Abstract
Objective
This study aimed to collect, organize, and conduct a meta-analysis of the literature on the expression of silent information regulator two homolog 1 (SIRT1) in the placental tissue and plasma of patients with pre-eclampsia.
Methods
The enrolled patients were divided into two groups: the pre-eclampsia group and the healthy group. This study summarized and analyzed the demographic characteristics of the two groups, including pregnancy age, gestational weeks, parity, gravidity, blood pressure, Body Mass Index, newborn weight, placental weight, and SIRT1 expression in placental tissue and maternal plasma.
Results
Eleven studies were included in this research, with 586 cases in the pre-eclampsia group and 479 cases in the control group. Three research studies are reporting immunohistochemistry tests, among which the pre-eclampsia group had a positivity rate of 30.24% (62/205), while the control group had 58.02% (76/131); the two groups have a significant difference (p < 0.05). Two research studies reported the results of ELISA tests, with 107 cases in the pre-eclampsia group and 125 cases in the control group. A comparison of the SIRT1 test results showed a statistically significant difference between the two groups (p < 0.05). Pre-eclampsia group patients had lower gestational weeks, newborn birth weight, and placental weight compared to the healthy control group (all p < 0.05). However, systolic and diastolic blood pressures were higher in the pre-eclampsia group than in the control group (p < 0.05).
Conclusion
SIRT1 expression is downregulated in pre-eclampsia patients’ plasma and placental tissue. Further research is needed to validate this conclusion.
Background
Pre-eclampsia is a pregnancy-specific disorder characterized primarily by high blood pressure, often accompanied by proteinuria and maternal organ dysfunction [Citation1, Citation2]. Worldwide, an estimated 4 million women are diagnosed with pre-eclampsia annually, resulting in the deaths of over 70,000 women and 500,000 babies [Citation3–5]. Pre-eclampsia is one of the leading causes of increased maternal and perinatal mortality rates and its specific pathogenesis are unclear. Some studies have shown that the pathogenesis may be related to increased oxidative stress in the patient’s body, immature nourishing layers, and SARS-CoV-2 infection [Citation6–8]. Based on its clinical presentation and prognosis, placental dysfunction is considered a critical factor in the pathological process of pre-eclampsia [Citation9].
Abnormal apoptosis of placental trophoblast cells is closely related to the pathogenesis of pre-eclampsia [Citation10]. Research has shown that Silent Information Regulator Two Homolog 1 (SIRT1), a protein deacetylase, is crucial in regulating various physiological processes such as cell metabolism, aging, proliferation, and apoptosis [Citation11]. Research indicates that SIRT1 is expressed to varying degrees in human placental syncytiotrophoblast and cytotrophoblast cells [Citation12]. In mammalian cells, SIRT1 can inhibit apoptosis by reducing the acetylation of p53 [Citation13]. Current research suggests that decreased expression of SIRT1 may be associated with the occurrence of pre-eclampsia, and its expression levels are negatively correlated with the severity of pre-eclampsia. A study by Pei et al. [Citation14] showed that pregnant mice with SIRT1 knockdown exhibited typical symptoms of pre-eclampsia, and the expression levels of SIRT1 in the serum and placenta of pre-eclampsia patients were relatively low. Tong Jun Ge et al. [Citation15] divided 100 cases of pre-eclampsia into mild and severe groups and measured the expression of SIRT1 in plasma. The results showed that the expression of SIRT1 in the severe pre-eclampsia group was significantly lower than that in the mild group, and there was a statistically significant difference between the two groups (p < 0.05). Furthermore, serum SIRT1 expression and uterine artery hemodynamics were closely related to fetal growth restriction (p < 0.001). These studies indicate that SIRT1 may be involved in the development and progression of pre-eclampsia in pregnant women.
This study aims to conduct a meta-analysis of the expression of SIRT1 in the plasma and placental tissue of pre-eclampsia pregnant women to provide additional research directions for the pathogenesis of pre-eclampsia.
Materials and methods
Literature search
This study conducted keyword searches in medical databases and relevant websites from January 2023 to June 2023. The keyword search included [(“Pre-eclampsia” or “Preeclampsia” or “hypertensive disorder of pregnancy”) and (“SIRT1” or “Sirtuin1” or “silence information regulator two homologs 1”) and/or “placenta”]. The search databases used in this study included Google Scholar, PubMed, Web of Science, ScienceDirect, EBSCO, Wiley, China National Knowledge Internet, and Wanfang database. As this study involves the summary and analysis of other studies, it does not involve medical ethics approval or patient-informed consent.
Inclusion and exclusion criteria
The inclusion criteria for the literature were as follows: (1) All studies included in the literature met the criteria of the "Guidelines for the Diagnosis and Treatment of Hypertensive Disorders in Pregnancy" for the diagnosis of pre-eclampsia [Citation16–18]; (2) Detection of placental tissue or plasma in the study population; (3) Natural conception; (4) Visual or statistically measurable experimental results. The exclusion criteria were as follows: (1) Artificial conception; (2) Incomplete data or failure to provide data on critical diagnostic indicators; (3) Severe autoimmune diseases, renal diseases, liver diseases, long-term chronic hypertension, etc.; (4) Complications such as gestational diabetes in pre-eclampsia; (5) Duplicate studies with overlapping data.
Main testing indicators
After the delivery of the placenta, a certain amount of placental tissue was collected to detect SIRT1 mRNA or protein (using immunohistochemistry and Western Blot methods). ELISA Kit was used to detect SIRT1 in the plasma of the pre-eclampsia group and the healthy control group. This study summarized and analyzed the demographic characteristics of the two groups, including maternal age, gestational weeks, parity, gravidity, blood pressure, BMI (kg/m^2), neonatal weight (g), placental weight (g), and the expression of SIRT1 in placental tissue and maternal plasma.
Statistical methods
In this study, data management and figure production were conducted using STATA 12.0 software. Forest plots for categorical data were generated using meta-analysis of binary variables, while meta-analysis of continuous variables (n, mean, and SD) was performed for analyzing continuous data. The statistical difference between two groups of continuous data depends on the overall difference (z-value and p-value). I^2 was used to assess heterogeneity among the included studies. Funnel plots were employed to assess publication bias for included studies reporting categorical data. Begg’s test and Egger’s test were used to detect publication bias for continuous data. A P/p-value <0.05 was considered statistically significant for detecting differences.
Results
Inclusion of the study
Through keyword searches, this meta-analysis initially identified 349 relevant studies. After reviewing the keywords, titles, and abstracts, 237 studies were excluded. From the remaining 112 studies, a rough overview of the article content led to excluding an additional 84 studies. Subsequently, a careful reading of the articles resulted in the exclusion of 14 more studies. Finally, considering factors such as the presence of a control group, data visualization, and study data duplication, the final three articles were excluded. Ultimately, this study included 11 relevant studies [Citation14, Citation19–28] on the correlation between SIRT1 and pre-eclampsia (). The pre-eclampsia group comprised 589 patients, while the healthy control group included 479 individuals. Detailed information regarding the authors, patient recruitment time, antibodies used, and experimental methods can be found in .
Table 1. The primary characteristics of the eligible studies.
Table 2. Basic information of the articles included in this study.
Analysis of baseline information
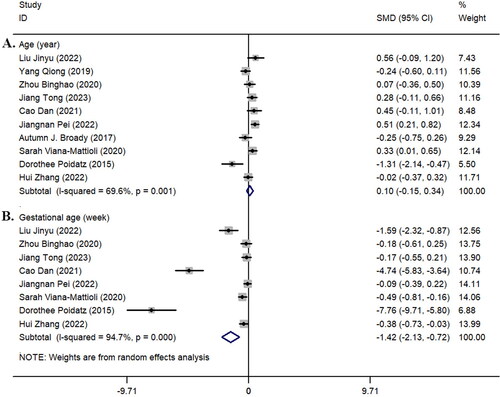
We investigated whether there was a statistically significant difference in maternal age between the pre-eclampsia and control groups. All the included studies compared the age of the two groups, but the results showed no statistically significant difference in age between the two groups (z = 0.77, p = 0.441; I^2 = 69.6%, p = 0.001, ). Additionally, we conducted a comparative analysis of the gestational age of pregnant women in 8 studies [Citation14, Citation19, Citation21, Citation22, Citation24, Citation26–28]. The results showed that the gestational age in the pre-eclampsia group was shorter than that in the healthy control group, and there was a statistically significant difference between the two groups (z = 3.94, p < 0.001; I^2 = 94.7%, p < 0.001, ). Detailed results can be seen in .
Figure 1. Forest plot of maternal age (year) and gestational age (week). A: Age (year); B: gestational age (week). SMD: Standardized mean difference; black dots: point estimates of effect sizes for each study; squares: weights of each study; line segment length: 95% confidence interval for each study’s effect size; diamond: summary effect size from meta-analysis; width of diamond: 95% confidence interval for the summary effect size.
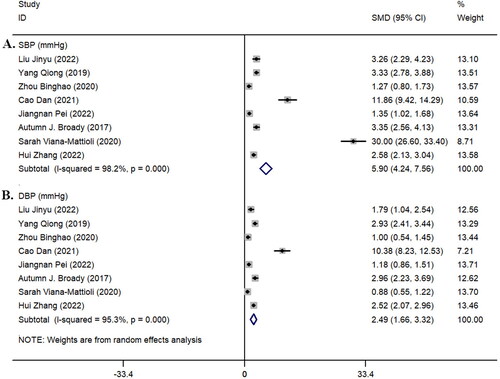
Among the eight studies [Citation14, Citation19–21, Citation24–26, Citation28] that included blood pressure data for both groups, the systolic blood pressure (z = 6.96, p < 0.001; I^2 = 98.2%, p < 0.001, ) and diastolic blood pressure (z = 5.90, p < 0.001; I^2 = 95.3%, p < 0.001, ) in the pre-eclampsia group were significantly higher than those in the healthy control group ().
Figure 2. Forest plot of systolic blood pressure (SBP) and diastolic blood pressure (DBP). A: SBP; B: DBP.
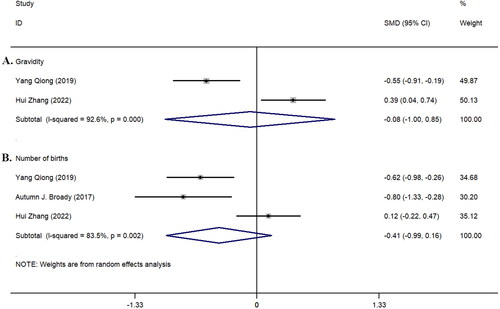
Furthermore, we studied the parity and gravidity between the two groups in the included studies (). The analysis of parity included three studies [Citation20, Citation25, Citation28], but no statistically significant difference was found between the two groups (z = 0.16, p = 0.870; I^2 = 92.6%, p < 0.001, ). The analysis of gravidity included two studies [Citation20, Citation28]; similarly, no statistically significant difference was observed between the two groups (z = 1.41, p = 0.158; I^2 = 83.5%, p = 0.002, ).
Figure 3. Forest plot of parity and gravidity in the included study population. A: Gravidity; B: Number of births.
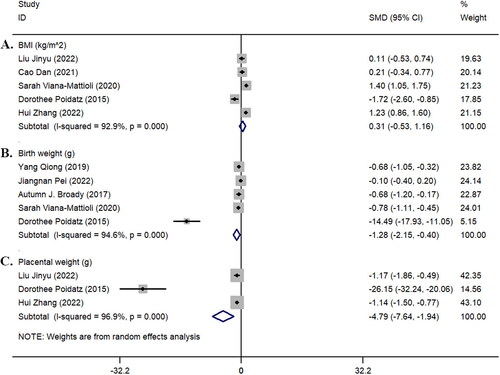
We summarized and analyzed the BMI (kg/m^2) of the two groups of pregnant women, the birth weight of newborns, and the placental weight. The results showed no statistically significant difference in BMI between the two groups of pregnant women (z = 0.73, p = 0.468; I^2 = 92.9%, p < 0.001). However, there were statistically significant differences between the two groups in terms of the birth weight of newborns (z = 2.86, p = 0.004; I^2 = 94.6%, p < 0.001) and placental weight (z = 3.30, p = 0.001; I^2 = 96.9%, p < 0.001) ().
Detection results of SIRT1 by different detection methods
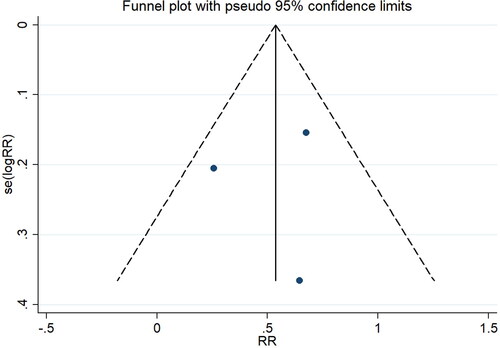
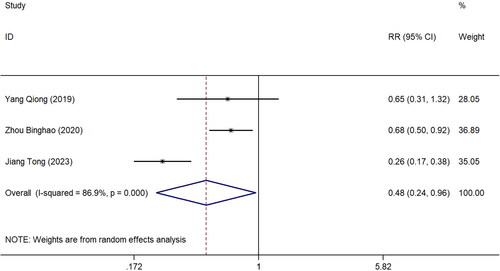
In this study, three articles [Citation20–22] used the immunohistochemistry staining method to detect the expression of SIRT1 in the placental tissue of patients in the pre-eclampsia and healthy control groups. The results showed that the positive rate in the pre-eclampsia group was 30.24% (62/205). In comparison, the positive rate in the healthy control group was 58.02% (76/131), indicating a statistically significant difference between the two groups (z = 2.07, p = 0.038; I^2 = 86.9%, p < 0.001) ().
Figure 5. Forest plot of immunohistochemistry detection of placental tissue in the included study population. RR: Relative risk.
Two studies [Citation14, Citation25] reported the expression of SIRT1 in the plasma of patients in the pre-eclampsia group and healthy control group using ELISA. The results showed that the expression level of SIRT1 in the pre-eclampsia group was significantly lower than that in the control group (z = 2.22, p = 0.026; I^2 = 78.7%, p = 0.030, ). Although five studies [Citation19, Citation21, Citation23, Citation24, Citation27] and three studies [Citation19, Citation24, Citation28] reported the expression of SIRT1 mRNA () and WB (), respectively, there was no statistically significant difference in the expression of SIRT1 between the two groups in both mRNA and protein levels (both p > 0.05) (). Detailed results of the different detection methods for SIRT1 are shown in .
Figure 6. Forest plot of ELISA detection of plasma, qRT-PCR detection of mRNA, and Western Blotting (WB) protein detection in the included study population. A: ELISA detection; B: qRT-PCR detection; C: WB detection.
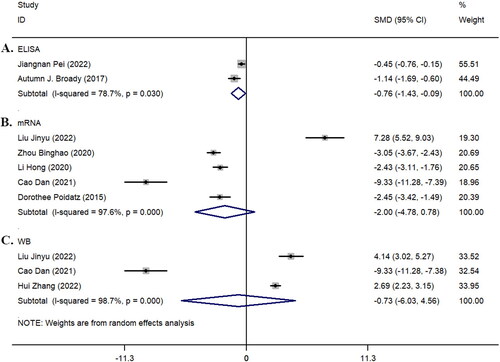
Table 3. Different detection methods were used to assess the expression of SIRT1 in the two groups.
Publication bias
The funnel plot results showed no significant publication bias among the studies using the immunohistochemistry method (). The analysis of studies investigating SIRT1 mRNA expression also indicated no significant publication bias, with Pr > |z| = 1.000 in Begg’s test and P > |t| = 0.740 in Egger’s test.
Discussion
Pre-eclampsia is a significant cause of high maternal and neonatal mortality rates and maternal multi-organ dysfunction. The probability of pre-eclampsia patients developing cardiovascular diseases within 15 years postpartum increases by 2–7 times [Citation29]. Current research suggests that the onset of pre-eclampsia may be related to insufficient infiltration of extravillous trophoblast cells in the placental basal plate and vascular endothelial injury and remodeling disorders caused by the activation of inflammatory factors [Citation3, Citation30]. As an essential regulator of cellular metabolism, the SIRT1 protein is involved in energy homeostasis regulation, mitochondrial biogenesis, and oxidative-antioxidant stress [Citation31–33]. SIRT1 has therapeutic potential in diseases associated with endothelial dysfunction and vascular abnormalities.
Further studies have shown that SIRT1 can participate in developing pre-eclampsia by regulating trophoblast cell apoptosis, placental blood flow perfusion, and maternal oxidative stress levels [Citation34–36]. Additionally, SIRT1 has been found to regulate the synthesis of various transcription factors and pro-inflammatory mediators through processes such as histone deacetylation, thus participating in the pathogenesis of pre-eclampsia [Citation37]. A study show that under ROS induction, SIRT1 can positively regulate apoptosis, autophagy, and mitochondrial function in embryonic stem cells, suggesting that modulating the expression level of SIRT1 may serve as a new therapeutic target to prevent oxidative threats to female fertility and counteract ROS-induced damage [Citation34].
This study summarized and analyzed the correlation between SIRT1 expression in placental tissue and plasma of pre-eclampsia patients. The results of immunohistochemistry detection showed that the positive rate of SIRT1 expression was lower in the pre-eclampsia group (30.24%, 62/205) compared to the healthy control group (58.02%, 76/131), and there was a statistically significant difference between the two groups (p < 0.05). This trend was further confirmed in the ELISA detection of SIRT1 in pre-eclampsia patients’ plasma (p = 0.026). However, there was no statistically significant difference in the expression of SIRT1 mRNA and protein detected by qRT-PCR and WB between the two groups (p > 0.05). This may be due to the inconsistent SIRT1 expression observed in the study by Liu Jinyu et al. [Citation19] compared to the other four studies. Their research showed significantly higher mRNA expression of SIRT1 in the pre-eclampsia group compared to the healthy control group (p < 0.05). The higher expression of SIRT1 in the pre-eclampsia group might be related to the triggering of compensatory mechanisms in response to tissue damage. We believe this discrepancy may be due to different immune responses of SIRT1 in the syncytiotrophoblast of the placenta at different stages of pregnancy, which was further confirmed in the study by YuJia Wang et al. [Citation38] Their research showed relatively higher immunoreactivity of SIRT1 in the syncytiotrophoblast of the placenta in early pregnancy, which decreased in the mid-pregnancy stage (p = 0.03).
This study also analyzed the blood pressure of the two groups and found statistically significant differences in systolic and diastolic blood pressure between the groups (p < 0.05), with the dynamic changes in systolic blood pressure being higher than diastolic blood pressure in both groups. Although there was no statistically significant difference in the age of pregnancy between the two groups, the gestational age of the pre-eclampsia group was significantly shorter than that of the healthy control group (p < 0.05), which is consistent with pre-eclampsia being prone to clinical preterm birth. There was no statistically significant difference in the parity and gravidity between the two groups, which ruled out the impact of these two indicators on the occurrence of pre-eclampsia. There was no statistically significant difference in BMI between the two groups. However, the birth weight of newborns in the pre-eclampsia group and the placental weight of pregnant women were significantly lower than those in the healthy control group.
This study conducted a detailed analysis of the expression of SIRT1 in pre-eclampsia patients and healthy control groups using meta-analysis. However, this study still has some limitations, such as the need for more collection and organization of clinical sample data from a more significant number of pre-eclampsia patients, the relatively lower quality of included studies, and inconsistencies in the use of testing reagents or diagnostic criteria. These factors may reduce the reliability of the study.
Conclusion
This study shows that the expression of SIRT1 in the placenta and plasma of pre-eclampsia patients is lower than that in the healthy control group, indicating that SIRT1 may be involved in the development of pre-eclampsia. Increasing the in vivo expression activity of SIRT1 in the future may have a beneficial effect on improving the symptoms of pre-eclampsia, providing new insights for the clinical treatment of pre-eclampsia.
Contributions
Hongling Wang: Study design, Data collection, Data analysis, Manuscript writing.
Wenwen Liang: Study design, Data analysis, Manuscript editing.
Weihua Su: Data collection, Data analysis.
Hong Cui: Data collection, Data analysis.
Huifeng Wang: Study design, Data analysis, Manuscript editing.
All the authors have read and approved the final manuscript.
Ethics approval and consent to participate
As this study involves the summary and analysis of other studies, it does not involve medical ethics approval or patient-informed consent.
| Abbreviation | ||
| SIRT1 | = | Silent information regulator two homolog 1 |
| BMI | = | Body Mass Index |
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Tochio A, Obata S, Saigusa Y, et al. Does pre‐eclampsia without proteinuria lead to different pregnancy outcomes than pre‐eclampsia with proteinuria? J Obstet Gynaecol Res. 2019;45(8):1–9. doi: 10.1111/jog.14017.
- Guida JP, Parpinelli MA, Surita FG, et al. The impact of proteinuria on maternal and perinatal outcomes among women with pre‐eclampsia. Int J Gynaecol Obstet. 2018;143(1):101–107. doi: 10.1002/ijgo.12487.
- Dimitriadis E, Rolnik DL, Zhou W, et al. Pre-eclampsia. Nat Rev Dis Primers. 2023;9(1):8. doi: 10.1038/s41572-023-00417-6.
- Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynecol Obstet. 2015;131:S173–S211. doi: 10.1016/S0020-7292(15)30007-2.
- Magee LA, Brown MA, Hall DR, et al. The 2021 international society for the study of hypertension in pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022;27:148–169. doi: 10.1016/j.preghy.2021.09.008.
- Tossetta G, Fantone S, Piani F, et al. Modulation of NRF2/KEAP1 signaling in preeclampsia. Cells. 2023;12(11):1545. doi: 10.3390/cells12111545.
- Fantone S, Mazzucchelli R, Giannubilo SR, et al. At-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem Cell Biol. 2020;154(3):339–346. doi: 10.1007/s00418-020-01892-8.
- Tossetta G, Fantone S, Balercia G, et al. Preeclampsia and severe acute respiratory syndrome coronavirus 2 infection: a systematic review. J Hypertens. 2022;40(9):1629–1638. doi: 10.1097/HJH.0000000000003213.
- Griffin M, Heazell AE, Chappell LC, et al. The ability of late pregnancy maternal tests to predict adverse pregnancy outcomes associated with placental dysfunction (specifically fetal growth restriction and pre-eclampsia): a protocol for a systematic review and meta-analysis of prognostic accuracy studies. Syst Rev. 2020;9(1):78. doi: 10.1186/s13643-020-01334-5.
- Liu M, Yin Y, Yu H, et al. Laminins regulate placentation and pre-eclampsia: focus on trophoblasts and endothelial cells. Front Cell Dev Biol. 2020;8:754. doi: 10.3389/fcell.2020.00754.
- Vaiciulis P, Liutkeviciene R, Liutkevicius V, et al. Association of SIRT1 single gene nucleotide polymorphisms and serum SIRT1 levels with laryngeal squamous cell carcinoma patient survival rate. Cancer Biomark. 2022;34(2):175–188. doi: 10.3233/CBM-210264.
- Lappas M, Mitton A, Lim R, et al. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84(1):167–178. doi: 10.1095/biolreprod.110.086983.
- Zhao X, Wu Y, Li J, et al. JNK activation-mediated nuclear SIRT1 protein suppression contributes to silica nanoparticle-induced pulmonary damage via p53 acetylation and cytoplasmic localisation. Toxicology. 2019;423:42–53. doi: 10.1016/j.tox.2019.05.003.
- Pei J, Liu Z, Wang C, et al. Progesterone attenuates sirt1-deficiency-mediated pre-eclampsia. Biomolecules. 2022;12(3):422. doi: 10.3390/biom12030422.
- Ge TJ, Kong JY. Clinical value of serum SIRT1 combined with uterine hemodynamics in predicting disease severity and fetal growth restriction in preeclampsia. Evidence-Based Complementary and Alternative Med. 2023;2023:1–12. doi: 10.1155/2023/1744625.
- Yang Z, Zhang W. Guidelines for the diagnosis and treatment of hypertensive disorders in pregnancy. Chin J Obst Gynecol. 2020;55:227–238.
- Magee LA, Pels A, Helewa M, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4(2):105–145. doi: 10.1016/j.preghy.2014.01.003.
- Sinkey RG, Battarbee AN, Bello NA, et al. Prevention, diagnosis, and management of hypertensive disorders of pregnancy: a comparison of international guidelines. Curr Hypertens Rep. 2020;22(9):66. doi: 10.1007/s11906-020-01082-w.
- Liu J-Y, Chen C, Yang X-Y, et al. Silence information regulator 2 homolog 1 participates in the pathogenesis of preeclampsia by regulating vascular endothelial growth factor A. Chinese J Family Plan Gynecotokol. 2023;14(3):31–35.
- Yang Q, Yuan Y-C, Tang X-H. Expression of SIRT1 and SIRT3 in placental tissues of pre-eclampsia women and its correlation with maternal prognosis. Modern Med J. 2020;48(9):1124–1129.
- Zhou B-H, Qiu J, Tian Y-J, et al. Expressions and significance of MiR-34a and SIRT1 in placenta of patients with preeclampsia. Hebei Med J. 2020;42(8):1144–1147.
- Jiang T, Xue X-Z. Expressions and clinical significances of silent information regulator 1 and nod like receptor protein 3 in placenta tissues of pregnant women with preeclampsia. Chinese J Family Plan. 2023;31(3):658–661.
- Li H, Wang P, Huang C-X, et al. Inhibition of SIRT1 expression by miR-34a regulates proliferation and apoptosis of human trophoblast cell. Acta Universitatis Medicinalis Anhui. 2020;55(12):1915–1920.
- Cao D, Tang X-F, Li M-H. miR-155-5p influences the occurrence of preeclampsia through targeted inhibition of Sirt1. J Practical Med. 2021;37(4):451–457.
- Broady AJ, Loichinger MH, Ahn HJ, et al. Protective proteins and telomere length in placentas from patients with pre-eclampsia in the last trimester of gestation. Placenta. 2017;50:44–52. doi: 10.1016/j.placenta.2016.12.018.
- Viana-Mattioli S, Nunes P, Cavalli R, et al. Analysis of SIRT1 expression in plasma and in an in vitro model of preeclampsia. Oxid Med Cell Longev. 2020;2020:4561083–4561087. doi: 10.1155/2020/4561083.
- Poidatz D, Dos Santos E, Duval F, et al. Involvement of estrogen-related receptor-γ and mitochondrial content in intrauterine growth restriction and preeclampsia. Fertil Steril. 2015;104(2):483–490. doi: 10.1016/j.fertnstert.2015.05.005.
- Zhang H, Wei X, Li M. The expression of Sirt1/FoxO1 pathway in the placenta of patients with preeclampsia and its connection with prognosis. J Obstet Gynaecol. 2022;42(8):3514–3521. doi: 10.1080/01443615.2022.2151347.
- Ma L-Y, Chen W-W, Gao R-L, et al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol: JGC. 2020;17(1):1.
- Fodor P, White B, Khan R. Inflammation—the role of ATP in pre‐eclampsia. Microcirculation. 2020;27(1):e12585. doi: 10.1111/micc.12585.
- de Knegt VE, Hedley PL, Kanters JK, et al. The role of leptin in fetal growth during pre-eclampsia. Int J Mol Sci. 2021;22(9):4569. doi: 10.3390/ijms22094569.
- Mahmoud SA, Abdel-Aziz MM, Khafaga RH, et al. The pre-conception maternal exposure to sofosbuvir affects the mitochondrial biogenesis in prenatal fetal tissues: experimental study on rats. Mol Med. 2023;29(1):1–14. doi: 10.1186/s10020-023-00666-x.
- Bramora P, Borymska W, Zych M, et al. Effect of naringenin on oxidative stress in the heart tissue of type 1 diabetic wistar rats. Acta Poloniae Pharmaceutica. 2022;79(5).
- Wang P, Huang C-X, Gao J-J, et al. Resveratrol induces SIRT1-Dependent autophagy to prevent H2O2-Induced oxidative stress and apoptosis in HTR8/SVneo cells. Placenta. 2020;91:11–18. doi: 10.1016/j.placenta.2020.01.002.
- Pankiewicz K, Issat T. Understanding the role of chemerin in the pathophysiology of Pre-Eclampsia. Antioxidants. 2023;12(4):830. doi: 10.3390/antiox12040830.
- Yin L, Xu L, Chen B, et al. SRT1720 plays a role in oxidative stress and the senescence of human trophoblast HTR8/SVneo cells induced by D-galactose through the SIRT1/FOXO3a/ROS signalling pathway. Reprod Toxicol. 2022;111:1–10. doi: 10.1016/j.reprotox.2022.05.001.
- Munro SK, Balakrishnan B, Lissaman AC, et al. Cytokines and pregnancy: potential regulation by histone deacetylases. Mol Reprod Dev. 2021;88(5):321–337. doi: 10.1002/mrd.23430.
- Wang Y, Zhang Y, Wu Y, et al. SIRT1 regulates trophoblast senescence in premature placental aging in preeclampsia. Placenta. 2022;122:56–65. doi: 10.1016/j.placenta.2022.04.001.