Abstract
Background: In recent years, new combined oral contraceptives (COCs) have become available, representing an advance in terms of individualization and compliance by users.
Objective: To provide recommendations regarding COCs: formulations, use, efficacy, benefits and safety.
Method: For these recommendations, we have used the modified Delphi methodology and carried out a systematic review of studies found in the literature and reviews performed in humans, published in English and Spanish in Pubmed, Medline and advanced medicine and computer networks until the year 2021, using the combination of terms: ‘oral contraceptives’, ‘estroprogestins’ and ‘combined oral contraceptives’.
Results: Regarding the estrogen component, initially switching from mestranol (the pro-drug of ethinylestradiol) to ethinylestradiol (EE) and then reducing the EE dose helped reduce side effects and associated adverse events. Natural estradiol and estradiol valerate are already available and represent a valid alternative to EE. The use of more potent 19-nortestosterone-derived progestins, in order to lower the dose and then the appearance of non-androgenic progestins with different endocrine and metabolic characteristics, has made it possible to individualize the prescription of COC according to the profile of each woman.
Conclusion: Advances in the provision of new COCs have improved the risk/benefit ratio by increasing benefits and reducing risks. Currently, the challenge is to tailor contraceptives to individual needs in terms of safety, efficacy, and protection of female reproductive health.
Introduction
Since 1960, the use of combined oral contraceptives (COCs) has expanded exponentially, providing simple, safe and effective protection against pregnancy [Citation1]. COCs are the most commonly used contraceptive method in industrialized countries and the third most common in developing countries [Citation1]. COCs prevent ovulation by inhibiting gonadotropin secretion through an effect on the pituitary and hypothalamus. The progestin primarily inhibits the function of the luteinizing hormone (LH), with subsequent inhibition of ovulation, while estrogen inhibits follicle-stimulating hormone (FSH) secretion, preventing the formation of the dominant follicle. Even if the follicle was not sufficiently inhibited, the effect of the progestin prevents the LH surge and therefore ovulation. There are studies showing that show COCs have a mechanism of action, at least partially, at a central level in the kisspeptin-neurokinin B axis and in the response of gonadotropins to these neuropeptides [Citation2].
The progestin also makes the endometrium not receptive to the implantation of the fertilized ovum and the cervical mucus becomes thick and impermeable to sperm transport. The mission of the estrogen will be to stabilize the endometrium and enhance the action of progestins, by increasing the concentration of progesterone receptors [Citation3]. Contraceptive and non-contraceptive benefits are well established () [Citation4]; as are their risks () [Citation5]. In order to reduce the negative effects associated with COCs, doses have been reduced, new progestogens have appeared, new combinations with estradiol, estradiol valerate, and estetrol, and new delivery mechanisms have been developed over the years.
Table 1. Advantages of oral contraceptives.
Table 2. Risks or side effects of COCs. Non-beneficial effects of oral contraceptives.
The challenge of contraception continues to be the improvement of the efficacy, safety and compliance of COCs in order to make individualized prescriptions that fit the profile of our patient. The aim of this document is to provide recommendations regarding COCs: formulations, use, efficacy, benefits and safety.
Method
For these recommendations, the modified Delphi methodology [Citation6] was used. A structured methodology was used to systematically collect judgments from a panel of experts in order to solve a series of questions. For this, we carried out a systematic review of the literature of studies and reviews in humans, published in English and Spanish in Pubmed, Medline and advanced medicine and computer networks until the year 2021, based on relevance criteria for these recommendations, using the following combination of terms: ‘oral contraceptives’, ‘estroprogestins’ and ‘combined oral contraceptives’.
Different estrogens used in COCs
Estrogen options currently available in COCs include ethinylestradiol (EE), and the natural estrogens: estradiol valerate (E2V), 17-beta estradiol (E2), and estetrol (E4) [Citation7]. Estrogens play an important role in COCs [Citation7,Citation8]. The most widely used estrogen in COCs is EE due to its good oral bioavailability (38–48%) compared to E2 (5%) [Citation8]). It is 15 to 20 times more active than estradiol after oral administration. However, given its metabolic characteristics and related risks, attempts have been made to reduce the dose to minimize thromboembolic and cardiovascular risks. However, dose reduction has produced an increase in irregular bleeding (amenorrhea or infrequent bleeding, prolonged withdrawal, spotting). Available COCs contain 20 15 to 35 mcg of EE. COCs with 20 mcg of EE were introduced as an option for individuals who could not tolerate 30–35 mcg and with the idea of reducing risks, mainly thromboembolic [Citation4,Citation5]. These reductions were possible thanks to the availability of gestagens with high anti-gonadotrophic activity and to new administration regimens [Citation8,Citation9].
Exogenously administered estradiol is chemically identical to endogenous E2 [Citation10]. In the past, a major obstacle to the use of E2 in hormonal contraceptives was its relative inactivity when administered orally [Citation3]. The first natural estrogen introduced in hormonal contraception was E2V associated with dienogest (DNG) in a quadriphasic regimen in which the dose of estrogen and progestin followed the physiological pattern of the ovarian and endometrial cycle for 26 days plus 2 days of placebo (COCs containing E2V/DNG) [Citation11].
Recently, natural estradiol (1.5 mg) was associated with nomegestrol acetate (2.5 mg) in a monophasic formulation with a 24/4-day regimen. This combination is better tolerated at the metabolic level and with less impact on coagulation parameters. It also provides good contraceptive efficacy and cycle control, although there has not been sufficient practical experience with its use [Citation12].
Finally, we have the appearance in the market of a low-dose combination of E4 15 mg and drospirenone (3 mg). This combination has showed high acceptability, tolerability and user satisfaction; effectively inhibiting ovulation with a similar effect on endometrial thickness as COCs containing EE. This combination could also theoretically lead to a lower risk of venous thromboembolism (VTE) when compared to COCs containing EE [Citation13].
Activity of the different gestagens
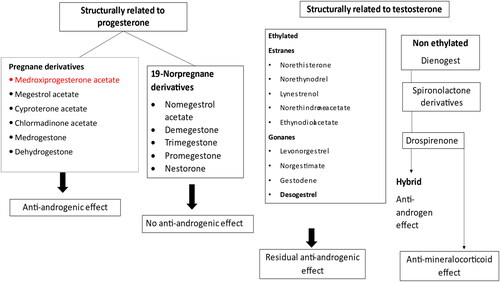
Progestogens can be natural (natural progesterone) or synthetic (gestagens). Gestagens can be classified into 2 large groups: (i) according to its molecule of origin [Citation14] (); and (ii) according to the sequence by which they were developed [Citation14]. Since progesterone is a steroid hormone, progestogens may have, in addition to the progestogenic effect, other steroidal side effects [Citation14]. Some of the most commonly used gestagens are described below.
Levonorgestrel (LNG)
Levonorgestrel (LNG) is a second generation gestagen derived from testosterone, widely used in C OCs. It is the progestogen by which the different COCs are compared when addressing VTE since, apparently, it has been less involved in this adverse event. Due to the fact that it is derived from testosterone, it does not have an anti-androgenic effect, hence, it lacks benefits on skin and hair, although, due to its origin, it is recommended when there is loss of libido. It has no glucocorticoid, mineralocorticoid or anti-mineralocorticoid effects [Citation15].
Desogestrel (DSG)
Desogestrel (DSG) is converted to etonogestrel (3-ketodesogestrel) and, through this active metabolite, it has progestogenic activity, anti-gonadotropic effects, very weak androgenic activity, and very weak glucocorticoid activity [Citation16]. Etonogestrel has approximately 300% of the affinity of progesterone for progesterone receptors and it is considered a very potent progestogen, inhibiting ovulation at very low doses [Citation16].
Gestodene (GSD)
GSD is an androgenically neutral gestagen, that has a minimal impact on blood glucose levels and lipid profile, with slight mineralocorticoid activity and excellent anti-estrogenic activity. Since it probably has no first hepatic pass effect, GSD has 100% bioavailability [Citation17].
Cyproterone acetate (CPA)
Although CPA has an important progestational action, it is prescribed as an anti-androgenic drug, since it displaces endogenous androgens (Testosterone and dihydrotestosterone [DHT]) from their interaction with the receptor and, therefore, its net effect is generally the reduction of physiological androgenic activity for which it is considered the reference, compared to other progestogens, in the treatment of hirsutism and ovarian hyperandrogenism. However, it also represents the drug with the highest ratio of thrombotic events. It has high tissue affinity and accumulates in the adipose tissue [Citation18].
Chlormadinone acetate (CAC)
It is a molecule similar to progesterone, with a moderate anti-androgenic effect related to the increase of sex hormone-binding globulin (SHBG) levels and to the inhibition of 5α-reductase, an enzyme that converts testosterone to the more potent 5α-dihydrotestosterone [Citation19].
Drospirenone (DRSP)
It is derived from 17α-spirolactone, and it is a progestogen with strong anti-mineralocorticoid activity. Its affinity for mineralocorticoid receptors is five times greater than the affinity for aldosterone itself [Citation20]. This progestogen maintains the anti-androgenic properties of progesterone without androgenic, estrogenic, glucocorticoid, or anti-glucocorticoid activity. For this reason, DRSP can help prevent water retention, weight gain, and increased blood pressure that are sometimes associated with the use of COCs [Citation20]. It has no androgenic, estrogenic, glucocorticoid, or anti-glucocorticoid effects [Citation20].
Dienogest (DNG)
It is a fourth generation orally active progestogen, semisynthetic, non-ethinylated, derived from 19 nortestosterone; with anti-proliferative, anti-androgenic, anti-inflammatory and anti-angiogenic activity. It reduces the production of estradiol, prevents ovulation and alters the cervical mucus, and has great anti-estrogenic power in the endometrium [Citation21]. Its anti-androgenic potency is approximately 30% of that of CPA. DNG has no partial activity on glucocorticoid, anti-mineralocorticoid, or estrogen receptors [Citation21].
Nomegestrol acetate (NOMAC)
It is a highly selective 19 nor derived progestogen, with specific binding to progesterone receptors. It has anti-androgenic activity, without androgenic, mineralocorticoid, or glucocorticoid activity. Associated to estradiol it is used in contraception and menopausal hormone therapy [Citation22].
Risks related to the use of COCs and how to minimize them
COCs may adversely affect different biomarkers of cardiovascular risk and risk factors for metabolic disorders, and have an effect on serum levels of adiponectin and leptin, and on insulin sensitivity and lipid profiles [Citation23]. It has been known for more than 30 years that the use of high-dose COCs increases the risk of carbohydrate intolerance. Reduced doses of both components in current COC formulations are associated with a lower incidence of glucose intolerance. The effects of different pills on glucose metabolism appear to be determined by the combination of dose-dependent estrogen-induced insulin resistance and gestagen-induced changes in insulin half-life [Citation24]. A gestagen with androgenic properties causes a greater decrease in insulin sensitivity than a gestagen with anti-androgenic activity [Citation24]. The anti-mineralocorticoid effect of DRSP (8 times more potent than spironolactone) involves direct effects that affect the production of aldosterone by adipocytes and indirectly the activation of glucocorticoid receptors in the adipose tissue, which induced by the mineralocorticoid receptor, under general conditions, determines the gene activation of pro-inflammatory proteins. DRSP has a potent anti-adipogenic effect through transcriptional control of adipogenesis by its antagonistic action on the mineralocorticoid receptor; it decreases pre-adipocyte differentiation and triglyceride accumulation, making it a gestagen of choice in case of users with metabolic disturbances [Citation25].
VTE is the most common serious complication associated with the use of COCs. The relative risk of thrombosis among COC users is 3 to 5 times higher than in non-users [Citation26,Citation27]. This risk is an effect of the estrogenic component. There is a clear relationship between the magnitude of the risk and the estrogen dose, as well as to the type of used estrogen. In the most recent formulations, the traditionally used EE has been replaced by natural estrogens such as estradiol or estetrol with encouraging results [Citation28]. Several studies have addressed the differences in VTE risk between different formulations according to the type of progestin used in the pill. The risk appears to be higher among users of gestagen-containing preparations of the latest generations (i.e. DSG, GSD, and DRSP). This is most likely due to differences between the ability of different progestins to balance estrogen-dependent VTE risk and not to an effect of the progestin per se [Citation27,Citation28].
The risk of VTE associated with COCs is higher during the first 3 months of use [Citation29]. There are hereditary and acquired risk factors for VTE that may increase the risk alone or in combination. Risk is 35 times higher in users with a Leyden factor V mutation than in non-users [Citation26,Citation27]. These factors should be taken into account during contraceptive counseling and prescription of COCs. The risk of VTE increases with age and smoking [Citation28].
COCs have been associated with a small risk of arterial thrombosis (myocardial infarction or ischemic stroke) and are in direct relationship with estrogen dose [Citation10,Citation26].
Regarding breast cancer, a systematic review and meta-analysis of 44 published studies [Citation30] showed a significant increase in the borderline risk of breast cancer during continuous use of COCs (OR = 1.08; 95% CI: 1.00–1.17). No relationship was found between risk and duration of COC use (OR = 0.95; 95% CI: 0.83–1.09). The risk disappeared 10 years after cessation of use. This has been confirmed in a new analysis in which forty-two studies were identified that met the inclusion criteria and included a total of 110,580 women (30,778 in the breast cancer group and 79,802 in the control group; of which 15,722 and 38,334 were using COCs, respectively). COC use was associated with a significantly increased overall risk of breast cancer (OR = 1.15, 95% CI: 1.01–1.31, p = .0358) [Citation31]. In relation to time of exposure, a prospective Danish study, with a follow-up of 10.6 years and 19.6 million person-years, found in women an increased relative risk in current vs. never users of COCs (RR 1.2 95% CI: 1.14–1.26). The risk increased from 1.06 (95% CI: 0.96–1.23) among women with less than 1 year of use to 1.38 (95% CI: 1.26–1.51) in those with more than 10 years of use (p=.002). The risk decreased after discontinuation of the contraceptive, being non-significantly only slightly higher in users of more than 5 years [Citation32].
A significantly increased risk of cervical cancer has been reported in association with use of COCs for more than 5 years. As with breast cancer, the risk of cervical cancer decreases within 10 years after cessation of use. Recent analyses have revealed an independent relationship between time of COC use and cervical cancer, finding that hormonal factors may influence cervical carcinogenesis, with a relative risk of 1.6 for CIN3 and 1.8 for carcinoma in situ [Citation33]. The risk appears to be related to the persistence of high risk human papillomavirus (HPV) infection [Citation34]. The relative risk of cervical cancer was increased in ‘ever’ users of COCs versus ‘never’ users to 1.19 (95% CI: 1.10–1.29) and in current users of combined COCs to 1.40 (95% CI: 1.28–1.53), but not in gestagen-only users (RR: 0.91 95% CI: 0.78–1.07) and the risks were higher with longer duration of use, but decreased with discontinuation, with an absolute increase of 1 per 14,700 contraceptive-using women per year [Citation35].
Management of the most frequent clinical problems associated to COC use
Intermenstrual bleeding
These are more frequent at the beginning of treatment. The first thing to do is to reassure the patient; bleeding is not associated with loss of efficacy and usually resolves after the third month. These bleedings are often related to inconsistent use and smoking. Therefore, we should ask about both and give advice. Adding estrogens (i.e. 2 mg of estradiol per day) regardless of the time of the cycle or changing to COCs with a higher EE dose or with a more potent gestagen in the endometrium may help resolve the intermenstrual bleeding [Citation36].
Amenorrhea
Low doses of estrogen in some women are not sufficient to stimulate the endometrium. The effect of the gestagen dominates and endometrial atrophy occurs, which is not permanent and has no pathological consequences. Hence, the first thing to do is to reassure the patient. There is no specific treatment to prevent this amenorrhea, thus, the patient may be instructed to continue or switch to other formulations [Citation37].
Weight gain
Weight gain can be a major nuisance associated with COCs use. It may be secondary to fluid retention or to increased fat and/or muscle mass. There is a perception of weight gain with COCs use in many women; however, most studies have not demonstrated significant weight gain [Citation38]. Furthermore, discontinuation of pill use (due to weight gain) was not significantly different across groups [Citation37]. Therefore, the available evidence is insufficient to state whether hormonal contraceptives are associated with weight gain, although it seems unlikely that they have a specific effect on this matter [Citation38]. The use DRSP containing COCs, due to their anti-mineralocorticoid and anti-androgenic effect, confer clear compliance-enhancing properties [Citation39].
Mastalgia
Users with persistent breast hypersensitivity can switch to COCs containing lower doses of estrogen or another type of gestagen, such as LNG or DRSP, because of their anti-mineralocorticoid effect [Citation40,Citation41].
Changes in sexual function
There are no clear recommendations in relation to COCs use and sexual function. There is also no clear evidence as to which combined COCs affects sexuality the most and which affects sexuality the least. It is true that it has been documented that women who experience problems with desire or arousal may improve when they switch to a different hormonal formulation; however, it is disturbing not to know why [Citation42]. Not all formulations of combined COCs have been studied. Theoretically, COCs with androgenic progestins, such as LNG, would be preferred. There are other aspects of combined use that should be taken into account in relation to sexual function: breakthrough bleeding, breast tenderness and vaginal dryness, which may tip the balance toward the use of one type of combined COC or another [Citation43,Citation44].
Extends cycle oral contraceptive options currently available is specially indicated for women who do not wish to have a withdrawal bleeding [Citation45].
As mentioned, there are contraindications for the use of COCs and other methods exist today, among which is contraception with only gestagens, that has demonstrated high efficacy and safety [Citation46].
Conclusions and recommendations
It would be advisable that women with any cardiovascular risk factor, with an age above 35 years, or with any side effect related to the dose of EE, be given COCs with EE doses of 20 mcg or contraceptives combined with estradiol or estradiol valerate.
LNG is the gestagen that has reported the lowest risk of VTE, and is therefore considered to be the one conferring the greatest safety in relation to this adverse effect.
Sexuality should be dealt with more profoundly. As LNG is derived from testosterone, it is the recommended gestagen when there is a loss of libido.
CPA is considered the reference for comparison with other gestagens in the treatment of hirsutism and ovarian hyperandrogenism, being known to have a higher thrombogenic risk.
DRSP has strong anti-mineralocorticoid activity, thus, it may help prevent water retention, weight gain and increased blood pressure that are sometimes associated with the use of COCs.
Since DRSP has a potent anti-adipogenic effect, it is the gestagen of choice in cases of glucose intolerance.
When there is irregular bleeding or spotting, one should investigate the irregular use of the COC; especially if she is a smoker. If necessary, the dose of EE could be increased.
COC users with persistent breast hypersensitivity can switch to OCs containing lower doses of estrogen or another type of gestagen, such as LNG or DRSP.
In adolescents desiring COCs, it is preferable to use contraceptives with an EE content of 30 mcg, especially thinking about the maintenance or even the increase of bone mineral density.
All COCs with a 24/4-day regimen as well as those containing DRSP should be preferred in cases of premenstrual syndrome. The continuous use of the pill may be a valid option to control symptoms.
Revising the medical eligibility criteria for the use of hormonal contraceptives of the World Health Organization will always be helpful.
Disclosure statement
All the authors declare that they have no conflict of interest.
Data availability statement
Data sharing not applicable – no new data generated.
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Altshuler AL, Gaffield ME, Kiarie JN. The WHO’s medical eligibility criteria for contraceptive use: 20 years of global guidance. Curr Opin Obstet Gynecol. 2015;27(6):1–6. doi: 10.1097/GCO.0000000000000212.
- George JT, Anderson RA, Millar RP. Kisspeptin-10 stimulation of gonadotrophin secretion in women is modulated by sex steroid feedback. Hum Reprod. 2012;27(12):3552–3559. doi: 10.1093/humrep/des326.
- Speroff L, Fritz M. Anticoncepción hormonal en Endocrinología Ginecológica Clínica y Esterilidad. 7ma edición en Inglés. 72 edición en Español. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 861–948.
- ACOG Practice Bulletin. No110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol. 2010;115(1):206–218.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196(4):412.e1–412.e7. doi: 10.1016/j.ajog.2006.12.015.
- Boulkedid R, Abdoul H, Loustau M, et al. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6(6):e20476. doi: 10.1371/journal.pone.0020476.
- Beltz AM, Hampson E, Berenbaum SA. Oral contraceptives and cognition: a role for ethinyl estradiol. Horm Behav. 2015;74:209–217. doi: 10.1016/j.yhbeh.2015.06.012.
- De Leo V, Musacchio MC, Cappelli V, et al. Hormonal contraceptives: pharmacology tailored to women’s health. Hum Reprod Update. 2016;22(5):634–646. doi: 10.1093/humupd/dmw016.
- Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. 2013;27(1):3–12. doi: 10.1016/j.beem.2012.11.004.
- Stanczyk FZ, Archer DF, Bhavnani BR. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception. 2013;87(6):706–727. doi: 10.1016/j.contraception.2012.12.011.
- Palacios S, Wildt L, Parke S, et al. Efficacy and safety of a novel oral contraceptive based on oestradiol (oestradiol valerate/dienogest): a phase III trial. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):57–62. doi: 10.1016/j.ejogrb.2009.11.001.
- Mansour D, Verhoeven C, Sommer W, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. Eur J Contracept Reprod Health Care. 2011;16(6):430–443. doi: 10.3109/13625187.2011.614029.
- Fruzzetti F, Fidecicchi T, Montt Guevara MM, et al. Estetrol: a new choice for contraception. J Clin Med. 2021;10(23):5625. doi: 10.3390/jcm10235625.
- Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2003;46(Suppl 1):S7–S16. doi: 10.1016/j.maturitas.2003.09.014.
- Lachnit-Fixson U. The role of triphasic levonorgestrel in oral contraception: a review of metabolic and hemostatic effects. Gynecol Endocrinol. 1996;10(3):207–218. doi: 10.3109/09513599609027990.
- Stone SC. Desogestrel. Clin Obstet Gynecol. 1995;38(4):821–828. doi: 10.1097/00003081-199538040-00017.
- Kuhl H, Jung-Hoffmann C, Wiegratz I. Gestodene-containing contraceptives. Clin Obstet Gynecol. 1995;38(4):829–840. doi: 10.1097/00003081-199538040-00018.
- Neumann F, Töpert M. Pharmacology of antiandrogens. J Steroid Biochem. 1986; Nov25(5B):885–895. doi: 10.1016/0022-4731(86)90320-1.
- Raudrant D, Rabe T. Progestogens with antiandrogenic properties. Drugs. 2003;63(5):463–492. doi: 10.2165/00003495-200363050-00003.
- Krattenmacher R. Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception. 2000;62(1):29–38. doi: 10.1016/s0010-7824(00)00133-5.
- Foster RH, Wilde MI. Dienogest. Drugs. 1998;56(5):825–833. doi: 10.2165/00003495-199856050-00007.
- Ruan X, Seeger H, Mueck AO. The pharmacology of nomegestrol acetate. Maturitas. 2012;71(4):345–353. doi: 10.1016/j.maturitas.2012.01.007.
- Bastianelli C, Farris M, Rosato E, et al. Pharmacodynamics of combined estrogen-progestin oral contraceptives: 1. Effects on metabolism. Expert Rev Clin Pharmacol. 2017;10(3):315–326.
- Cagnacci A, Ferrari S, Tirelli A, et al. Insulin sensitivity and lipid metabolism with oral contraceptives containing chlormadinone acetate or desogestrel: a randomized trial. Contraception. 2009;79(2):111–116. doi: 10.1016/j.contraception.2008.09.002.
- Caprio M, Antelmi A, Chetrite G, et al. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology. 2011;152(1):113–125. doi: 10.1210/en.2010-0674.
- Dinger J, Do Minh T, Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception. 2016;94(4):328–339. doi: 10.1016/j.contraception.2016.06.010.
- Dragoman MV, Tepper NK, Fu R, et al. A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. Int J Gynaecol Obstet. 2018;141(3):287–294. doi: 10.1002/ijgo.12455.
- Shapiro S, Dinger J. Risk of venous thromboembolism among users of oral contraceptives: a review of two recently published studies. J Fam Plann Reprod Health Care. 2010;36(1):33–38. doi: 10.1783/147118910790291037.
- Dinger J. Oral contraceptives and venous thromboembolism: old questions revisited. J Fam Plann Reprod Health Care. 2009;35(4):211–213. doi: 10.1783/147118909789587385.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1931–1943. doi: 10.1158/1055-9965.EPI-13-0298.
- Kanadys W, Barańska A, Malm M, et al. Use of oral contraceptives as a potential risk factor for breast cancer: a systematic review and Meta-Analysis of Case-Control studies up to 2010. Int J Environ Res Public Health. 2021;18(9):4638. doi: 10.3390/ijerph18094638.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239. doi: 10.1056/NEJMoa1700732.
- Roura E, Travier N, Waterboer T, et al. The influence of hormonal factors on the risk of developing cervical cancer and Pre-Cancer: results from the EPIC cohort. PLoS One. 2016;11(1):e0147029. doi: 10.1371/journal.pone.0147029.
- Longatto-Filho A, Hammes LS, Sarian LO, et al. Hormonal contraceptives and the length of their use are not independent risk factors for high-risk HPV infections or high-grade CIN. Gynecol Obstet Invest. 2011;71(2):93–103. doi: 10.1159/000320742.
- Iversen L, Fielding S, Lidegaard Ø, et al. Contemporary hormonal contraception and cervical cancer in women of reproductive age. Int J Cancer. 2021;149(4):769–777. doi: 10.1002/ijc.33585.
- Bradley LD, Gueye NA. The medical management of abnormal uterine bleeding in reproductive-aged women. Am J Obstet Gynecol. 2016;214(1):31–44. doi: 10.1016/j.ajog.2015.07.044.
- Hillard PA. Menstrual suppression: current perspectives. Int J Womens Health. 2014;6:631–637. doi: 10.2147/IJWH.S46680.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014;2014(1):CD003987.
- Bitzer J, Paoletti AM. Added benefits and user satisfaction with a low-dose oral contraceptive containing drospirenone: results of three multicentre trials. Clin Drug Investig. 2009;29(2):73–78. doi: 10.2165/0044011-200929020-00001.
- Risser WL, Gefter LR, Barratt MS, et al. Weight change in adolescents who used hormonal contraception. J Adolesc Health. 1999;24(6):433–436. doi: 10.1016/s1054-139x(98)00151-7.
- Meade TW. Risk and mechanism of cardiovascular events in users of oral contraceptives. Am J Obstet Gynecol. 1988;158(6 Pt 2):1646–1652. doi: 10.1016/0002-9378(88)90203-7.
- Wilde MI, Balfour JA. Gestodene. A review of its pharmacology, efficacy and tolerability in combined contraceptive preparations. Drugs. 1995;50(2):364–395. doi: 10.2165/00003495-199550020-00010.
- Palacios S, Lilue M. Función sexual femenina y anticoncepción hormonal. Ginecol Obstet Mex. 2020;88(Supl 1):S178–S188.
- Tracy EE. Contraception: menarche to menopause. Obstet Gynecol Clin North Am. 2017;44(2):143–158. doi: 10.1016/j.ogc.2017.02.001.
- Burness CB. Extended-Cycle levonorgestrel/ethinylestradiol and low-dose ethinylestradiol (seasonique(®)): a review of its use as an oral contraceptive. Drugs. 2015; Jun75(9):1019–1026. doi: 10.1007/s40265-015-0407-9.
- Palacios S, Regidor PA, Colli E, et al. Oestrogen-free oral contraception with a 4 mg drospirenone-only pill: new data and a review of the literature. Eur J Contracept Reprod Health Care. 2020;25(3):221–227. doi: 10.1080/13625187.2020.1743828.