Abstract
Objective
To investigate the expression of HMGB1 and toll-like receptor 4 (TLR4) in adenomyosis eutopic/ectopic endometrium.
Methods
Twenty patients with adenomyosis and 20 controls, all undergoing laparoscopy, were recruited from September 2015 to July 2016. Samples were collected from the endometrium without adenomyosis (CE), the eutopic endometrium with adenomyosis (EuE), and the ectopic endometrium with adenomyosis (EE). The mRNA and protein expression of HMGB1 and TLR4, and interleukin-6 (IL-6) and interleukin-8 (IL-8) RNA expression levels were measured.
Results
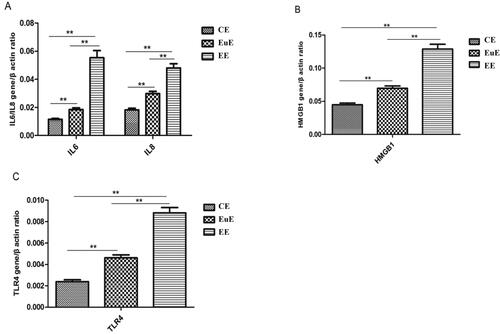
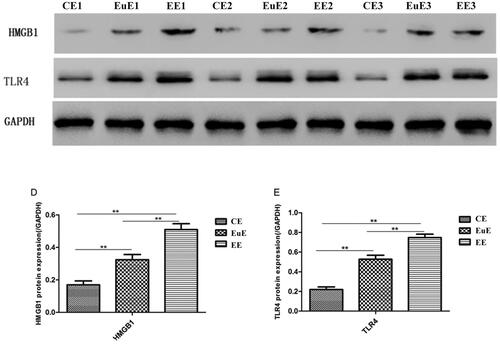
The average age of the adenomyosis women was 43.4 ± 5.3 years; their BMI was 23.3 ± 2.3 kg/m2. The control group included women aged 38.8 ± 9.8 years, with BMI 22.2 ± 3.4 kg/m2. The mRNA expression levels of HMGB1, TLR4, IL-6, and IL-8 in the EE and EuE groups were higher than those in the CE group (p < .01), and those in the EE group were higher than those in the EuE group (p < .01). The protein expression levels of HMGB1 and TLR4 in the EE and EuE groups were higher than those in the CE group (p < .01); they were higher in the EE group than the ones in the EuE group (p < .01). HMGB1 mRNA was significantly positively correlated with TLR4 in EuE and EC patients (r = 0.538 and r = 0.916, p < .01), as well as with IL-6 (r = 0.470 and r = 0.976, p < .01) and IL-8 (r = 0.574 and r = 0.650, p < .01).
Conclusions
The overexpression of HMGB1 and TLR4 in EuE and EE is positively correlated with IL-6 and IL-8 expression. The HMGB1 signaling-mediated immune-inflammatory system might be involved in the development of adenomyosis.
Introduction
Adenomyosis is a common gynecological disease in women of childbearing age, with clinical manifestations of menorrhagia, secondary dysmenorrhea, and infertility, which sometimes severely affect patients’ quality of life [Citation1]. This disease is most frequently diagnosed (in 70–80% of the reported cases) in 40–50-year-old women [Citation2,Citation3]. Nevertheless, the actual incidence is largely unknown [Citation2], with a widely ranging reported prevalence (1–70%) [Citation2–4].
The precise etiology of adenomyosis is still unknown [Citation5]. The following theories for the possible causes or contributing factors have been proposed: increased estrogen exposure (early menarche, short menstrual cycles, and elevated body mass index), exposure to tamoxifen (which binds to selective estrogen receptors and may stimulate both normal and ectopic endometrial tissue), and endometrial trauma (including prior spontaneous abortion, dilatation and curettage, and cesarean delivery) [Citation2]. Previous studies have shown that adenomyosis is a sometimes chronic immune-inflammatory disease involving various immune cells and inflammatory cytokines (e.g. interleukin-1β (IL-1β), IL-6, IL-8, tumor necrosis factor-α (TNF-α), etc.) in the focal tissues [Citation6]. Still, the inflammatory mechanisms contributing to adenomyosis pathogenesis remain unelucidated.
The high-mobility group box chromosomal protein (HMGB) family consists of nonhistone chromosomal proteins widely distributed in eukaryotic cells [Citation7,Citation8]. HMGB1 is an important HMGB family member which binds to specific sites of DNA in the nucleus and participates in the regulation of replication, transcription, recombinant, and repair of DNA and nucleosome stabilization [Citation8]. Under normal physiological conditions, HMGB1 is stably localized in the cell nucleus; however, under oxidation/inflammatory stress conditions, it escapes from the nucleus into the cytoplasm and is then secreted into the extracellular matrix (via a process called exocytosis) [Citation9,Citation10].
As an important danger-associated molecular pattern (DAMP) molecule, extracellular matrix HMGBl acts as an endogenous ligand of molecules, such as TLR4 to stimulate the activation of TLR4-mediated cellular inflammatory signaling and participate in the inflammatory response [Citation11,Citation12]. The HMGB1-TLR4-mediated inflammatory signaling system has been proved to participate in the formation of multiple tumor inflammatory pathological microenvironments and is involved in tumor inflammatory proliferation, invasion and metastasis, and other pathological mechanisms [Citation13,Citation14]. Nevertheless, the role of the HMGB1-TLR4-mediated signaling pathway in the formation of adenomyosis pathological inflammatory microenvironment remains unclear. Guo et al. [Citation15] reported that TLR4-mediated inflammatory signals induced and regulated the inflammatory response of adenomyosis interstitial cells and the secretion of IL-6, transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), matrix metalloproteinase 2 (MMP2), and other inflammatory factors that were involved in the inflammatory pathological mechanism of adenomyosis. Notably, the proliferation of mesenchymal cells was also induced resulting in the formation of an invasive growth phenotype [Citation15].
This study aimed to detect the expression of HMGB1, TLR4, IL-6, and IL-8 in CE, EuE, and EE tissue groups and explore the role of HMGB1 in the formation and development of inflammatory pathophysiology of adenomyosis.
Methods
Sample and data collection
From September 2015 to July 2016, 20 patients with adenomyosis were admitted to Yangpu Hospital affiliated to Tongji University School of Medicine, Shanghai, China. Adenomyosis was confirmed by pathological examination at the Department of Pathology of Shanghai Yangpu Central Hospital, Shanghai, China. The endometrium without adenomyosis (CE) samples was isolated from the healthy women of childbearing age. The specimens were collected using a small curette in areas of endometrial hyperplasia before implanting an intrauterine device or after removing it. The ectopic endometrium with adenomyosis (EE) samples were isolated from freshly collected specimens during surgery (total hysterectomy or partial adenomyosis excision); the eutopic endometrium with adenomyosis (EuE) samples were isolated from freshly collected specimens during surgery (total hysterectomy or diagnostic curettage); adenomyosis was confirmed by pathological examination. The control group included 20 healthy women of childbearing age who did not take hormones within three months. All included individuals had a history of one or two births.
Gene expression determination by RT-PCR
The specimen was cut into pieces. Total RNA was extracted using Trizol (#1596-026, Invitrogen Inc., Carlsbad, CA), during which low-temperature operations were ensured. The concentration and purity of the RNA were determined. cDNA was prepared using a reverse transcription kit (#K1622, Fermentas, Burlington, Canada), and the target fragment was amplified by PCR. Data were analyzed using the ABI Prism 7300 SDS Software (Applied Biosystems, Foster City, CA). The expression levels of the target fragments were compared using β-actin as an internal reference. The primer sequences, annealing temperature, and product size for HMGB1, IL-6, and IL-8 are presented in .
Table 1. RT-PCR primers.
HMGB1 protein expression by Western blot
The specimens were cut into pieces and homogenized with lysate containing protease and phosphatase inhibitors until their complete lysis was achieved. Next, proteins were extracted by centrifugation at 4 °C and ×12,000 rpm for 15 min, followed by BCA quantification and storage at −80 °C. The protein samples were then mixed with 1× SDS gel buffer and boiled for 5 min for denaturation. The samples were loaded at 20 μg/well, followed by 10% SDS-PAGE electrophoresis. Further, they were transferred to a PVDF membrane, sealed, and incubated with diluted primary antibodies against HMGB1 (1:300; Ab77302, Abcam, Cambridge, UK), CATB1 (1:800; Ab58802, Abcam), ALOX-5 (1:1000; Ab169755, Abcam), and GAPDH (1:2000; 5174; Cell Signaling Technology, Inc., Danvers, MA) overnight at 4 °C. After rinsing, HRP-labeled secondary antibody was added (goat anti-rabbit HRP-conjugated secondary antibody, A0208; donkey anti-goat HRP-conjugated secondary antibody, A0181; goat anti-mouse HRP conjugated secondary antibody, A0216; all 1:1000; all from Beyotime, Beijing, China), incubated at room temperature for 1 h, and developed using ECL. The gray value of the band was determined using the TANON-5200 imaging system (Tanon Science & Technology Co. Ltd., Shanghai, China). HMGB1 was observed at 29 kDa, and GAPDH at 37 kDa.
Statistical analysis
The data were analyzed using SPSS version 20.0 (IBM, Armonk, NY). Continuous data are expressed as means ± standard deviation and analyzed using Student’s t-test. Correlations were tested by Pearson correlation analysis. p < .05 was considered to indicate a statistically significant difference. Finally, graphs were made with GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA).
Results
Patient data
The adenomyosis group included 20 women with a disease course of 4.0 ± 3.1 years. Their average age was 43.4 ± 5.3 years, and their BMI was 23.3 ± 2.3 kg/m2. The control group included healthy women of childbearing age who underwent intrauterine device implantation or removal; they were aged 38.8 ± 9.8 years, and their BMI was 22.2 ± 3.4 kg/m2. No differences in age and BMI were observed between the two groups.
Comparison of target gene expression among the CE, EuE, and EE tissue groups
RT-PCR showed that the expression levels of IL-6 and IL-8 mRNA in the EE and EuE groups were higher than those in the CE group (p < .01); those in the EE group were also higher than the ones in the EuE group (p < .01) (). The levels of HMGB1 mRNA expression in the EE and EuE groups were higher than that in the CE group (p < .01); that in the EC group was also higher than the one in the EuE group (p < .01) (). TLR4 mRNA expression in the EE and EuE groups was higher than that in the CE group (p < .01) and higher in the EE group than in the EuE group (p < .01) ().
HMGB1 and TLR4 protein expression in the CE, EuE, and EE groups
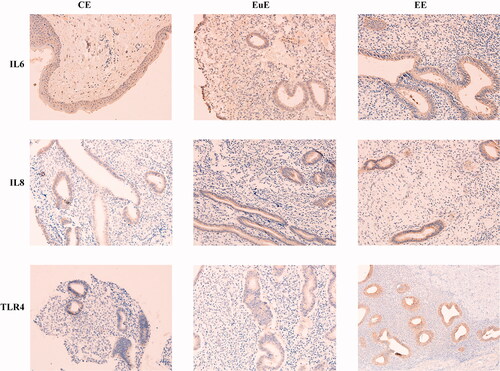
Western blot revealed that the protein expression levels of HMGB1 and TLR4 in the EE and EuE groups were higher than that in the CE group (p < .01); those in the EE groups were higher than the ones in the EuE group (p < .01) (). Immunohistochemistry revealed that the protein expression levels of TLR4 in the EE and EuE groups were higher than that in the CE groups; those in the EE groups were higher than the ones in the EuE groups ().
Correlations between HMGB1 expression and TLR4, IL-6, and IL-8 in the EuE and EE groups
HMGB1 mRNA was significantly positively correlated with TLR4 in the EuE and EE groups (r = 0.538 and r = 0.916, p < .01), as well as with IL-6 (r = 0.470 and r = 0.976, p < .01) and IL-8 (r = 0.574 and r = 0.650, p < .01) (). displays the correlations of the mRNA expression levels of HMGB1 with those of IL-6 and IL-8, as well as that of TLR4 with the ones of IL-6 and Il-8, and of HMGB1 with that of TLR4.
Figure 4. Correlation of mRNA HMGB1 expression with TLR4, IL-6, and IL-8 (A), TRL4 with IL-6 and IL-8 (B), and TLR4 with HMGB1 (C) in the EuE and EE groups.
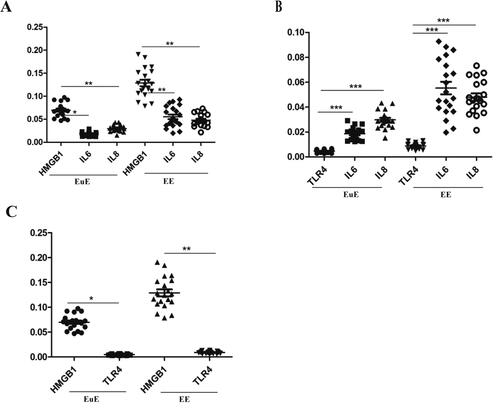
Table 2. Correlation between HMGB1 expression and IL-6 and IL-8 in EuE and EE.
Discussion
In this study, we aimed to investigate the expression of HMGB1 and TLR4 in EE and EuE in adenomyosis. The overexpression of HMGB1 and TLR4 in EuE and EE was positively correlated with the expression of IL-6 and IL-8. Hence, HMGB1 signaling-mediated immune-inflammatory system might be involved in adenomyosis development.
The four main theories for the pathogenesis of adenomyosis are as follows:
Direct invasion of the endometrium into the myometrium, possibly caused by myometrial weakness due to pregnancy or surgery, which enables active endometrial tissue to grow into the injured lining. the immunologic activity at the endometrial myometrial interface is altered, while macrophage-activated T and B cells produce antibodies and stimulate cytokines altering the junction zone of the endomyometrium, and the elevated prolactin levels promote myometrial cell degeneration and invasion of the endometrial stroma into the myometrium and the progression to adenomyosis;
Embryologic development due to misplaced pluripotent Mullerian remnants;
Invagination of the basalis along with the intramyometrial lymphatic system through altered or absent junctional zone [Citation16];
Endometrial repopulation driven by bone marrow-derived stem cells, which fosters the development of the endometrium within the musculature of the uterus, leading to adenomyosis [Citation2,Citation3].
The first theory involves immune activation and inflammatory activation, which has been supported by recent evidence [Citation17–20]. Still, what exactly triggers this inflammatory reaction remains unknown since women with adenomyosis often have a history of pregnancy or uterine trauma, but the injury itself has been caused much earlier in the past, whereas the symptoms and signs are still present.
HMGB1 is a chromosomal protein, first isolated from bovine thymocytes by Goodwin et al. [Citation21]. Under normal physiological conditions, HMGB1 is located in the nucleus but is actively or passively released out of the cells under stress conditions, such as oxidative stress, TNF-α activities, and bacterial lipopolysaccharide (LPS) exposure [Citation9,Citation10,Citation12]. Further, extracellular HMGB1 binds to TLR4 and activates the TLR4-dependent inflammatory pathway. Indeed, as an endogenous ligand molecule of TLR4, extracellular matrix HMGB1 is identified by TLR4 to activate the inflammatory signaling pathway and induce the release of inflammatory factors in target cells, forming an inflammatory pathological environment that has been observed in various diseases [Citation11,Citation12,Citation22–25]. The HMGB1/TLR4 signal system has been evidenced to participate in the inflammatory pathological process of the development of malignant tumors, such as ovarian cancer [Citation26]. It also promotes the formation of the tumor inflammatory microenvironment and promotes inflammation-related tumor proliferation, anti-apoptosis, invasion, metastasis, and angiogenesis [Citation27,Citation28]. In this study, the expression levels of HMGB1, TLR4, IL-6, and IL-8 were elevated, but the study had not been designed to determine causal relationships. The findings in the literature cited above indicate that inflammation might cause and sustain adenomyosis through a vicious cycle. Nonetheless, which molecular event causes the initial activation of the HMGB1-TLR4 axis remains unknown.
There are several immune cells and inflammatory cytokines in adenomyosis lesions, of which IL-6 and IL-8 have been used as representatives in adenomyosis inflammatory pathology studies [Citation29,Citation30]. Luo et al. [Citation31] found that the expression levels of TLR4 in EuE and of EE in endometriosis were higher than those in the normal endometrium. TLR4 signal activation promoted the secretion of IL-8, and IL-8 enhanced the invasion and proliferation of endometrial stromal cells via the FAK signal pathway, which disappeared after the addition of the anti-IL-8 neutralizing antibody or a FAK inhibitor [Citation31]. These results constitute a stepping stone in understanding the pathogenesis of adenomyosis. Further research is necessary to determine the exact chains of events leading to adenomyosis formation.
Combined with the previous work and the review of basic literature, this study proposed the following possible pathogenesis of adenomyosis: The HMGB1/TLR4 signal system might participate in the inflammatory pathological process of the development of adenomyosis. It generates local inflammatory response and activates the specific immune system of the body, builds and maintains a sustained inflammatory microenvironment in the local lesion, and forms the local inflammatory pathology of adenomyopathy, which might involve the pathogenesis of adenomyopathy. This study might provide a new idea for the treatment of the adenomyosis in the future.
In this work, due to the limited number of samples in the control group, some of them were found to be endometrial tissues after removing the intrauterine device employed. Thus, the influence of inflammatory reaction on IUDs could not be completely excluded. Therefore, the number of specimens in future studies should be increased, and endometrium tissues of women in whom no IUD has been placed should be selected and included as a control group.
In conclusion, the results of this article show increased inflammation in adenomyosis lesions compared to endometrium in the presence of adenomyosis and endometrium in the absence of adenomyosis meiosis. Therefore, this study indicates that the HMGB1 signaling-mediated immune-inflammatory system might be involved in the development of adenomyosis.
Author contribution
All authors contributed to patient management and report writing. Xiu-Ni Liu wrote the main manuscript text and prepared the figures. Zhong-Ping Cheng guided the overall manuscript preparation. All authors reviewed and agreed with the final version of the manuscript.
Ethics approval
This work was performed in compliance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the Ethics Committee of Yangpu Hospital [22KN195], and all patients provided written informed consent.
What this study adds to the clinical work
These findings indicate that the high-mobility group box-1 (HMGB1) signaling-mediated immune-inflammatory system can serve as a novel biomarker and a potential target for adenomyosis.
Consent to publish
N/A.
Acknowledgments
We would like to gratefully acknowledge the contribution of the Corresponding author Zhong-Ping Cheng.
Disclosure statement
All authors declare that they have no competing interests.
Additional information
Funding
References
- Chen J, Porter AE, Kho KA. Current and future surgical and interventional management options for adenomyosis. Semin Reprod Med. 2020;38(2–03):1–7. doi:10.1055/s-0040-1718921.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164–185. doi:10.1016/j.jmig.2015.09.018.
- Senturk LM, Imamoglu M. Adenomyosis: what is new? Womens Health (Lond). 2015;11(5):717–724. doi:10.2217/whe.15.60.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)-pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68–81. doi:10.1016/j.bpobgyn.2016.09.006.
- Donnez J, Stratopoulou CA, Dolmans MM. Uterine adenomyosis: from disease pathogenesis to a new medical approach using GnRH antagonists. Int J Environ Res Public Health. 2021;18(19):9941.
- Bourdon M, Santulli P, Jeljeli M, et al. Immunological changes associated with adenomyosis: a systematic review. Hum Reprod Update. 2021;27(1):108–129. doi:10.1093/humupd/dmaa038.
- Malarkey CS, Churchill ME. The high mobility group box: the ultimate utility player of a cell. Trends Biochem Sci. 2012;37(12):553–562. doi:10.1016/j.tibs.2012.09.003.
- Mandke PP, Vasquez KM. Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: implications in DNA repair and immune responses. DNA Repair (Amst). 2019;83:102701. doi:10.1016/j.dnarep.2019.102701.
- Yu Y, Tang D, Kang R. Oxidative stress-mediated HMGB1 biology. Front Physiol. 2015;6:93. doi:10.3389/fphys.2015.00093.
- Kwak MS, Kim HS, Lee B, et al. Immunological significance of HMGB1 Post-Translational modification and redox biology. Front Immunol. 2020;11:1189. doi:10.3389/fimmu.2020.01189.
- Yang H, Antoine DJ, Andersson U, et al. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93(6):865–873. doi:10.1189/jlb.1212662.
- Diener KR, Al-Dasooqi N, Lousberg EL, et al. The multifunctional alarmin HMGB1 with roles in the pathophysiology of sepsis and cancer. Immunol Cell Biol. 2013;91(7):443–450. doi:10.1038/icb.2013.25.
- Nan K, Han Y, Fang Q, et al. HMGB1 gene silencing inhibits neuroinflammation via down-regulation of NF-κB signaling in primary hippocampal neurons induced by Aβ(25-35). Int Immunopharmacol. 2019;67:294–301. doi:10.1016/j.intimp.2018.12.027.
- Qu D, Ling Z, Tan X, et al. High mobility group protein B1 (HMGB1) interacts with receptor for advanced glycation end products (RAGE) to promote airway smooth muscle cell proliferation through ERK and NF-κB pathways. Int J Clin Exp Pathol. 2019;12(9):3268–3278.
- Guo J, Chen L, Luo N, et al. LPS/TLR4-mediated stromal cells acquire an invasive phenotype and are implicated in the pathogenesis of adenomyosis. Sci Rep. 2016;6(1):21416. doi:10.1038/srep21416.
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35(5):592–601. doi:10.1016/j.rbmo.2017.06.016.
- Liang S, Shi LY, Duan JY, et al. Celecoxib reduces inflammation and angiogenesis in mice with adenomyosis. Am J Transl Res. 2021;13(4):2858–2866.
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38(2–03):129–143. doi:10.1055/s-0040-1716687.
- Kobayashi H. Endometrial inflammation and impaired spontaneous decidualization: insights into the pathogenesis of adenomyosis. Int J Environ Res Public Health. 2023;20(4):3762. doi:10.3390/ijerph20043762.
- Ying P, Li H, Jiang Y, et al. Qiu’s neiyi recipe regulates the inflammatory action of adenomyosis in mice via the MAPK signaling pathway. Evid Based Complement Alternat Med. 2021;2021:9791498–9791410. doi:10.1155/2021/9791498.
- Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38(1):14–19. doi:10.1111/j.1432-1033.1973.tb03026.x.
- Min HJ, Kim SJ, Kim TH, et al. Level of secreted HMGB1 correlates with severity of inflammation in chronic rhinosinusitis. Laryngoscope. 2015;125(7):E225–30.
- Shen X, Li WQ. High-mobility group box 1 protein and its role in severe acute pancreatitis. World J Gastroenterol. 2015;21(5):1424–1435. doi:10.3748/wjg.v21.i5.1424.
- Shimizu S, Kouzaki H, Kato T, et al. HMGB1-TLR4 signaling contributes to the secretion of interleukin 6 and interleukin 8 by nasal epithelial cells. Am J Rhinol Allergy. 2016;30(3):167–172. doi:10.2500/ajra.2016.30.4300.
- Yasuda T, Ueda T, Takeyama Y, et al. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33(4):359–363. doi:10.1097/01.mpa.0000236741.15477.8b.
- Wang X, Gao S, Song L, et al. Astragaloside IV antagonizes M2 phenotype macrophage polarization-evoked ovarian cancer cell malignant progression by suppressing the HMGB1-TLR4 axis. Mol Immunol. 2021;130:113–121. doi:10.1016/j.molimm.2020.11.014.
- Zhou LY, Shi LY, Xiao Y. Changes of HMGB1 expression on angiogenesis of ovarian cancer and its mechanism. J Biol Regul Homeost Agents. 2016;30(1):233–238.
- Rapoport BL, Steel HC, Theron AJ, et al. High mobility group box 1 in human cancer. Cells. 2020;9(7):1664. doi:10.3390/cells9071664.
- Ulukus EC, Ulukus M, Seval Y, et al. Expression of interleukin-8 and monocyte chemotactic protein-1 in adenomyosis. Hum Reprod. 2005;20(10):2958–2963. doi:10.1093/humrep/dei154.
- Mikhaleva LM, Davydov AI, Patsap OI, et al. Malignant transformation and associated biomarkers of ovarian endometriosis: a narrative review. Adv Ther. 2020;37(6):2580–2603. doi:10.1007/s12325-020-01363-5.
- Luo XZ, Zhou WJ, Tao Y, et al. TLR4 activation promotes the secretion of IL-8 which enhances the invasion and proliferation of endometrial stromal cells in an autocrine manner via the FAK signal pathway. Am J Reprod Immunol. 2015;74(6):467–479. doi:10.1111/aji.12425.