Abstract
Objective
This study is aimed to determine the efficacy of a cocktail style treatment by combining GnRH-antagonist, letrozole, and mifepristone on the prevention of ovarian hyperstimulation syndrome (OHSS) in high-risk women.
Methods
This prospective, randomized controlled clinical trial was performed between January 2018 and December 2018. A total of 170 women who identified as high risk of OHSS during the ovarian hyperstimulation and underwent cryopreservation of whole embryos. On the day of oocyte retrieval, the combination group received 0.25 mg Cetrorelix for 3 d, 5 mg letrozole for 5 d, and 50 mg mifepristone for 3 d, the mifepristone group received 50 mg mifepristone for 3 d. A total of 156 cases were included in final analysis. All the frozen embryo transfer (FET) cycles were followed up until December 2021.
Results
The combination group showed significantly decreased incidence of moderate and severe OHSS than mifepristone group (20.5% vs. 42.3%), with remarkably reduced serum estradiol level on hCG + 3 and + 5 d, decreased ovarian diameter, and shortened luteal phase. Oocyte retrieval number, levels of estradiol on hCG + 0 and VEGF, and ovarian diameter on hCG + 5 were associated with the severity of the symptoms. There was no significant difference in cumulative live birth rates (LBRs) between the combination and mifepristone group (74.4% vs. 76.9%).
Conclusions
The combination treatment effectively reduces the incidence of moderate/severe OHSS in high-risk women.
Introduction
Ovarian hyperstimulation syndrome (OHSS) is one of the most serious complications associated with controlled ovarian hyperstimulation (COH) in assisted reproductive technology (ART). Women who develop to OHSS experience different levels of gastrointestinal discomfort, liver and renal malfunction, edema, oliguria, ascites, and hydrothorax [Citation1, Citation2]. In extreme cases, although rare, the patients suffer from life-threatening situations because OHSS can cause venous or arterial thromboembolic events, including stroke, and loss of perfusion of an extremity [Citation3, Citation4].
Modified COH protocols, such as low Gonadotropin dose, GnRH antagonist (GnRH-ant) protocol, GnRH agonist/hCG dual trigger, and coasting, has been widely used to minimize the incidence of OHSS in IVF cycles. The incidence of moderate and severe OHSS ranges from 0.2 to 2% of all COH cycles, our previous clinical study shows the incidence of severe OHSS is 1.2% (55/4735) in all COH cycles in our center [Citation5]. However, moderate and severe OHSS has been calculated to occur in 15–45.8% in high-risk women, even with above-mentioned modified protocols [Citation5–7]. Therefore, secondary interventions after oocyte retrieval are needed to minimize the incidence and severity of OHSS in high-risk women.
OHSS can be classified as early-onset and late-onset based on the time of onset. Early onset OHSS arises between 3 and 7 d following human chorionic gonadotropin (hCG) injection and relates to the exogenous hCG dosage; late-onset OHSS is related to endogenous hCG elevation due to pregnancy [Citation8]. Early onset cases are promptly mitigated with the arrival of the menses, but last longer if the patient becomes pregnant. On the other hand, late-onset cases caused by pregnancy are intend to be more severe and the symptoms might last for over a month, suggesting that ovarian corpus luteum may play an essential role in the progression of OHSS.
Indeed, hCG is the main hormone for maintaining the activity of corpus luteum [Citation9], and the pro-luteolysis effect induced by GnRH-ant may decrease the production of vasoactive factors in the ovaries, resulting in the regression of severe OHSS [Citation10]. Letrozole, an aromatase inhibitor, has been originally applied in the ovarian stimulation. Recently, letrozole treatment after oocyte retrieval shows a prevention effect on moderate and severe OHSS due to the reduction of serum E2 level [Citation6]. Progesterone is produced by corpus luteum after ovulation and essential for corpus luteum function, and progesterone withdrawn triggers corpus luteum regression and menstruation. Mifepristone (also termed as RU486), a progesterone receptor antagonist, is clinically used for pregnancy termination due to its luteolysis effect [Citation11], but the application of mifepristone for OHSS prevention is limited in clinical studies. Some other adjuvant drugs are also used to prevent or minimize the severity of OHSS during ovarian stimulation or after oocyte retrieval, such as dopamine agonists and aspirin [Citation12, Citation13]. However, hitherto it is hard to draw a conclusion that single protocol or drug can completely prevent OHSS. We theorize that a cocktail style treatment by combining GnRH-ant, letrozole, and mifepristone could effectively prevent OHSS in high-risk women. Here we design a prospective, randomized, clinical trial to determine whether this drug regimen decrease the incidence of OHSS in women who identified as high-risk status during the COH.
Materials and methods
Study design
This is a prospective randomized controlled trial (Registration number: ChiCTR-INR-17014174) registered at the China Clinical Trials Registry (www.chictr.org.cn) on 2017-12-27 (indicated as Date of Registration on the Registration page), which was approved by Ethics Committee of Clinical Trials in Wuhan University Renmin Hospital (Reference number: 2018K-C001), the first participant was enrolled on 2018-01-01. All participants signed informed consent before the enrollment.
Study participants
The participants were enrolled from the Center for Reproductive Medicine, Wuhan University Renmin Hospital from January 2018 to December 2018. Inclusion criteria: infertile women aged 20–38 underwent all embryo freezing during their first IVF/ICSI cycle, had at least one of the following high-risk criteria: serum E2 ≥ 4000 pg/mL on the day of hCG administration day (trigger day, hCG + 0); number of oocytes retrieval ≥ 18; ultrasonography proved ovarian hyperstimulation on the day of oocyte retrieval. Exclusion criteria: allergic to mifepristone, cetrorelix, or letrozole; liver or kidney diseases; received any medicine affecting the permeability of blood vessel during IVF/ICSI cycle; coasting and any other interventions to prevent OHSS during ovulation stimulating; or possibility of pregnancy.
Sample size calculation: our preliminary experimental results showed that the incidence of moderate to severe OHSS was 50% (pc) in mifepristone group and 26% (pe) in combination group, respectively. According to the following formula [10], n = [(uα+ uβ)2 (1 + 1/k) p (1 − p)]/(pe − pc)2, p = (pe + k × pc)/(1 + k)], the sample size is calculated by comparing the two sample rates. Assume that the ratio of participants in two groups is 1:1, then k = 1. To achieve 80% power and α 0.05 significant level (adopting two-tailed test, uα = 1.96, uβ = 0.8417), at least 69 participants should be included in each group. If the follow-up loss rate is 10%, a total of at least 152 participants would be required. A total of 156 participants were included for final analysis in this study.
Protocols for controlled ovary hyperstimulation (COH)
The COH protocols were conducted according to the Guidelines in our center [Citation5]. Briefly, in luteal GnRH agonist (GnRH-a) protocol, daily administration of GnRH-a (IPSEN PHARMA BTOTECH, Paris, France, 0.1 mg) at mid-luteal phase for at least 14 d before starting Gonadotropin stimulation. After 2–3 weeks, patients received ovarian stimulation with gonadotropin (Gn, Gonal-F, Merck-Serono, Aubonne, Switzerland) of 150–225 IU daily. The Gn dose was adjusted according to the hormonal level and the diameter of the oocytes. In follicular phase long GnRH-a protocol, the patients received a subcutaneous injection of 3.75 mg of a long-acting GnRH agonist (Diphereline, IPSEN PHARMA BIOTECH) on the 2nd day of menstruation. Patients were monitored to determine whether pituitary downregulation was achieved 30–40 d later, with E2 < 25 pg/mL, LH < 5 U/L, and the diameter of the antral follicle < 5 mm, then received ovarian stimulation with gonadotropin as long luteal GnRH agonist protocol. In GnRH-ant protocol, Gn was used on the 2nd or 3rd day of menstruation, and 0.25 mg/d Cetrorelix was added on the 5th or 6th day of ovarian stimulation until hCG trigger day. 2000–10,000 IU hCG (Lizhu Pharmaceutical Co., Ltd., Guangdong, China) with or without 0.2 mg GnRH agonist (Decapeptyl, Ferring Pharmaceuticals, Saint-Prex, Switzerland) was administered for triggering once at least 3 follicles reached to 18 mm or at least 2 follicles reached to 20 mm, the oocytes were retrieved and fertilized by IVF/ICSI 36–40 h after triggering.
Grouping and medication regimen
The consolidated standards of reporting trials (CONSORT) flow diagram is presented in . A total of 170 infertile women undergoing IVF/ICSI treatment were assessed for eligibility. The ‘Serial number’ and patient information was registered according to screening order. The screening process was initiated at any time during the controlled ovarian stimulation, the participants were informed about the risk of OHSS, and all agreed to the cancelation of fresh embryo transfer and signed the inform consent. A specialized person who is not involved in the recruitment process was authorized to generate a ‘Random number’ table by EXCEL Office Software based on the ‘Serial number’. Randomization was performed by a study clinician at consultation, according to computer generated random numbers in sealed envelopes. The participants were randomly allocated into ‘Combination group’ or ‘Mifepristone group’ and received medication on the day of oocyte retrieval. Doctors who assess the patients’ condition of OHSS were blinded to the grouping and the assignments throughout this trial. We followed all participants for their frozen embryo transfers (FETs) after their first IVF/ICSI cycles until December 2021.
Figure 1. Flow diagram for enrollment, randomization, follow-up, and analysis of study participants.
In mifepristone group, participants received 25 mg mifepristone (Beijing Famostat Pharmaceutical Technology Co, Ltd., Beijing, China) orally twice for 3 consecutive days. In combination group, participants received 0.25 mg Cetrorelix (Merch Serono S.p.A, Lyon, France) subcutaneously injection per day for 3 consecutive days, 5 mg Letrozole tablets (Jiangsu Hengrui Pharmaceutical Co, Ltd., Jiangsu, China) orally per day for 5 consecutive days, and 25 mg mifepristone tablet orally twice for 3 consecutive days.
The classification of OHSS
presents the classification of OHSS was based on clinical and laboratory features as previously described [Citation6, Citation14]. Patients demonstrating any feature of severe or critical OHSS should be classified in that category. However, those only with ovarian enlargement without any clinical manifestations or abnormal laboratory indicators will be allocated to mild group.
Table 1. Classification of OHSS.
Observational outcome measures
The main outcome measure was the incidence and severity of OHSS. The outcomes included no OHSS, mild, moderate, severe, and critical OHSS. The secondary outcomes included serum E2, progesterone, and VEGF levels, ovarian size (maximum diameter), the duration of luteal phase after oocyte retrieval. Other outcome measures included the incidence of thoraco-abdominal puncture, blood concentration, abnormal liver and kidney function, and abnormal coagulation function, the total volume of pleural or peritoneal effusion, and etc. The pregnancy outcomes for all FET cycles were followed up until December 2021, including the number of FET cycles, embryo transfer type, clinical pregnancy rate (CPR), abortion rate, implantation rate, and live birth rate (LBR).
Statistical analysis
Date analysis was processed by SPSS version 22.0 statistical software (SPSS Inc, Chicago, IL). Metrological data were presented as means ± SEM. Independent sample t test was adopted when normal distribution and homogeneity of variance were met, otherwise Mann–Whitney U was performed. Rate was compared using the chi-square test, Fisher’s Exact Test was applied when necessary. No critical OHSS was recorded in this study, we used four categories of OHSS (no OHSS, mild, moderate, and severe OHSS) to serve as dependent variables, and ordinal logistic regression analysis was used to identified the factors that associated with the severity of OHSS. p < .05 is considered statistical significance.
Results
Participants
From January 2018 to December 2018, 170 women were screened for eligibility and assigned with Serial number, 169 women were consented to participate in this trial and randomly allocated into 2 groups to receive either combination (89 women) or mifepristone (80 women) treatment. A total of 11 women were quitted from the combination group (7 were prescribed with traditional Chinese medicine and 4 withdrew the consent from this trial), and 2 women were quitted from mifepristone group (2 were prescribed with GnRH-ant and mifepristone simultaneously). Ultimately, 156 women (78 women in combination group and 78 in mifepristone group) were included in this trial, and the follow-up rate for primary outcome was 100% ().
Basal clinical characteristics between the two groups
The basal characteristics of participants between the combination and mifepristone groups were similar, with no significant differences in age, body mass index (BMI), antral follicle count (AFC), basal FSH, LH, and E2 levels on day 2 of the menstrual cycle, duration and causes of infertility, COH protocols, total dosage and duration of Gn, E2 and progesterone (P) levels on hCG trigger day (hCG + 0), the number of oocytes retrieved and good-quality embryos (p > .05) ().
Table 2. Basal characteristics of the participants.
Incidence of OHSS in the two groups
After 5 d of treatment, the incidence of OHSS was comparable between the combination and mifepristone groups (80.5 vs. 84.6%, p = .526). However, the proportion of mild OHSS was significantly higher in the combination group than that in mifepristone group (60.3 vs. 55.1%, p = .024), and the incidence of moderate and severe OHSS was remarkably decreased in the combination group than mifepristone group (20.5% vs. 42.3%, p = .003) (). During the classification, patients with enlarged ovaries (diameter in 7–8 cm), but without any symptoms and abnormalities in laboratory tests, were allocated into mild group. The proportion of participants without any symptoms or discomforts was significantly higher in the combination group than that in mifepristone group (37.2% vs. 20.5%, p = .033). The incidence of moderate and severe OHSS showed no significant difference among luteal long GnRH-a, GnRH-ant, and follicular phase long GnRH-a protocol in both two groups ().
Table 3. Incidence of OHSS in two groups.
Table 4. Incidence of moderate and severe OHSS in different COH protocols.
Serum E2, P, and VEGF alteration in the two groups
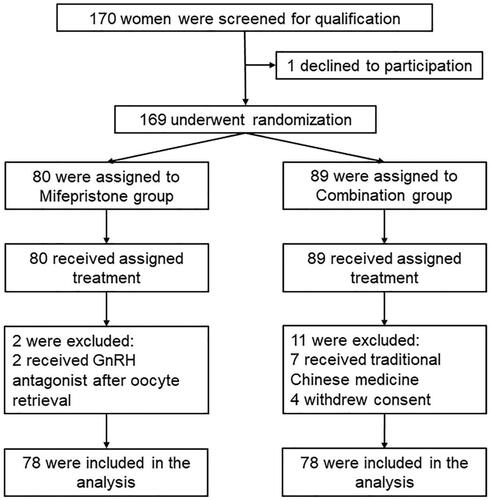
The comparison of serum E2 and P levels between the combination and mifepristone groups is shown in . There was no difference in serum E2 level on hCG + 0 between the two groups. However, the serum E2 level was dramatically reduced on hCG +3 (110.9 ± 63.5 vs. 2857.2 ± 1111.4, p < .001) and hCG + 5 (173.3 ± 132.6 vs. 3758.4 ± 2029.2, p < .001) in the combination group than that in mifepristone group (). The serum P4 levels on hCG + 0, +3, and +5 showed no statistical difference between the two groups ().
Figure 2. Alterations of serum E2, P, and VEGF level between the combination and mifepristone groups. (A) Comparison of serum E2 level on hCG + 0, +3, and +5. (B) Comparison of serum P level on hCG + 0, +3, and +5.
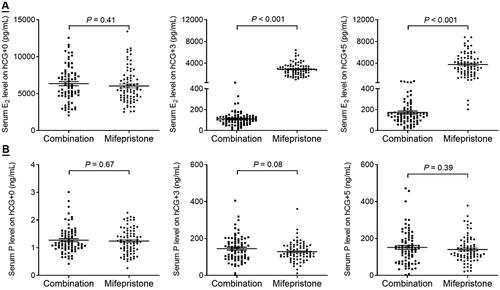
VEGF has been identified as a key regulator in increased vascular permeability in the pathogenesis of OHSS [Citation15]. As shown in , the serum VEGF levels on hCG + 3 and + 5 were comparable between the combination group and mifepristone group. However, the VEGF level on hCG + 5 was significantly increased in patients who developed to OHSS, and the VEGF level was positively associated with the severity of OHSS ().
Figure 3. Comparison of serum VEGF level between the combination and mifepristone groups. (A) Comparison of serum VEGF level on hCG + 3 and +5. (B) The relationship between serum VEGF level on hCG + 3 and the severity of OHSS. (C) The relationship between serum VEGF level on hCG + 5 and the severity of OHSS.
Clinical and laboratory features comparison between the two groups
The clinical and laboratory features regarding to OHSS are summarized in . The ovarian diameter was significantly decreased in the combination group when compared with mifepristone group on hCG + 5 (8.7 ± 1.1 vs. 9.1 ± 1.5, p = .021). The luteal phase (the duration between oocyte retrieval and next menstruation) was shorter in the combination group than that in mifepristone group (7.5 ± 2.6 vs. 9.6 ± 1.8, p < .001), and the incidence of luteal phase < 5 d was increased in the combination group than mifepristone group (19.2 vs. 1.3%, p < .001). There was no statistical difference in ascites index (the total depth of ascites measured by ultrasound) and the incidence of ascites puncture and drainage between the two groups. The combination group showed decreases in white blood cell count (7.6 ± 2.0 vs. 9.6 ± 2.4, p < .001) and the incidence of hemoconcentration (3.9 vs. 12.8%, p = .043) than mifepristone group on hCG + 3 d. The combination group also had decreased alanine transaminase (ALT, 59.6 ± 39.4 vs. 74.2 ± 41.9, p = .026) level and increased albumin (ALB, 34.3 ± 3.5 vs. 32.4 ± 3.5, p = .001) level than mifepristone group.
Table 5. Clinical and laboratory features between combination and mifepristone groups.
Ordinal multinomial logistic regression analysis
Ordinal multinomial logistic regression analysis was performed to predict the relationship between the incidence and severity of OHSS and patients’ clinical features, in which the four categories of OHSS (no OHSS, mild, moderate, and severe OHSS) served as dependent variables, and patient’s age, AFC, Gn duration and dosage, oocyte retrieved number, interventions (combination and mifepristone), serum E2 on hCG + 0, and serum VEGF on hCG + 5 were served as independent variables. As shown in , the oocyte retrieved number (odds ratio 1.09, 95% confidence interval 1.00 − 1.19, p = .05), intervention (odds ratio 0.31, 95% confidence interval 0.15–0.63, p = .001), serum E2 level on hCG + 0 (odds ratio 1.00, 95% confidence interval 1.00–1.00, p = .03), serum VEGF on hCG + 5 (odds ratio 1.03, 95% confidence interval 1.02–1.04, p < .001), and ovarian diameter on hCG + 5 (odds ratio 3.63, 95% confidence interval 2.35–5.60, p < .001) were associated with the severity of OHSS.
Table 6. Ordinal multinomial logistic regression analysis.
Cumulative pregnancy outcomes
The total FET cycles with embryo transfer were 98 and 106 in combination and mifepristone groups, respectively. There was no difference in the number of FET attempts, the proportion of blastocyst transfers, and the embryo transfer number. The total LBR in all FET was 59.2% in combination group, which was comparable to mifepristone group (56.6%, p = .71). No significant difference in CPR, implantation rate and abortion rate were observed between the two groups. Regarding to cumulative pregnancy outcome, all the FET cycles after this entire IVF/ICSI cycle were analyzed. There was no significant difference in cumulative LBR per randomized between the combination and mifepristone groups (74.4 vs. 76.9%, p = .71) ().
Table 7. Cumulative pregnancy outcomes after freeze-all cycle and all subsequent frozen embryo transfer cycles between combination and mifepristone groups.
Discussion
This is a prospective clinical randomized trial to investigate the efficacy of a cocktail style treatment by combining GnRH-ant, letrozole, and mifepristone on the prevention of OHSS in high-risk women compared with the monotherapy of mifepristone. Although the total incidence of OHSS is similar between the combination group and mifepristone group, the incidence of moderate and severe OHSS is remarkably decreased in the combination group (20.5 vs. 42.3%, p = .003), with significantly reductions in serum E2 level, ovarian diameter, and luteal phase. We suggest that this drug regimen can be used in clinical to minimize the severity of OHSS in high-risk women.
OHSS is a potentially life-threatening complication in COH treatments, which results from the release of vasoactive substances from the multiple corpora lutea, such as estrogens and VEGF, due to the ovarian hyperstimulation after oocyte maturation trigger. The incidence of OHSS shows a gradual decrease as the incessant sculpture of ovarian stimulation protocols, however, certain patients are at high risk of OHSS, such as PCOS and high responsive patients [Citation16]. The identification of patients susceptible to elicit a hyper-response to standard COH protocols would allow clinician to adjust their treatment, at a large extent, to minimize the severity of the symptoms. In clinical practice, all the symptoms, even with the severe OHSS symptoms, are vanished with the arrival of the menstruation. Therefore, medications that promote luteolysis are theoretically effective for the prevention or treatment of OHSS.
Recently, GnRH-ant administration during the luteal phase offers another therapeutic method for patients who developed to severe OHSS after oocyte retrieval. Treatment of 0.25 mg GnRH-ant for 3 d (from hCG + 5 to +7) significantly accelerates patients’ recovery from severe OHSS symptoms and shows similar pregnant outcomes with the control group [Citation17]. Another study shows that 0.25 mg GnRH-ant administration from days 5 to 8 after oocyte retrieval for women who manifested early severe OHSS symptoms is associated with rapid regression of the syndrome and accelerated luteolysis [Citation18]. Although these two studies suggest a valid therapeutic effect of GnRH-ant on severe OHSS, the effect of GnRH-ant on OHSS prevention during the luteal phase remains undefined. Letrozole manifests promptly E2 reduction effect, two clinical trials show that letrozole treatment during the luteal phase effectively decreases the incidence of moderate and severe OHSS due to its luteolysis effect [Citation6], and the incidence of OHSS is decreased as the increases in letrozole dose [Citation19]. Progesterone is secreted by corpora lutea and functions to maintain the corpora lutea, and progesterone withdraw is the trigger for luteal regression and menses initiation. Mifepristone is a well-identified progesterone receptor antagonist, which is clinically used for emergency contraception by inhibiting embryo implantation and early pregnancy termination. Although the clinical application of mifepristone in OHSS treatment is limited, animal models show that progesterone is a key regulator for increased vascular permeability in mouse uterine vessels, and mifepristone blocks the effect of progesterone on vascular permeability [Citation20]. In hCG induced OHSS rat model, mifepristone could significantly reduce the ovarian enlargement and VEGF production [Citation21, Citation22], suggesting the luteolysis effect of mifepristone is potentially effective for minimize the risk of OHSS.
We have previously used a rat model to determine the effect of GnRH-ant, letrozole, and mifepristone, alone or in combination, on the prevention of OHSS [Citation23]. The results have shown that GnRH-ant, letrozole, and mifepristone are all beneficial to prevent the progression of OHSS through different luteolytic mechanisms, and the combination group shows enhanced synergistic effect on preventing the progression of OHSS. Therefore, we proposed this prospective, randomized study to evaluate the effect of combination of GnRH-ant, letrozole, and mifepristone on preventing OHSS in high-risk women. The combination group shows decreases in the proportion of moderate and severe OHSS than that in mifepristone group, the clinical and laboratory index comparison demonstrates that the combination treatment accelerates luteolysis process and improves the participants’ hemoconcentration and liver function. These results suggest that the combination treatment by GnRH-ant, letrozole, and mifepristone is effectively to reduce the severity of OHSS in high-risk women.
GnRH-ant protocol has been recommended for PCOS women to reduce the incidence of OHSS. A meta analysis reported that GnRH-ant group does not reduce the incidence of severe OHSS, but significantly reduce the incidence of moderate and severe OHSS when compared with GnRH-a group [Citation24]. Another study showed that GnRH-ant protocol significantly lower OHSS rates in general IVF patients and PCOS women. However, the ongoing pregnancy rate was significantly lower in GnRH-ant group than GnRH-a group in general IVF patients [Citation25]. In China, luteal long GnRH-a and follicular phase long GnRH-a protocols are the preferred options for first IVF cycle patients in most IVF centers. In this study, tubal factor is the primary etiology in included participants, and the proportions of luteal GnRH-a, GnRH-ant, and follicular phase GnRH-a protocols are not differed between combination and mifepristone groups. GnRH-ant protocol does not lower the incidence of moderate and severe OHSS in combination group or mifepristone group. These results suggest that COH protocols may not be associated with the severity of the symptoms.
In this study, oocyte retrieval number, serum E2 level on hCG + 0, VEGF level and ovarian diameter on hCG + 5 are associated with the severity of the symptoms. E2 level has been served as a reliable predictor for identifying high-risk women of OHSS during the COH, and estrogens are shown to increase vascular permeability in uterine and ovarian circulation. However, some cases of OHSS are reported in women with low E2 level and natural conceived pregnancy [Citation26], and large doses of E2 treatment could not reproduce OHSS in animal model, indicating that E2 alone is not able to initiate OHSS. VEGF is strongly implicated as a vasoactive factor and plays important role in regulating angiogenesis and vascular permeability. VEGF is also involved in hCG-dependent ovarian angiogenesis, and increased VEGF mRNA and protein levels are detected in serum and peritoneal fluids in women with high risk of OHSS [Citation27]. Cabergoline, a dopamine agonist with anti-VEGF activity, has been demonstrated to be an effective and safe drug for the prevention of OHSS [Citation28]. E2 itself does not exhibit direct vasoactive effects, but E2 induces endometrial VEGF expression and stimulates microvessel endothelial cell proliferation and vascular expansion [Citation29], suggesting that E2 and VEGF are both essential for the initiation of OHSS.
Previous study shows letrozole treatment, although decrease the incidence of moderate and severe OHSS, could increase VEGF level on hCG + 7 [Citation6], indicating that letrozole-induced E2 reduction is not able to suppress VEGF expression. Follicular E2 and VEGF levels are decreased in PCOS women received GnRH-ant protocol [Citation30], and serum VEGF level is down-regulated by both of GnRHa and GnRH-ant, which may partially explain the decreases in OHSS in GnRHa trigger and GnRH-ant protocol [Citation31]. Ordinal multinomial logistic regression analysis also shows serum E2 level on hCG + 0 and VEGF level on hCG + 5 are associated with the severity of OHSS, therefore, targeting in reduction of serum E2 and VEGF levels is theoretically for OHSS prevention. In this study, serum E2 level on hCG + 0 is comparable between the two groups. However, serum E2 levels on hCG + 3 and + 5 are dramatically decreased in the combination group. Serum VEGF levels on hCG + 3 and + 5 are similar between the two groups, but the VEGF level on hCG + 5 is significantly higher in OHSS cases than that in non-OHSS women and positively related to the severity of OHSS.
OHSS is characterized as enlarged ovaries and the categories of OHSS are diagnosed partially based on the ovarian diameter. In this study, the ovarian diameter on hCG + 5 is significantly reduced in the combination group than that in mifepristone group, and logistic regression analysis shows that the ovarian diameter is associated with the severity of OHSS. The luteal phase in combination group is shorter than mifepristone group, and the proportion of women with < 5 d of luteal phase is higher in combination group. During the clinical observational frame after oocyte retrieval, the proportion of patients without any OHSS related symptoms is significantly increased in the combination group, and most of patients in combination group are recovered and back to normal life more quickly than mifepristone group. These results suggest that the synergistic effect of GnRH-ant, letrozole, and mifepristone could accelerate luteolysis effect and prevent the occurrence of moderate and severe OHSS.
Strengths and limitations
This is a prospective and randomized clinical trial, and the evaluation on participants’ symptoms is blinded to the grouping. This study has a major limitation in control group design regarding the monotherapy of GnRH-ant or letrozole as a prospective randomized study. Our previous animal OHSS model showed that the combination group has a synergistic effect than monotherapy; Mifepristone has been used as the primary preventive therapy to accelerate luteolysis for high risk women in our center. However, the effect of mifepristone on OHSS prevention remains unknown. Therefore, the combination treatment was set as experiment group, and mifepristone was set as control group in this clinical trial. The incidence of moderate and severe OHSS reached to 20.5% in combination group and 42.3% in mifepristone group, possibly due to the high proportion of GnRH agonist protocols in this study. The reported incidence of moderate and severe OHSS in high risk women were 25% and 45.1% in letrozole and aspirin treatment groups, respectively [Citation6]. The total incidence of severe OHSS was 8.97% in this study, which was similar to 10.61% in GnRH agonist protocols from 2021 ESHER Maribor consensus [Citation32].
Conclusions
The combination treatment by combining GnRH-ant, letrozole, and mifepristone after oocyte retrieval is an effective drug regimen for reducing the severity of OHSS in high-risk women. This cocktail style treatment prevents moderate and severe OHSS syndrome may partially through targeting E2 reduction and luteolysis.
Institutional review board statement
This is a prospective randomized controlled trial (Registration number: ChiCTR-INR-17014174) registered at the China Clinical Trials Registry (www.chictr.org.cn) on 2017-12-27 (indicated as Date of Registration on the Registration page), which was approved by Ethics Committee of Clinical Trials in Wuhan University Renmin Hospital (Reference number: 2018K-C001), the first participant was enrolled on 2018-01-01.
Informed consent statement
All participants signed informed consent before the enrollment.
Consent for publication
Not applicable.
Authors’ contribution
Conceptualization, Qingzhen Xie and Qianrong Qi; methodology, Yaqin Wang; software, Yi Xia; validation, Qianrong Qi, Yi Xia. and Jin Luo; formal analysis, Qianrong Qi; investigation, Yaqin Wang; resources, Qingzhen Xie; data curation, Yi Xia; writing – original draft preparation, Qianrong Qi; writing – review and editing, Qingzhen Xie; supervision, Yaqin Wang; project administration, Qingzhen Xie; funding acquisition, Qianrong Qi and Qingzhen Xie; All authors have read and agreed to the published version of the manuscript.
Clinical trial registration information
Date of registration: 12-27-2017
Date of initial participant enrollment: 01-01-2018
Clinical trial identification number: ChiCTR-INR-17014174
URL of the registration site: www.chictr.org.cn
Acknowledgments
We thank all the staffs in the Center for Reproductive Medicine, Renmin Hospital of Wuhan University for their help in clinical works.
Disclosure statement
The authors declare no conflict of interest.
Availability of data and materials
Not applicable.
Additional information
Funding
References
- Practice committee of the American society for reproductive medicine. Electronic address aao, practice committee of the American society for reproductive M: prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1–9.
- Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS). Hum Reprod Update. 2003;9(1):77–96. doi: 10.1093/humupd/dmg005.
- Braat DD, Schutte JM, Bernardus RE, et al. Maternal death related to IVF in The Netherlands 1984-2008. Hum Reprod. 2010;25(7):1782–1786. doi: 10.1093/humrep/deq080.
- Cluroe AD, Synek BJ. A fatal case of ovarian hyperstimulation syndrome with cerebral infarction. Pathology. 1995;27(4):344–346. doi: 10.1080/00313029500169273.
- Wang YQ, Luo J, Xu WM, et al. Can steroidal ovarian suppression during the luteal phase after oocyte retrieval reduce the risk of severe OHSS? J Ovarian Res. 2015;8(1):63. doi: 10.1186/s13048-015-0190-y.
- Mai Q, Hu X, Yang G, et al. Effect of letrozole on moderate and severe early-onset ovarian hyperstimulation syndrome in high-risk women: a prospective randomized trial. Am J Obstet Gynecol. 2017;216(1):42 e41–42 e10.
- Toftager M, Bogstad J, Bryndorf T, et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31(6):1253–1264. doi: 10.1093/humrep/dew051.
- Humaidan P, Nelson SM, Devroey P, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31(9):1997–2004. doi: 10.1093/humrep/dew149.
- Casper RF, Yen SS. Induction of luteolysis in the human with a long-acting analog of luteinizing hormone-releasing factor. Science. 1979;205(4404):408–410. doi: 10.1126/science.377491.
- Kol S. Luteolysis induced by a gonadotropin-releasing hormone agonist is the key to prevention of ovarian hyperstimulation syndrome. Fertil Steril. 2004;81(1):1–5. doi: 10.1016/j.fertnstert.2003.05.032.
- McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev. 1999;79(2):263–323. doi: 10.1152/physrev.1999.79.2.263.
- Ferrero H, Garcia-Pascual CM, Gaytan M, et al. Dopamine receptor 2 activation inhibits ovarian vascular endothelial growth factor secretion in an ovarian hyperstimulation syndrome (OHSS) animal model: implications for treatment of OHSS with dopamine receptor 2 agonists. Fertil Steril. 2014;102(5):1468–1476 e1461. doi: 10.1016/j.fertnstert.2014.07.1240.
- Elmahdy M, Abdelsalam EA, Maghraby HA. Combining several interventions to reduce the incidence of OHSS: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2017;212:110–114. doi: 10.1016/j.ejogrb.2017.03.016.
- Golan A, Weissman A. Symposium: update on prediction and management of OHSS. A modern classification of OHSS. Reprod Biomed Online. 2009;19(1):28–32. doi: 10.1016/s1472-6483(10)60042-9.
- Ajonuma LC. Is vascular endothelial growth factor (VEGF) the main mediator in ovarian hyperstimulation syndrome (OHSS)? Med Hypotheses. 2008;70(6):1174–1178. doi: 10.1016/j.mehy.2007.11.004.
- Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol. 2012;10(1):32. doi: 10.1186/1477-7827-10-32.
- Lainas GT, Kolibianakis EM, Sfontouris IA, et al. Pregnancy and neonatal outcomes following luteal GnRH antagonist administration in patients with severe early OHSS. Hum Reprod. 2013;28(7):1929–1942. doi: 10.1093/humrep/det114.
- Lainas GT, Kolibianakis EM, Sfontouris IA, et al. Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: an observational cohort study. Reprod Biol Endocrinol. 2012;10(1):69. doi: 10.1186/1477-7827-10-69.
- He Q, Liang L, Zhang C, et al. Effects of different doses of letrozole on the incidence of early-onset ovarian hyperstimulation syndrome after oocyte retrieval. Syst Biol Reprod Med. 2014;60(6):355–360. doi: 10.3109/19396368.2014.957879.
- Goddard LM, Murphy TJ, Org T, et al. Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell. 2014;156(3):549–562. doi: 10.1016/j.cell.2013.12.025.
- Ohba T, Ujioka T, Ishikawa K, et al. Ovarian hyperstimulation syndrome-model rats; the manifestation and clinical implication. Mol Cell Endocrinol. 2003;202(1-2):47–52. doi: 10.1016/s0303-7207(03)00061-3.
- Ishikawa K, Ohba T, Tanaka N, et al. Organ-specific production control of vascular endothelial growth factor in ovarian hyperstimulation syndrome-model rats. Endocr J. 2003;50(5):515–525. doi: 10.1507/endocrj.50.515.
- Luo J, Qi Q, Chen Y, et al. Effect of GnRH-antagonist, mifepristone and letrozole on preventing ovarian hyperstimulation syndrome in rat model. Reprod Biomed Online. 2021;42(2):291–300. doi: 10.1016/j.rbmo.2020.10.006.
- Pundir J, Sunkara SK, El-Toukhy T, et al. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod Biomed Online. 2012;24(1):6–22. doi: 10.1016/j.rbmo.2011.09.017.
- Lambalk CB, Banga FR, Huirne JA, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23(5):560–579. doi: 10.1093/humupd/dmx017.
- Sridev S, Barathan S. Case report on spontaneous ovarian hyperstimulation syndrome following natural conception associated with primary hypothyroidism. J Hum Reprod Sci. 2013;6(2):158–161. doi: 10.4103/0974-1208.117164.
- Wang TH, Horng SG, Chang CL, et al. Human chorionic gonadotropin-induced ovarian hyperstimulation syndrome is associated with up-regulation of vascular endothelial growth factor. J Clin Endocrinol Metab. 2002;87(7):3300–3308. doi: 10.1210/jcem.87.7.8651.
- Alvarez C, Marti-Bonmati L, Novella-Maestre E, et al. Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. J Clin Endocrinol Metab. 2007;92(8):2931–2937. doi: 10.1210/jc.2007-0409.
- Aberdeen GW, Wiegand SJ, Bonagura TW, Jr., et al. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology. 2008;149(12):6076–6083. doi: 10.1210/en.2008-0521.
- Vrtacnik-Bokal E, Virant Klun I, Verdenik I. Follicular oestradiol and VEGF after GnRH antagonists or GnRH agonists in women with PCOS. Reprod Biomed Online. 2009;18(1):21–28. doi: 10.1016/s1472-6483(10)60420-8.
- Cerrillo M, Rodriguez S, Mayoral M, et al. Differential regulation of VEGF after final oocyte maturation with GnRH agonist versus hCG: a rationale for OHSS reduction. Fertil Steril. 2009;91(4):1526–1528. doi: 10.1016/j.fertnstert.2008.08.118.
- Group ECPW, Vlaisavljevic V, Apter S, et al. The maribor consensus: report of an expert meeting on the development of performance indicators for clinical practice in ART. Hum Reprod Open. 2021;2021(3):hoab022.
