Abstract
Objective
An increasing number of research have emerged to compare the pregnancy outcomes between the natural cycle and the hormone replacement therapy (HRT) cycle in preparing the endometrium for frozen-thawed embryo transfer (FET), but the results are controversial. This prospective randomized controlled study was hence designed to obtain more solid evidence.
Materials and methods
In this study, patients with regular menstrual cycle length (21–35 days) who underwent FET between January 2010 to December 2017 were recruited for this study. Upon further filtering with the selection criteria of patients being, a total of 405 patients were recruited and randomized. Finally, analysis was performed on 384 patients: 178 belonged to the natural cycle group whereas the remaining 206 were in the HRT group. The primary outcome was live birth rate, while the secondary outcomes were implantation rate, clinical pregnancy rate, early miscarriage rate, late miscarriage rate, multiple birth rate and low birth weight rate.
Results
The live birth rate (37.6% vs 30.1%, p = 0.119) of natural cycle group were higher than those of the hormone replacement therapy group, although the difference was not significant. The secondary outcomes were not found to differ significantly between the two groups. Nonetheless, the endometrium was found to be thicker in the natural cycle group (10.75 mm) than the HRT group (9.00 mm) (p < 0.001).
Conclusion
No significant differences were observed between the pregnancy outcomes of the natural cycle group and the HRT group which comprised of patients with regular menstrual cycle length.
Introduction
The first human frozen-thawed blastocyst was transferred in 1983 [Citation1]. Since then, the landscape of assisted reproductive technology (ART) has shifted dramatically, with frozen embryos gaining prominence, resulting in a large number of surplus human embryos being frozen. The advancement of vitrification technology and the adoption of single embryo transfer (SET) have significantly increased the proportion of frozen embryo transfer (FET) [Citation2]. FET allows maximized usage of available embryos in each oocyte retrieval cycle, reduces embryo wastage, improves the cumulative pregnancy rate[Citation3], reduces the incidence of ovarian hyperstimulation syndrome (OHSS) and multiple pregnancy [Citation4, Citation5], and reduces the physical sufferings, mental distraught and economic burden brought by superovulation and oocyte retrieval to patients [Citation6]. Although FET has many benefits, there is currently insufficient high quality evidence to suggest an optimal endometrial preparation regimen for FET based on pregnancy outcomes.
Many endometrial preparation regimens for frozen embryo transfer (FET) have been established with the aim of synchronizing endometrial and embryo development [Citation7–9], among which the natural cycle (NC) and the hormone replacement therapy (HRT) protocols are most commonly used.
The natural cycle protocol does not require the use of exogenous hormones. The changes in the endometrium are in line with the natural physiological process and the procedure is relatively simple to implement [Citation10, Citation11]. In this method, repeated hormone measurements and ultrasound monitoring are required to accurately monitor the luteinizing hormone (LH) peak and ovulation. As for the hormone replacement cycle protocols, it directly stimulates endometrial growth with exogenous hormones and inhibits the spontaneous development of follicles. Endometrial thickness is the only variable that must be monitored in this procedure, saving the cost of ultrasound examination and blood tests for ovulation monitoring. HRT comes with several drawbacks of its own. In the case of a successful pregnancy, estrogen and progesterone must be continuously released until placental autonomy is established to replace the missing corpus luteum which needs more costs. Besides being more expensive, drugs used in HRT such as gonadotrophin-releasing hormone agonists (GnRHa), may adversely affect and delay the recovery of spontaneous ovulation should FET fail [Citation7].
A recent Cochrane Review [Citation7] analyzed previous results of different endometrial preparation schemes and suggested that there is currently insufficient evidence to indicate which endometrial preparation regimen is more advantageous for FET. However, there is a limitation of the conclusions due to a lack of randomized controlled trials (RCTs). Moreover, there are very few RCTs that report live birth rate as the primary outcome. This prospective randomized controlled trial was thus designed to observe the effect of two endometrial preparation programs (namely NC and HRT) on pregnancy outcomes especially live birth rate after FET in patients with regular menstrual cycle length in order to address this significant gap in the literature.
Materials and methods
Trial design
This study is a prospective randomized controlled trial conducted in the Sun Yat-sen Memorial Hospital of Sun Yat-sen University, and was registered at the Clinical Trial Web site(https://clinicaltrials.gov/) with a designation NCT01780558. The study protocol was approved by the Reproductive Medicine Ethics Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University, and signed informed consent was obtained from all patients.
Participants
A total of 405 patients who had undergone at least one cycle of IVF/ICSI and had preserved frozen embryos at the Reproductive Medicine Center of the Obstetrics and Gynecology Department of Sun Yat-sen Memorial Hospital from January 2010 to December 2017 were enrolled. Inclusion criteria included: aged ≤ 40 years old, regular menstrual cycle length (21–35 days), and ≥ 3 frozen embryos. Meanwhile, exclusion criteria included: patients suffering from conditions such as chocolate cyst, adenomyosis of uterus, uterine scar, clear hydrosalpinx and intrauterine adhesion, repeated implantation failure (≥ 3 times), and endometrial thickness of < 7 mm before ovulation. Patients canceled the cycle if no dominant follicle was found after NC + FET.
Protocol
All patients receiving IVF/ICSI at the reproductive medicine center of our hospital during the stated period of time can be included in this study, regardless of the superovulation protocol used. This includes long protocol, short-acting long-term protocol, short protocol, ultra-long protocol, micro-stimulation protocol and antagonist protocol. Our center generally transfers cleavage-stage embryos on the third day and blastocyst embryos on the fifth day after insemination. The remaining embryos can be frozen after embryo transfer in fresh cycles. Embryos can also be frozen in canceled cycles for reasons such as high OHSS risk and endometrial problems. Our center used slow-freeze cryopreservation to freeze the surplus embryos. The morphological score of frozen embryos should meet the standards set by the center. Resuscitated embryos will be re-scored. A successful embryo recovery is defined by at least 50% living cells in a thawed embryo. Thawed embryos are cultured for at least 2 - 24 h, and a few are further cultured into blastocysts before transfer. The selection criteria for embryos transfer are grade I or II cleavage stage embryos, certain grade III cleavage embryos and all blastocysts that were assessed as ‘good’ or ‘reasonable’ based on Chinese expert consensus on human embryo morphological assessment. The maximum number of embryos which can be transferred is 2 - 3 depending on the patient’s previous assisted pregnancy condition and the regulations of the Ministry of Health of the People’s Republic of China.
In NC-FET protocol, the first B-ultrasound monitoring was performed from the 10th day of the menstrual period onwards, and was done every 2 - 3 days to monitor follicular development. When the diameter of the dominant follicle reached ≥ 14 mm, urine LH was then measured every day until either the LH peak or when B-ultrasound indicates ovulation (D0). In the case that no urinary LH peak was observed even when dominant follicle diameter has grown to > 18 mm, intramuscular injection of human chorionic gonadotropin (HCG) 5000 - 10000 IU was performed. Day-3-cleavage stage embryos were transplanted either on the fourth day after LH peak/injection of HCG or on the third day after ovulation. Blastocyst embryos were transplanted on the sixth day after LH peak/injection of HCG or on the fifth day after ovulation. Luteal support was provided by HCG 2000 U every 3 days from the embryo transfer (ET) date. If the development of dominant follicle was not observed up until the 20th day of the menstrual cycle or 5 days after the estimated ovulation period, the cycle will be canceled.
In HRT-FET protocol, patients with regular menstrual cycle were given 2 mg/d of estrogen orally for 5 days from the third day of the menstrual cycle, and then increased to 4 mg/d orally for 3 days, followed by the first B-ultrasound monitoring. If the endometrium did not reach 7 mm, the dosage of estrogen was gradually increased by 2 mg/d until the endometrium was thicker than 7 mm under the monitoring of B-ultrasound. Progesterone was administered intramuscularly at 40 - 60 mg/d on the transition day (D0) to transform the endometrium, and the dosages of progesterone and estrogen remained unchanged at the current levels. Day-3-cleavage stage embryos were transferred on the fourth day and blastocyst embryos were transplanted on the sixth day after progesterone administration. If no pregnancy was observed 14 days after ET, the progesterone and estrogen regimens should be discontinued; if pregnancy was observed, the drug should be continued until the eighth week of pregnancy. The hormone dosages can be gradually reduced until fully discontinued if the pregnancy progressed without abnormalities. If the endometrium does not reach 7 mm after 20 days of estrogen administration, the cycle was canceled; if the dominant follicle appeared during treatment and the thickness of the endometrium reached 7 mm before ovulation, the HRT cycle was carried on; if ovulation occurred during treatment, then the cycle was performed according to the natural cycle procedure and this cycle was excluded from this study.
Outcomes
The primary outcome was live birth rate, while the secondary outcomes were implantation rate, clinical pregnancy rate, multiple pregnancy rate, early miscarriage rate, late miscarriage rate, preterm birth rate and low birth weight rate. All pregnancy outcomes were calculated by per 100 cycles. Live birth rate was defined as a delivery that resulted in at least one live birth expressed per 100 cycles. Clinical pregnancy was diagnosed with ultrasound evidence of a gestational sac on the week 6 and elevation in hCG. Implantation rate was defined as the number of gestational sacs per 100 transferred embryos. Multiple birth was defined as the delivery of more than one fetus by a woman after 28 complete weeks of gestational age. Miscarriage was defined as the fetal loss of a clinical pregnancy before 28 completed weeks of gestational age. Early miscarriage was defined as the termination of pregnancy before12 weeks of gestational age. Late miscarriage was defined as the termination of pregnancy between 12 and 28 weeks of gestational age. Preterm delivery was defined a fetal delivery between 28 and 37 weeks of gestational age. Low birth weight was defined as a birth weight of less than 2500 g.
Randomization
The patients were divided into groups A (NC-FET) and B (HRT-FET) randomly by using a computer to generate random numbers. The completed randomization protocol was concealed within sequentially numbered opaque envelopes, and the content was the endometrial preparation protocol accepted by the numbered subjects. Patients who met the inclusion criteria opened up the numbered envelope that corresponds to their order of visit and received the corresponding treatment plan.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation if the data had a normal distribution, and variables were expressed as medians with interquartile ranges if the data had a skewed distribution. The independent-samples Student’s t-test was carried out if continuous variables in each experimental group presented a normal distribution. The Mann-Whitney U-test was done if the data was not normally distributed. Categorical variables were compared using the χ2 test or the Fisher’s exact test. A P-value of < 0.05 was considered statistically significant. All data analysis was performed by SPSS version 25.0 (SPSS Inc, Chicago, IL, USA).
Results
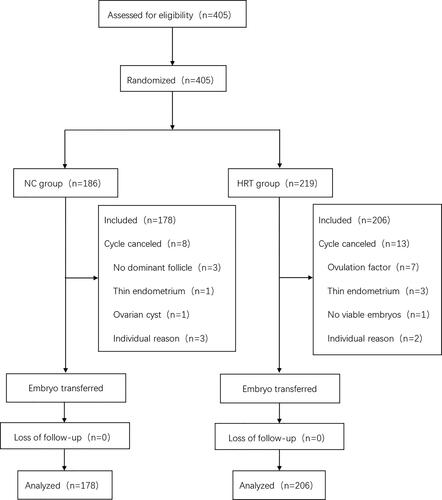
presents a flow chart of this study. A total of 405 patients who were treated at the reproductive medicine center of Sun Yat-sen Memorial Hospital from January 2010 to December 2017, and met the inclusion criteria for FET were recruited. There were 186 patients in group A, 8 of whom canceled the cycle due to the absence of dominant follicles, thin endometrium, ovarian cysts and other individual reasons. Meanwhile, there were 219 people in group B, 13 of whom canceled the cycle because of ovulation factor, thin endometrium, the absence of viable embryos, and other individual factors. No significant difference was observed between the cycle cancelation rate of both groups. All patients were not lost to follow-up.
The general characteristics of groups A and B are shown in . The demographic characteristics of the two groups, including age of freeze, age of study, BMI, basal hormones levels, No. of prior IVF attempts, prior fresh cycle pregnancy rate, infertility duration, and infertility factors, were comparable. The basal LH and testosterone levels were different between groups A and B, but the median values in both groups were within the normal range. Differences were not observed in the number, the quality and the type of transferred embryos between groups A and B. However, the endometrial thickness of group A was significantly thicker than that of group B (10.75 vs 9.00, p < 0.001) on the day of transition. No significant difference (37.64% vs 30.10%, p = 0.119) was noticed in the live birth rate between both groups too. No significant differences were identified among the secondary outcomes between groups A and B ().
Table 1. General characteristics of the two groups.
Table 2. Comparison of clinical outcomes between the two groups.
A stratified analysis was performed to understand the pregnancy outcomes of different embryos in the two protocols (). Both groups were first stratified based on the presence or absence of good-quality embryos. However, no significant differences were observed between groups A and B for the primary and secondary outcomes regardless of the status of good-quality embryos. Secondly, the two groups were stratified according to whether there were blastocysts transferred. In terms of the blastocyst transfer cycle, no significant differences were observed between groups A and B for the primary and secondary pregnancy outcomes too. In terms of cleavage stage embryo transfer cycles, no significant differences were reported in the pregnancy outcomes as well.
Table 3. Subgroup analysis of pregnancy outcomes between the two groups.
Lastly, both groups were stratified according to whether the endometrial thickness achieved at least ≥ 10 mm (). In the cycle with endometrial thickness of < 10 mm or ≥ 10 mm on transition day, no significant difference was reported in the primary nor secondary outcomes between group A and group B.
Table 4. Subgroup analysis of pregnancy outcomes between the two groups.
Discussion
Results from this study show that the NC group had a higher live birth rate than the HRT group (37.6% vs 30.1%, p = 0.119), whereas the HRT group yielded a slightly higher rate of miscarriage than the NC group (19.8% vs 14.8%, p = 0.398), although the differences were not significant. To our knowledge, few previous randomized controlled studies have included live birth rate as the primary outcome when comparing the natural cycle and HRT cycle. Our study provides a high quality evidence.
Consistent with our findings, some prospective randomized controlled studies [Citation12, Citation13] also reported no significant difference in live birth rate, while a retrospective cohort study conducted by Li, C et al. [Citation14] showed that the HRT method achieved a lower live birth rate than the NC and ovarian stimulation (OS) methods. HCG can promote angiogenesis of the uterine vasculature to increase the blood supply to the placenta and developing fetus and inhibit the macrophage function to prevent rejection of the fetal and placental tissue [Citation15]. This may explain the lower live birth rate and slightly higher miscarriage rate in the HRT cycle as compared to that of the NC cycle. In addition, the endometrial thickness of the HRT cycle depends on the amount of estrogen present, with higher endometrial thickness requiring a higher amount of estrogen. However, high estrogen levels may affect endometrial receptivity [Citation16–18], thus explaining the lower live birth rates and slightly higher miscarriage rates in the HRT cycles than the NC cycles.
No significant difference in clinical pregnancy rates between the two groups was observed. This is consistent with previous randomized studies of patients with regular menstrual cycles [Citation13, Citation19]. In another randomized study that involved women with irregular menstrual cycles, oligomenorrhea, or amenorrhea, the pregnancy rate of the NC group was similar to that of the HRT group [Citation10]. Meanwhile in some retrospective studies, such as the one published in 2020, no difference was found in clinical pregnancy rates between natural cycles (n = 561) and artificial cycles (n = 585), but clinical pregnancy rates were lower in natural and artificial cycles compared to the modified natural cycles (n = 1749) in which ovulation was triggered by hCG. However, several other retrospective cohort studies have shown higher clinical pregnancy rates in the NC group as compared to that of HRT groups [Citation20–22].
In the HRT cycle, exogenous estrogen and progesterone were used for the preparation of the endometrium while inhibiting ovulation. Therefore, the ovarian corpus luteum does not exist. The ovarian corpus luteum produces hormones including estradiol and progesterone which are important in embryo implantation and pregnancy maintenance. Additionally, some other vasoactive products such as vascular endothelial growth factor and relaxin are thought to be critical for initial placenta formation as well [Citation23, Citation24]. These factors in the HRT cycle may be detrimental to embryo implantation and increase the risk of obstetric complications. The research by Hu et al. found that HRT cycles are also associated with increased risk of preterm birth rate, low birth weight rate, increased odds of macrosomia, premature rupture of membranes, and gestational hypertensive disorders [Citation25]. However, this study finds no significant difference between the NC and HRT cycles in terms of preterm birth rate and low birth weight rate during follow-ups for obstetric complications. A retrospective study in 2020 found that poor quality blastocyst transfer was associated with a decrease in a singleton birthweight [Citation26]. It was found that the rate of good quality of embryo in both groups was approximately the same. This can be used to explain the similarity in low birth rate between the two groups. Future studies should compare perinatal outcomes of endometrial preparation protocols for frozen embryo transfer with good quality embryos. A large observational study of more than 9 million women in China found that preterm birth rate is positively correlated with maternal age [Citation27]. The average age of the patients we recruited for our study was under 35 years which is considered the threshold of advanced age. This may be the reason why there was no difference in preterm birth rate between the two groups.
Embryo quality is a major factor affecting live birth and pregnancy rates in FET [Citation28, Citation29], so a stratified analysis was performed. No significant differences in the primary and secondary pregnancy outcomes were noticed between the NC and HRT groups, regardless of the quality of embryo and the presence or absence of blastocyst during transfer. These results suggest that the quality of embryos may not affect pregnancy outcomes both groups.
Additionally, endometrial thickness was noticed to be thicker on transition day in the NC group compared with the HRT group (10.75 vs 9.00, p < 0.001). Dikey et al. [Citation30, Citation31] concluded that it is difficult to obtain clinical pregnancy when the thickness of endometrium is less than 6 mm, whereas the pregnancy rate increases significantly when the thickness of endometrium reaches 9 mm. Furthermore, they found that the rate of biochemical pregnancy rate increases when the endometrium is thinner. The study by Check et al. [Citation32, Citation33] showed that the endometrium thickness correlates positively with the pregnancy rate, and pregnancy rate significantly improves when the endometrial thickness reaches more than 10 mm. In a large retrospective cohort study, it was found that live birth rate rates increase significantly until an endometrial thickness of 10–12 mm, while in FET cycles live birth rates plateau after 7–10 mm [Citation34]. Another retrospective study suggested that the endometrium thickness correlates positively with the live birth rate [Citation35]. In this present study, endometrial thickness on the transition day was stratified with 10 mm as the boundary to analyze the pregnancy outcomes of both the NC group and the HRT group. It was found that both the NC and HRT groups obtained the similar pregnancy outcomes regardless of the endometrial thickness being less than 10 mm or greater than 10 mm on transition day. This may be attributed to the satisfactory endometrial thickness obtained by most of the cycles in both the groups on transition day.
In conclusion, no significant differences were noticed in terms of pregnancy outcomes between patients of the NC and the HRT groups with regular menstrual cycle which resonates the findings of some other prospective randomized controlled studies [Citation12, Citation13]. This study is one of the few randomized controlled studies that compared the live birth rate between the NC and the HRT groups. We have provided a high quality evidence that can help to fill this significant gap in the literature. Physicians and patients can flexibly choose a suitable endometrial preparation regimen based on individual circumstances. In the future, more large multicenter prospective randomized controlled studies are needed.
Authors’ contributions
Jianyun Huang and Xuedan Jiao contributed equally to this study. Jianyun Huang drafted the manuscript; Qingxue Zhang, Jianyun Huang and Xuedan Jiao contributed in the conception and design of this study; Jianyun Huang and Xuedan Jiao were in charge of data analysis; Haiyan Lin, Yingchen Wu and You Yang checked the analysis and critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank Ph.D. Xiaohui Ji for her assistance with data analysis. We thank all involved clinical staff for the collection of data.
Disclosure statement
The authors have no competing interests to declare.
Additional information
Funding
References
- Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983 Oct 20-26; 1983 Oct 20-26305(5936):1–7. doi: 10.1038/305707a0.
- Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014; Jul102(1):19–26. doi: 10.1016/j.fertnstert.2014.05.027.
- Kolibianakis EM, Zikopoulos K, Devroey P. Implantation potential and clinical impact of cryopreservation–a review. Placenta. 2003; Oct24 Suppl B(Suppl B):S27–S33. doi: 10.1016/s0143-4004(03)00133-4.
- Groenewoud ER, Cohlen BJ, Macklon NS. Programming the endometrium for deferred transfer of cryopreserved embryos: hormone replacement versus modified natural cycles. Fertil Steril. 2018; May109(5):768–774. doi: 10.1016/j.fertnstert.2018.02.135.
- Tiitinen A, Halttunen M, Härkki P, et al. Elective single embryo transfer: the value of cryopreservation. Hum Reprod. 2001; Jun16(6):1140–1144. doi: 10.1093/humrep/16.6.1140.
- Dor J, Rudak E, Davidson A, et al. Endocrine and biological factors influencing implantation of human embryos following cryopreservation. Gynecol Endocrinol. 1991; Sep5(3):203–211. doi: 10.3109/09513599109028442.
- Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2017; Jul 57(7):Cd003414.
- Mackens S, Santos-Ribeiro S, van de Vijver A, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. 2017; Nov 132(11):2234–2242. doi: 10.1093/humrep/dex285.
- Yarali H, Polat M, Mumusoglu S, et al. Preparation of endometrium for frozen embryo replacement cycles: a systematic review and meta-analysis. J Assist Reprod Genet. 2016; Oct33(10):1287–1304. doi: 10.1007/s10815-016-0787-0.
- Sathanandan M, Macnamee MC, Rainsbury P, et al. Replacement of frozen-thawed embryos in artificial and natural cycles: a prospective semi-randomized study. Hum Reprod. 1991; May6(5):685–687. doi: 10.1093/oxfordjournals.humrep.a137407.
- Levi Setti PE, Cirillo F, De Cesare R, et al. Seven years of vitrified blastocyst transfers: comparison of 3 preparation protocols at a single ART center. Front Endocrinol (Lausanne). 2020;11:346. doi: 10.3389/fendo.2020.00346.
- Peeraer K, Couck I, Debrock S, et al. Frozen-thawed embryo transfer in a natural or mildly hormonally stimulated cycle in women with regular ovulatory cycles: a RCT. Hum Reprod. 2015; Nov30(11):2552–2562. doi: 10.1093/humrep/dev224.
- Groenewoud ER, Cohlen BJ, Al-Oraiby A, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod. 2016; Jul31(7):1483–1492. doi: 10.1093/humrep/dew120.
- Li C, He YC, Xu JJ, et al. Perinatal outcomes of neonates born from different endometrial preparation protocols after frozen embryo transfer: a retrospective cohort study. BMC Pregnancy Childbirth. 2021; Apr 2921(1):341. doi: 10.1186/s12884-021-03791-9.
- Smitz J, Platteau P. Influence of human chorionic gonadotrophin during ovarian stimulation: an overview. Reprod Biol Endocrinol. 2020; Aug 618(1):80. doi: 10.1186/s12958-020-00639-3.
- Pan Y, Li B, Wang Z, et al. Hormone replacement versus natural cycle protocols of endometrial preparation for frozen embryo transfer. Front Endocrinol (Lausanne). 2020;11:546532. doi: 10.3389/fendo.2020.546532.
- Ma WG, Song H, Das SK, et al. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003; Mar 4100(5):2963–2968. doi: 10.1073/pnas.0530162100.
- van der Linden M, Buckingham K, Farquhar C, et al. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015; Jul 72015(7):Cd009154.
- Mounce G, McVeigh E, Turner K, et al. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertil Steril. 2015; Oct104(4):915–920.e1. doi: 10.1016/j.fertnstert.2015.07.1131.
- Lin J, Zhao J, Hao G, et al. Maternal and neonatal complications after natural vs. Hormone replacement therapy cycle regimen for frozen single blastocyst transfer. Front Med (Lausanne). 2020;7:338. doi: 10.3389/fmed.2020.00338.
- Levron J, Yerushalmi GM, Brengauz M, et al. Comparison between two protocols for thawed embryo transfer: natural cycle versus exogenous hormone replacement. Gynecol Endocrinol. 2014; Jul30(7):494–497. doi: 10.3109/09513590.2014.900032.
- Saito K, Kuwahara A, Ishikawa T, et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod. 2019; Aug 134(8):1567–1575. doi: 10.1093/humrep/dez079.
- Novak J, Danielson LA, Kerchner LJ, et al. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001; Jun107(11):1469–1475. doi: 10.1172/JCI11975.
- Singh B, Reschke L, Segars J, et al. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril. 2020; Feb113(2):252–257. doi: 10.1016/j.fertnstert.2019.12.007.
- Hu KL, Zhang D, Li R. Endometrium preparation and perinatal outcomes in women undergoing single-blastocyst transfer in frozen cycles. Fertil Steril. 2021; Jun115(6):1487–1494. doi: 10.1016/j.fertnstert.2020.12.016.
- Zhang J, Huang J, Liu H, et al. The impact of embryo quality on singleton birthweight in vitrified-thawed single blastocyst transfer cycles. Hum Reprod. 2020; Feb 2935(2):308–316. doi: 10.1093/humrep/dez287.
- Deng K, Liang J, Mu Y, et al. Preterm births in China between 2012 and 2018: an observational study of more than 9 million women. Lancet Glob Health. 2021; Sep9(9):e1226–e1241. doi: 10.1016/S2214-109X(21)00298-9.
- Cimadomo D, Capalbo A, Levi-Setti PE, et al. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Hum Reprod. 2018; Nov 133(11):1992–2001. doi: 10.1093/humrep/dey291.
- Tiegs AW, Sun L, Patounakis G, et al. Worth the wait? Day 7 blastocysts have lower euploidy rates but similar sustained implantation rates as day 5 and day 6 blastocysts. Hum Reprod. 2019; Sep 2934(9):1632–1639. doi: 10.1093/humrep/dez138.
- Dickey RP, Olar TT, Curole DN, et al. Endometrial pattern and thickness associated with pregnancy outcome after assisted reproduction technologies. Hum Reprod. 1992; Mar7(3):418–421. doi: 10.1093/oxfordjournals.humrep.a137661.
- Dickey RP, Olar TT, Taylor SN, et al. Relationship of biochemical pregnancy to pre-ovulatory endometrial thickness and pattern in patients undergoing ovulation induction. Hum Reprod. 1993; Feb8(2):327–330. doi: 10.1093/oxfordjournals.humrep.a138045.
- Check JH, Nowroozi K, Choe J, et al. Influence of endometrial thickness and echo patterns on pregnancy rates during in vitro fertilization. Fertil Steril. 1991; Dec56(6):1173–1175. doi: 10.1016/s0015-0282(16)54736-0.
- Check JH, Nowroozi K, Choe J, et al. The effect of endometrial thickness and echo pattern on in vitro fertilization outcome in donor oocyte-embryo transfer cycle. Fertil Steril. 1993; Jan59(1):72–75. doi: 10.1016/s0015-0282(16)55617-9.
- Mahutte N, Hartman M, Meng L, et al. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. 2022; Apr117(4):792–800. doi: 10.1016/j.fertnstert.2021.12.025.
- Eleftheriadou A, Francis A, Wilcox M, et al. Frozen blastocyst embryo transfer: comparison of protocols and factors influencing outcome. J Clin Med. 2022; Jan 2911(3):737. doi: 10.3390/jcm11030737.