. One of the key features of PCOS is the presence of excess adiposity or obesity, which is capable of synthesizing and storing estrogen. But a cross-sectional study [Citation26] found that endotrophin could potentially contribute to the development of polycystic ovary syndrome (PCOS) more than other adipokines. The level of high-molecular-weight adiponectin was positively linked to SHBG [Citation27], and as adiponectin modulates follicular growth and maturation [Citation28], further investigation is needed to explore the correlation between adiponectin and follicles. But in our study, there was no obvious linear relationship between E2 and ovulation. Previous studies have also suggested that a high androgen microenvironment inhibits granulosa cell proliferation and alters cell identity [Citation29] granulosa cell proliferation was found to be increased in anovulatory polycystic ovaries compared with both normal and ovulatory polycystic ovaries [Citation30], and AQP-9 mRNA levels in granulosa cells of patients with PCOS were significantly correlated with SHBG levels in follicular fluid [Citation31]. While the conventional understanding that the primary function of SHBG involves regulating the concentrations of free sex steroids in plasma, it was suggested that there was a more direct impact of SHBG through the mediation of intracellular signaling cascades facilitated by membrane-bound SHBG receptors [Citation32]. These novel insights into mechanisms of SHBG implied a potentially more significant role than the previous assumptions. Therefore, we concluded that the prediction of SHBG for ovulation was independent of testosterone and estradiol, supporting the previous finding that SHBG had a stronger predictive ability compared with T and E2.
Furthermore, in this study, SHBG was also found to be associated with lower conception rate, pregnancy rate, and a probably lower live birth rate when increasing the sample size. Another secondary analysis of two trials conducted among PCOS women found that SHBG was positively associated with conception, pregnancy, and live birth, which is consistent with our results [Citation9]. One reason for this result was that low SHBG levels were associated with a low ovulation rate, which corresponds to decreased conception and live birth rates. Another reason was that previous studies have found a correlation between low SHBG levels and various pregnancy complications, which may also contribute to lower live birth rates. Firstly, SHBG has been identified as a marker in predicting gestational diabetes mellitus (GDM) [Citation33,Citation34] and preeclampsia [Citation35] in PCOS subjects. Additionally, SHBG could be used as an integrated biomarker to predict an adverse cardio-metabolic profile in pregnant women with pregestational plus gestational obesity. Overall, these studies indicated that women with PCOS who have low SHBG levels had a lower chance of achieving pregnancy rate, a lower live birth rate, and a higher incidence of several pregnancy complications, which could have adverse effects on both maternal and child health. Therefore, if PCOS women with low SHBG levels desire to have children, they should take measures to increase their SHBG levels in order to improve their ovulation rate.
The study is based on a large multicenter, randomized, double-blind, placebo-controlled design with a representative sample of PCOS population. The clinical implications of our study, which, in contrast to previous studies, focused primarily on the associations between normal sexual hormones and ovulation, include SHBG as a marker for assessing ovulation. This is the first study to systematically assess and demonstrate the correlation between SHBG levels and reproductive outcomes. Higher baseline SHBG levels are associated with increased ovulation and serve as a relatively independent predictive marker. However, there are several limitations of this study. Firstly, this study is a secondary analysis of PCOSAct, making it challenging to obtain certain data, such as information regarding normal controls, and enrollment of a larger number of subjects. Secondly, the study did not assess the relationship between SHBG and ovulation for each menstrual cycle. Further research is needed to clarify the role of SHBG in determining reproductive outcomes.
Conclusions
Higher baseline SHBG was associated with higher ovulation compared with other baseline sexual hormones.
Compliance with ethics guidelines
All authors (Hui Chang, Hang Ge, Qi Wu, Yanli Zhang, Jian Li, Mengyi Zhu, Xi Luo, Yanhua Han, Yong Wang, Chi Chiu Wang, Xiaoke Wu) declare that they have no conflict of interest or financial conflicts to disclose.
Preprint
A preprint has previously been published [Citation36].
Acknowledgements
We gratefully acknowledge the 21 collaborating sites and the following members of the PCOSAct study group: LH Hou, Zhen-Xing Hu, Xiao-Guang Shao, Jun Ge, Jin-Feng Zhang, Hui-Ying Xue, Xiao-Feng Xu, Rui-Ning Liang, Hong-Xia Ma, Hong-Wei Yang, WeiLi Li, Dong-Mei Huang, Yun Sun, Cui-Fang Hao, Shao-Min Du, Zheng-Wang Yang, Xin Wang, Ying Yan, Xiu-Hua Chen, Ping Fu, Cai-Fei Ding, Ya-Qin Gao, ZhongMing Zhou, Chi Chiu Wang, Tai-Xiang Wu, Jian-Ping Liu, Ernest HY Ng, Richard S Legro, and Heping Zhang. Dr. Xiaoke Wu had full access to all data in the study (including statistical reports and tables) and thus takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Dr Xiaoke Wu, Elisabeth Stener Victorin, Ernest HY Ng and Chi Chi-u-Wang. Drafting of the manuscript: Hui Chang and Hang Ge. Data collection and data interpretation: Hui Chang, Yanli Zhang, Mengyi Zhu, Xi Luo and Yanhua Han. Statistical analysis: Qi Wu, Jian Li, Dr Yong Wang and Dr Chi Chiu Wang. Supervision: Dr Xiaoke Wu. All authors critically reviewed the article and approved it for submission.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets generated analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):1. doi: 10.1038/nrdp.2016.57.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, R.E.A.-S.P. consensus workshop group. Revised 2003 consensus on diagnostic criteria and Long-Term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–6. doi: 10.1016/j.fertnstert.2003.10.004.
- Krentowska A, Kowalska I. Metabolic syndrome and its components in different phenotypes of polycystic ovary syndrome. Diabetes Metab Res Rev. 2022;38(1):e3464. doi: 10.1002/DMRR.3464.
- Zhu J L, Chen Z, Feng W J, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142–148. doi: 10.1016/J.CCA.2019.09.010.
- Li Y, Fang L, Yan Y, et al. Association between human SHBG gene polymorphisms and risk of PCOS: a meta-analysis. Reprod Biomed Online. 2021;42(1):227–236.
- Simons PIHG, Valkenburg O, Stehouwer CDA, et al. Sex hormone-binding globulin: biomarker and hepatokine? Trends Endocrinol Metab. 2021;32(8):544–553. doi: 10.1016/J.TEM.2021.05.002.
- Liu X, Wang N, Gao Z. β-Carotene regulates glucose transport and insulin resistance in gestational diabetes mellitus by increasing the expression of SHBG. Clin Exp Pharmacol Physiol. 2022;49(12):1307–1318. doi: 10.1111/1440-1681.13712.
- Xargay-Torrent S, Carreras-Badosa G, Borrat-Padrosa S, et al. Circulating sex hormone binding globulin: an integrating biomarker for an adverse cardiometabolic profile in obese pregnant women. PLoS One. 2018;13(10):e0205592. doi: 10.1371/journal.pone.0205592.
- Kuang H, Jin S, Hansen KR, et al. Identification and replication of prediction models for ovulation, pregnancy and live birth in infertile women with polycystic ovary syndrome. Hum Reprod. 2015;30(9):2222–2233. doi: 10.1093/humrep/dev182.
- Chen Z, Zhang Y, Liu J. Diagnosis of polycystic ovary syndrome: standard and guideline of Ministry of Health of People’s Republic Of China. Zhong Hua Fu Chan Ke Za Zhi. 2012;47:74–75.
- Li R, Qiao J, Yang D, et al. Epidemiology of hirsutism among women of reproductive age in the community: a simplified scoring system. Eur J Obstet Gynecol Reprod Biol. 2012;163(2):165–169. doi: 10.1016/j.ejogrb.2012.03.023.
- Zhao X, Ni R, Li L, et al. Defining hirsutism in Chinese women: a cross-sectional study. Fertil Steril. 2011;96(3):792–796. doi: 10.1016/j.fertnstert.2011.06.040.
- Cooper TG, Noonan E, Eckardstein S, et al. World health organization reference values for human semen. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048.
- The practice committee of the American Society for Reproductive Diagnostic evaluation of the infertile female : a committee opinion. Fertil Steril. 2012;98:302–307. doi: 10.1016/j.fertnstert.2012.05.032.
- Kuang H, Li Y, Wu X, et al. Acupuncture and clomiphene citrate for live birth in polycystic ovary syndrome : Study design of a randomized controlled trial. Evidence-Based Comple Alternative Med. 2013;2013:1–11. doi: 10.1155/2013/527303.
- Wu X-K, Stener-Victorin E, Kuang H-Y, et al. Effect of acupuncture and clomiphene in Chinese women with polycystic ovary syndrome a randomized clinical trial. JAMA. 2017;317(24):2502–2514. doi: 10.1001/jama.2017.7217.
- Legro RS, Brzyski RG, Diamond MP, et al. The pregnancy in polycystic ovary syndrome II study: baseline characteristics and effects of obesity from a multicenter randomized clinical trial. Fertil Steril. 2014;101(1):258–269.e8. doi: 10.1016/j.fertnstert.2013.08.056.
- Arat Ö, Devecı D, Özkan ZS, et al. What is the effect of the early follicular phase FSH/LH ratio on the number of mature oocytes and embryo development? Turk J Med Sci. 2020;50(2):420–425. doi: 10.3906/SAG-1910-234.
- Lu X, Khor S, Zhu Q, et al. Decrease in preovulatory serum estradiol is a valuable marker for predicting premature ovulation in natural/unstimulated in vitro fertilization cycle. J Ovarian Res. 2018;11(1):96. doi: 10.1186/s13048-018-0469-x.
- Zheng Y, Pan Y, Li P, et al. Ovarian sensitivity decreased significantly in patients with insulin resistance undergoing in vitro fertilization and embryo transfer. Front Physiol. 2021;12:809419. doi: 10.3389/FPHYS.2021.809419.
- Ciepiela P, Dulęba AJ, Kario A, et al. Oocyte matched follicular fluid anti-Müllerian hormone is an excellent predictor of live birth after fresh single embryo transfer. Hum Reprod. 2019;34(11):2244–2253. doi: 10.1093/HUMREP/DEZ186.
- Henríquez S, Kohen P, Xu X, et al. Significance of pro-angiogenic estrogen metabolites in normal follicular development and follicular growth arrest in polycystic ovary syndrome. Hum Reprod. 2020;35(7):1655–1665. doi: 10.1093/humrep/deaa098.
- Seyam E, Hefzy E. Tumor necrosis factor alpha versus LH and androstendione as a reliable predictor of spontaneous ovulation after laparoscopic ovarian drilling for women with clomiphene citrate resistance polycystic ovarian disease. Eur J Obstet Gynecol Reprod Biol. 2018;222:126–133. doi: 10.1016/J.EJOGRB.2018.01.011.
- Vaitsopoulou CI, Kolibianakis EM, Bosdou JK, et al. Expression of genes that regulate follicle development and maturation during ovarian stimulation in poor responders. Reprod Biomed Online. 2021;42(1):248–259. doi: 10.1016/j.rbmo.2020.05.012.
- Liu Y, Zhao XX, Hu XJ, et al. Effect of sex hormone–binding globulin polymorphisms on the outcome of in vitro Fertilization-Embryo transfer for polycystic ovary syndrome patients: a Case-Control study. J Cell Biochem. 2019;120(3):4675–4686. doi: 10.1002/jcb.27756.
- Guney G, Taskin MI, Baykan O, et al. Endotrophin as a novel marker in PCOS and its relation with other adipokines and metabolic parameters: a pilot study. Ther Adv Endocrinol Metab. 2021;12:20420188211049607. doi: 10.1177/20420188211049607.
- Shorakae S, Abell SK, Hiam DS, et al. High-molecular-weight adiponectin is inversely associated with sympathetic activity in polycystic ovary syndrome. Fertil Steril. 2018;109(3):532–539. doi: 10.1016/j.fertnstert.2017.11.020.
- Richards JS, Liu Z, Kawai T, et al. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil Steril. 2012;98(2):471–479.e1. doi: 10.1016/j.fertnstert.2012.04.050.
- McFee RM, Romereim SM, Snider AP, et al. A High-Androgen microenvironment inhibits granulosa cell proliferation and alters cell identity. Mol Cell Endocrinol. 2021;531:111288. doi: 10.1016/J.MCE.2021.111288.
- Stubbs SA, Stark J, Dilworth SM, et al. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab. 2007;92(11):4418–4426. doi: 10.1210/jc.2007-0729.
- Qu F, Wang FF, Lu XE, et al. Altered aquaporin expression in women with polycystic ovary syndrome: hyperandrogenism in follicular fluid inhibits aquaporin-9 in granulosa cells through the phosphatidylinositol 3-Kinase pathway. Hum Reprod. 2010;25(6):1441–1450. doi: 10.1093/humrep/deq078.
- Rosner W, Hryb DJ, Kahn SM, et al. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol. 2010;316(1):79–85. doi: 10.1016/J.MCE.2009.08.009.
- Li G, Huang W, Zhang L, et al. A prospective cohort study of early-pregnancy risk factors for gestational diabetes in polycystic ovarian syndrome. Diabetes Metab Res Rev. 2018;34(5):e3003. doi: 10.1002/dmrr.3003.
- Faal S, Abedi P, Jahanfar S, et al. Sex hormone binding globulin for prediction of gestational diabetes mellitus in pre-conception and pregnancy: a systematic review. Diabetes Res Clin Pract. 2019;152:39–52. doi: 10.1016/J.DIABRES.2019.04.028.
- Nevalainen J, Korpimaki T, Kouru H, et al. Performance of first trimester biochemical markers and mean arterial pressure in prediction of early-onset pre-eclampsia. Metabolism. 2017;75:6–15. doi: 10.1016/J.METABOL.2017.07.004.
- Chang H, Wu Q, Ge H, et al. High baseline SHBG, as an independent predictor, was associated with high ovulation : a secondary analysis of PCOSAct. 2021;1–17. doi:10.21203/rs.3.rs-146920/v1.