Abstract
Purpose
To illustrate the results achieved by genes in premature ovarian insufficiency (POI) and collaborations in the field, and to explore key themes and future directions.
Methods
Articles and reviews related to POI genes published between 1990 and 2022 were retrieved from the Web of Science core collection (WoSCC) for the total bibliometric analysis. Tools were analyzed for publication, country, institution, journal, authors, reference, keywords, subject categories, funding agencies, and research hotspots using a bibliometric online analysis platform, Bibliographic Co-occurrence Matrix Builder (BICOMB), CiteSpace V, and VOSviewer.
Results
A total of 2,232 papers were included in this study. Articles were published in 52 countries, with the United States publishing the most, followed by China. A total of 308 institutions contributed to relevant publications. Shandong University published the most papers. Qin Y’s team published the most relevant papers. Human reproduction and fertility and sterility are the two journals with the most papers. X-chromosome abnormalities, transcription factor mutations, and FMR1 genes are the directions of more POI, and DNA repair is the keyword of the research frontier in recent years.
Conclusions
This study summarizes the relevant literature on POI gene research for the first time and analyzes the current hotspots and future trends in this field. The findings can further reveal the etiology, diagnosis, and treatment of POI, which is beneficial for researchers to grasp the genetic dynamics of POI women.
Introduction
Premature ovarian insufficiency (POI) is a clinical syndrome in which women develop ovarian dysfunction (follicular depletion, endocrine function, and regression or loss of reproductive function) before the age of 40 years, with a diverse, complex, highly heterogeneous, and progressive clinical presentation [Citation1]. The name, definition, and exact diagnosis of POI have been controversial, during which many other terms have been used, such as primary ovarian insufficiency, premature ovarian failure, and premature menopause [Citation2]. The 2016 European Society of Human Reproduction and Embryology (ESHRE) guidelines interpret and recommend POI [Citation3]. POI is often designated as spontaneous or idiopathic POI because its etiology is largely undetermined. The identified causes of POI that may involve these mechanisms have been categorized into two groups: genetic and non-genetic causes. Genetic causes include various genetic abnormalities, and nongenetic causes include autoimmune and metabolic disorders, infections, environmental factors, and medical manipulations. Chromosomal abnormalities, genetic polymorphisms and single-gene mutations have long been recognized as causes of POI, with chromosomal abnormalities explaining 20–25% of POI cases [Citation4]. Despite intensive research on the genes responsible for ovarian decline, however, identifying the exact causative gene for POI has been a challenge. A comprehensive understanding of the influence and application of genes in POI is necessary to grasp a complex disease such as POI.
Researchers revealed gene copy number changes, gene sequence variations, and multiple candidate genes in patients with POI based on whole genome and whole exome sequencing. Most of the genes responsible for female infertility/low fertility are expressed in the ovary, except for those encoding gonadotropins [Citation5]. Based on whole-exome sequencing, a number of individual gene mutations as well as gene function in gene deletion pattern organisms have been identified in patients with POI [Citation6]. POI genes are usually associated with partial defects in individual gene function or loss of functions of a few genes. A large number of mutations in oocyte genes essential for meiosis and DNA repair have been identified in POI patients, and mutations and low expression of these genes are responsible for POI and follicular failure [Citation7]. As well as genomewide based advances, genetic studies have identified additional candidate genes responsible for POI and ovarian senescence. Candidate genes can be classified into ligand receptor signaling, meiosis and DNA repair, transcription factors, RNA metabolism, and enzymes [Citation8]. In addition, several POI genes, including several receptor genes (LHCGR, FSHR, IGF2R, and TGFBR1), and several ligand genes (FSHB, IGF1, and TNF) are also associated with female menopause and are causative genes for POI [Citation9]. The genetics research of POI is becoming more and more perfect, but there is a lack of systematic discussion on the research progress, research status, and future research frontiers in this field. For women with premature ovarian failure and fertility requirements, early diagnosis and intervention are necessary, so further construction of POI gene profile is of great value for the diagnosis and treatment of POI.
Bibliometrics is a scientific method based on the characteristics of the literature system and bibliometrics, which uses applied mathematics and statistics and other measurement methods to analyze the distribution structure, quantitative relationship and change law of the literature. We analyzed papers related to POI gene research in WoSCC, covering different countries, regions, and institutions. The trends and research directions of POI gene research were summarized and described by co-occurrence, citation and co-citation to provide a basis for the development of future POI clinical guidelines. This study is a timely, intuitive, and unbiased approach to qualitative and quantitative assessment of research trends and characteristics in the literature database using statistical methods and graphical visualization of the interactions. These analyses can provide obstetricians and gynecologists with a macroscopic understanding and microscopic characterization of the entire field of knowledge.
Materials and methods
Data source
The WoSCC Bibliographic Database is one of the largest and most comprehensive electronic scientific literature databases in the world [Citation10], containing over 12,000 influential journals. It is widely recognized as the most comprehensive and reliable database for bibliometric analysis compared to other databases such as Scopus and PubMed [Citation11]. The WoSCC database is currently used for a large number of bibliometric studies, and the documents retrieved from this database ensure that the conclusions are reliable and authoritative and contain all the fields analyzed by the software. Data sources for literature searches are from the Web of Science Core Collection (WoSCC), and citation indexes include Science Citation Index (SCI-Expanded)—1900-present, Social Science Citation Index (SSCI)-1900-present, Arts and Humanities Citation Index (AHCI)-1975-present, Conference Proceedings-Science (CPCI-S)- 1990-present, Conference Proceedings-Social Sciences and Humanities (CPCI-SSH) −1990-present, Emerging Sources Citation Index (ESCI)-1990-present, Conference Proceedings-Social Sciences and Humanities (CPCI-S)-1990-present, Science (CPCI-S)-1990-present, Conference Proceedings-Social Sciences and Humanities (CPCI-SSH)−1990-present, Citation Index to Emerging Sources (ESCI)-2017-present, (CCR-Expanded)-1985-present, (IC)−1993-present. The search date is August 10, 2022.
Search strategy
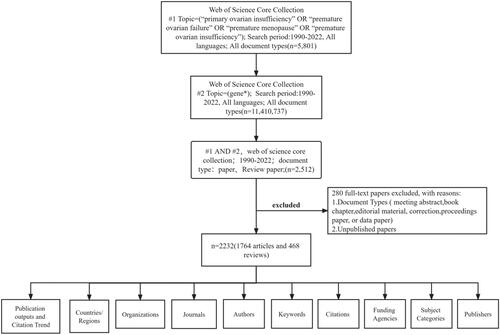
The data retrieval strategy was: Subject: (‘primary ovarian insufficiency’ OR ‘premature ovarian’ OR ‘premature ovarian failure’ OR ‘premature menopause’ OR ‘premature ovarian insufficiency’) and subject: (gene*). The literature was published from the date of creation (January 1, 1990) to July 03, 2022. An asterisk (*) was used to search for related/derived terms to increase the scope of the search. The above keywords were selected from the Medical Subject Headings (MeSHs) provided by the National Library of Medicine (NLM)/PubMed. The search is not limited to any category or language. shows the specific search process, as well as the final results obtained. The results were independently validated by two authors (ZX and SWY). We collected the following basic information for each article: title, abstract, author, institution, country/region, journal, keywords, and references.
Exclusion criteria
Exclusion criteria were (1) publications involving book chapters, conference proceedings, conference abstracts, editorial materials, web publications, errata files, data files, correspondence, news, and retracted publications; and (2) unpublished literature that did not have sufficient information for further analysis. A total of 280 papers were excluded.
Data analysis
The WoSCC database has built-in features for analyzing publication characteristics, including publication output, country, institution, author, keywords, and journal citation relationships. Data are extracted from the WoSCC database and exported as ‘tab-delimited text files’, ‘excel files’, ‘plain text’ for additional processing. Microsoft Excel was used to plot the literature searched in the WoSCC database, create trends in publication and citation charts, and rank countries, institutions, disciplines, and authors. In this study, we used VOSviewer (Leiden University, the Netherlands), CiteSpace V (Drexel University, Philadelphia, PA, USA), and the bibliometric online analysis platform (https://bibliometric.com/) for a joint analysis to construct a relevant knowledge graph.
CiteSpace V is the most widely used bibliometrics software, focusing on dynamic visualizations that reflect the evolution of bibliometric networks over time. In this study, it was used to analyze and visualize the most strongly cited co-cited references, co-cited authors, co-cited journals, cited reference bursts, keyword co-occurrences, keyword citation bursts, keyword clustering, co-cited references clustering, and timelines over a given period of time. CiteSpace parameter settings: time spanning January 1990 to September 2022, with a time slice of 1 year. Default values were followed for text processing, node type, link strength and range, and selection criteria. The node types are author, organization and keyword, TopN = 50, TopN%=10.0%, and the pruning methods are pathfinder and pruning sliced networks, while the rest of the parameters are kept at the default settings.
VOSviewer is a distance-based bibliometric tool that focuses on the visualization of bibliometric networks. VOSviewer software analyzes the types of Co-authorship, co-occurrence, Citation, co-citation, and the unit of analysis is Authors, Organizations, Countries, Documents, Sources, Author keywords, and cited references. Counting mode is full counting, set the minimum citation frequency, the minimum number of articles is 5, and the rest of the parameters are the default settings of the software. Generate the maps of authors, organizations, countries and co-occurrence of literature and distribution of journals.
The bibliometric online analysis platform provides bibliometric analysis of scientific citation data in an intuitive and user-friendly manner. Based on the platform, cooperation between countries was visualized and analyzed.
A node represents an element, such as country, institution, author, journal, reference, and keyword. The links between nodes reflect the partnerships between items. A larger link width between nodes indicates a stronger collaboration, while the size of a node represents the number, with a larger size indicating a larger number of publications. Nodes with high centeredness indicate turning points or critical points related to the domain. Clusters of network nodes are distinguished by color.
Results
Publication outputs and citation trend
In the WOS core library, there were 11,410,737 and 5,801 entries for genes and POI, respectively. After excluding 280 conference abstracts and other conference paper materials, a total of 2232 articles (70%) and 468 reviews (18%) were obtained.
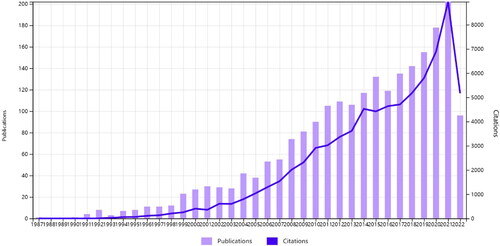
shows the trend in the number of publications and citations of literature related to POI genes. POI genetic research activity peaks in 2022. Research on POI and genes can be divided into four phases: the first phase, from 1992 to 2005, had a slow growth rate and was largely devoid of relevant studies. The second phase, from 2005 to 2011, showed a largely steady growth trend in the number of publications. In the third stage, from 2011 to 2017, the number of literature on genes fluctuated and remained stable at around 110. Publications on POI genes are in a steady state, accompanied by the maturation of sequencing technologies. The fourth stage of rapid growth from 2019 to 2022, with a peak in 2022 when all publications were cited 10,069 times, with an average of 22.33 citations per item and an h-index of 22.33.
Countries/regions
The co-authorship analysis of countries () shows that the United States, China, France, and the United Kingdom are the countries with more published POI genes, while Italy and Germany are emerging forces. The global geographic distribution of productivity () shows that 52 countries/regions are involved in the publication of POI genes. The top 10 countries/regions with the most publications in POI gene research are listed in . The most literature on the role of genes in POI is mainly from the United States (665), China (448), France (212), and the United Kingdom (188), and the United States also has the highest citation frequency (34883) and centrality (512). Although China has more literature on POI genes than France, it has lower citation frequency and centrality. As shown in the top 10 countries/regions with the strongest citation burst (), China had the highest burst intensity (29.83), but the late citation burst, appearing in 2019–2022, indicates that a large number of Chinese researchers joined the ranks of those studying POI genes. The citation outbreaks in the United Kingdom and the United States were 8.19 and 7.77, respectively, but both citation outbreaks occurred before 2000, indicating that they were the main countries that initially explored the role of genes in POI.
Figure 3. (A) Co-authorship analysis of countries. The analysis was performed using VOSviewer 1.6.14 with the method linlog/modularity, weighted by the number of citations, and scored as the average year of publication. The thickness of the lines indicates the strength of the relationship. The color indicates the average year of publication. Five occurrences were included only. (B) National/regional collaborations leading to POI-gene publication from 1990–2022. (C) Countries with the highest number of citations in POI-gene papers from 1990–2022.
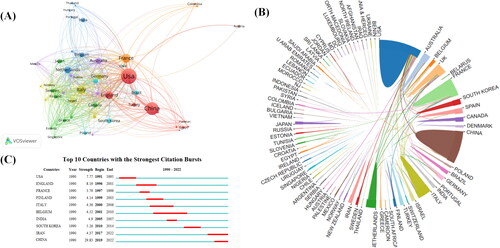
Table 1. Top 10 countries/regions and institutions with the most published POI-genes papers from 1990–2022, along with citation frequency and total linkage intensity.
Analysis of organizations
lists the top 10 high-producing institutions, with seven in the United States and one each in Italy, China, and Finland. Shandong University topped the list with 71 (20.88%) POI genes, with 2247 citations and 2883 total link strengths. shows that Shandong University is the institution with the most collaborations with other organizations. However, the total number of publications (210) and citations (18,951) of the seven institutions in the United States are much higher than that of Shandong University, with the University of California Davis being the best ().
Table 2. The top 10 organizations with the highest output in POI gene research from 1990 to 2022.
Table 3. Ten journals with the highest number of publications in POI-genes from 1990 to 2022.
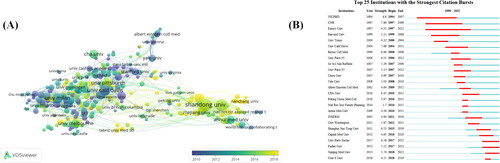
The co-occurrence relationship also showed that Shandong University, University of Milan, Fudan University, University of California Davis, and Emory University were the top 5 cited organizations (). These data indicate that institutions in Chinese, the United States, and Italy continue to dominate in POI genes. The yellow nodes indicate an average publication year of 2018, suggesting that organizations with yellow nodes may be emerging research institutions in the field, and Fudan University is the institution that recently published more articles on POI genes. For the institutional burst monitoring (), the top three items were University of California Davis bursts from 2004–2011, China bursts from 1997–2009, and Baylor College of Medicine bursts from 2006-2008.
Figure 4. Co-authorship analysis of organizations. (A) Analysis was performed using VOSviewer 1.6.14 with the method linlog/modularity, weight as a number of citations, and score as the average year of publication. The thickness of the lines indicates the strength of the relationship. The color indicates the average year of publication. Five occurrences were included only. (B) The 25 organizations with the strongest CiteSpace citation burst. ɣ:1.0, minimum duration:2.
Journals
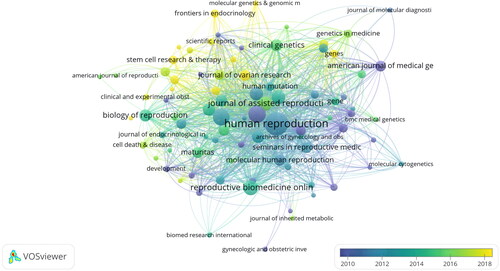
shows the main distribution of POI genes in different journals and the top 10 journals, the most numerous of which is HUMAN REPRODUCTION with 122 publications. The impact factors of these journals in 2022 ranged from 5.00 to 23.20; among them, HUMAN REPRODUCTION UPDATE, HUMAN MOLECULAR GENETICS, FERTILITY AND STERILITY, and HUMAN REPRODUCTION had the highest. By JCR partition analysis, Q1 is 20%, Q2 is 50% and Q3 is 30%. HUMAN REPRODUCTION is probably the most popular journal in terms of number of papers and impact factor ().
Figure 5. Citation analysis of journal. The analysis was performed using VOSviewer 1.6.14 with the method linlog/modular, weighted by citation volume, and scored as the mean year of publication. The thickness of the lines indicates the strength of this relationship. The color indicates the average year of publication. Five occurrences were included only.
Authors
The analysis of core authors helped to explore the distribution of the literature. Core authors were evaluated based on criteria including volume of published literature, total citations, and h-index. 10822 authors were involved in POI gene research.
lists the top 10 core authors in the field from 1990–2022. Qin Y and his colleague Chen Z of Shandong University were among the top three. Qin Y ranked first with 53 papers and 1365 total citations. However, Veitia RA from the University of Diderot ranked first in terms of total citations (1460), with only 25 papers. This indicates that Veitia RA's research in the field of POI genes has been recognized by more scholars. In addition, Rajkovic A and Veitia RA of the University of California have 25 papers respectively, with a total of 1213 and 1301 citations. They are also highly recognized teams.
Table 4. Ten core authors with the highest number of publications in POI-genes from 1990 to 2022.
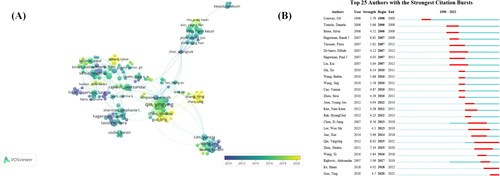
As shown in ., the overlay visualization shows the co-authorship of authors, with 224 authors. The visualization map shows that Qin Y, Zhao S and Guo T (Shandong University) collaborate closely, indicating that they work closely in the field, and the average publication year is 2018-2020, indicating that they are the rising force in the research field in recent years. In addition, some emerging researchers (Yellow Dot) and teams are starting to converge in the field of POI genetics, indicating that the field remains a hot spot. For author outbreak monitoring, the top one is Qin Y and Zhao S outbreak from 2015 to 2020 with the longest duration, followed by the Hagerman RJ outbreak from 2007 to 2011, and Ma X outbreak from 2010 to 2011.
Keywords
A total of 5951 author keywords were involved in 2232 documents, of which 256 reached the threshold (minimum number of documents for a keyword:5). network visualization graph shows the co-occurrence relationship of keywords. The size of the circles indicates the occurrence of keywords. Among them, POI, POF, menopause, infertility, fragile x syndrome, and ovary are high-frequency keywords, and only POI has an average publication year in 2018, while the rest of the keywords were used sequentially in the field from 2010–2016. shows the top 25 keywords with the strongest citation explosion. Excluding POI and follicle-stimulating hormone, genetics and oxidative stress had the highest burst intensity with 14 and 13.83, respectively. it can be seen that the burst of X chromosome, intellectual disability, premutation vector, FMRI messenger RNA, transcription factor FOXL2, bone morphogenetic protein, growth differentiation factor, chromosomal instability, whole exome sequencing, and DNA repair were also higher, which are all about POI gene studies. keyword burst shows that DNA repair, copy reduction and POI are the hot spots of research in recent years, and many scholars are devoted to this field. Whole-exome sequencing is a common test for POI genes. Transplantation is also the main treatment considered for POI patients in recent years.
Figure 7. Co-occurrence analysis of the authors keywords. (A) The analysis method is modularity in linlog/VOSviewer, with a weight of one event and a score of the average year of publication. The thickness of the lines indicates the strength of the relationship. The color indicates the average year of publication. (B) Top 25 keywords with the strongest CiteSpace citation burst. ɣ:1.0; minimum duration:2. (C) Relationships between genes and other authors’ keywords were performed by network visualization. Figure 8. Analysis of co-references. (A) Top 20 documents with the strongest CiteSpace citation burst. ɣ:1.0; minimum duration:2. Light color indicates the time range from 1990-2022 statistics, dark cyan indicates the time range from publication to the strongest citation burst, and red indicates the duration of the strongest citation burst. (B)The analysis method is modularity in linlog/VOSviewer with weights of citation counts. The thickness of the lines indicates the strength of the relationship. The purple part of the circle indicates the Central position of the document.
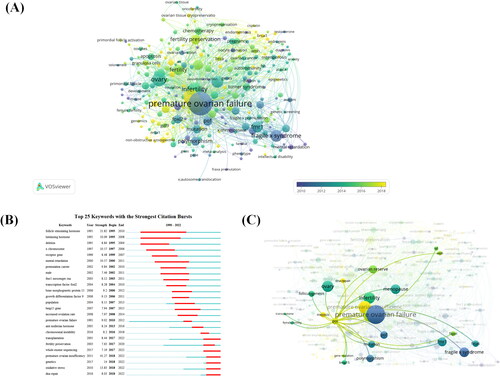
Figure 8. Analysis of co-references. (A) Top 20 documents with the strongest CiteSpace citation burst. ɣ:1.0; minimum duration:2. Light color indicates the time range from 1990-2022 statistics, dark cyan indicates the time range from publication to the strongest citation burst, and red indicates the duration of the strongest citation burst. (B)The analysis method is modularity in linlog/VOSviewer with weights of citation counts. The thickness of the lines indicates the strength of the relationship. The purple part of the circle indicates the Central position of the document.
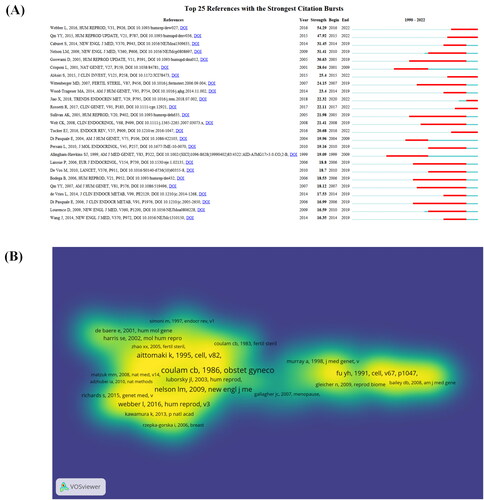
Cited references
According to the literature citation analysis, which reflects how the literature is cited, lists the top 10 most highly cited, with citations ranging from 147-225. ‘Clinical practice. primary ovarian insufficiency’ (2009), with 225 citations; K Aittomäki 1995, ‘Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure’ (1995), with 212 citations; and thirdly, ‘Premature ovarian failure’, with 197 citations. The next is about the exploration of the genetic mechanism of POI, and the results show that FMR1 repeat size and Fragile X premutation are important causes of POI. The top 25 papers with the strongest citation burst are given in . The article published by Webber L et al. showed the highest burst of 54.29 from 2016 to 2020, and the article published by Qin Y et al. also showed a good burst (47.92) from 2015–2022.
Table 5. Top 10 most cited POI-gene articles from 1990 to 2022.
Funding agencies
A total of 1709 funding institutions have funded some research in this field. lists the top ten funding agencies that provide the most funding for POI genes, with the United States Department of Health and Human Services (HHS), the National Institutes of Health (NIH), and the National Science Foundation (NSF) being the top three sources of funding. The United States provides the main funding for POI gene research.
Table 6. The 10 fund institutions that received the most funding for POI gene papers from 1990 to 2022.
Subject categories
In the disciplinary ranking (), POI genetic literature was concentrated in the disciplines of Obstetrics and Gynecology, Reproductive Biology, Genetic Genetics, and Endocrinology Metabolism. Obstetrics Gynecology published the most papers (667), accounting for 29.87%. This was followed by Reproductive Biology with about 546 papers, accounting for 24.45%.
Table 7. The 10 disciplines that published the most POI gene papers from 1990 to 2022.
Discussion
This study analyzed 2,232 SCI papers related to POI gene research published from 1990-2022. The number of annual publications on POI genes has increased consistently over the decades of involvement in this work and peaked in 2021. Based on the bibliometrics, it can be seen that there are fewer types of article reviews and insufficient generalization of genes, and more research is predicted to be conducted. Currently, the countries, organizations, authors, and journals that mainly study POI genes are dominated by the United States and China. This may be related to the Human Genome Project (HGP) initiated by the National Institutes of Health (NIH) in 1990 and the ‘Precision Medicine’ strategic plan in 2015 [Citation12]. Among them, HHS, NIH, and NSF, which are the three major health protection agencies in the United States, are the main sources of funding for POI gene research, thanks to the increasing investment in basic research in the United States since the 1950s, and they are also the agencies with the largest amount of funding from the U.S. government. The country with the second highest number of publications is China, which may be related to the emphasis placed on human genome research by China’s National Natural Science Foundation and National Key Research and Development Program. In the discipline rankings, the POI genetics literature is published primarily in obstetrics and gynecology and reproductive biology, which also suggests that POI females require lifelong management and are a disease that severely impacts women’s reproductive health.
According to the literature analysis, groups such as Qin Y's team, Chen ZJ's team and Rajkovic A's team are still leading in this field. There is a collaboration between Qin Y's team and Chen ZJ's team, working on the mechanisms of genetic mutations in POI patients [Citation13,Citation14], affecting oocyte-specific transcription factors [Citation15], and reporting on the genetic characteristics of many Chinese women with POI [Citation16,Citation17]. Likewise, Rajkovic A himself has made many elaborations on genetics, which are highly cited and internationally recognized, although the number of publications is low. Tassone F [Citation18] reported multiple cases of fragile x mutant women, with a total of 1301 citations, from which the researchers obtained a high reference value. Allingham Hawkins D J [Citation19] published an article in 1999 on the importance of fragile X chromosome pre-mutation as a risk factor for premature ovarian failure. This study was jointly completed by countries such as Canada, the United States, Brazil, the United Kingdom, Italy, Greece, etc. The article cited 147 references, providing researchers with great research ideas and references. Rajkovic A. and Tassone F. are both affiliated with the University of California but have not seen any relevant collaborations that could further strengthen ties. In France, the team of Touraine P is also a stalwart in interpreting the genetics of POI patients. Cao YX from Anhui Medical University is in close contact with Wang B from Peking Union Medical College and Wang J from Capital University of Medical Sciences, who are working together on POI genetics mapping. It is recommended that researchers from different countries continue to maintain close cooperation and share the latest research results in POI genetics.
In terms of research focus, studies on POI female genes have mainly concentrated on population, fertility preservation, and testing methods. The exploration of POI female genes by researchers from 1995–2010 was still in a relatively macro and superficial stage. Initially, a series of comparisons were made about the similarities and differences between POI female genes and normal healthy female genes. The impact of POI female genes from different populations was also a hot research topic during this period. Analyzing the top 10 most cited articles on POI genes, researchers first attempted to determine the genetics affecting women with POI, starting with the X chromosome. Experimental studies typically include gene knockouts, organic pathologic processes, and the effects of hormone therapy on the gene in women with POI. Observational studies typically include genetic studies of individual cases of POI patients, as well as landscapes of various pathogenic mutations. In addition, research testing techniques to study the genome of women with POI include genome sequencing analysis, whole exome sequencing, gene microarray, and fluorescence in situ hybridization, among which whole exome sequencing explodes in 2017–2022 and is an important and effective means of accurate diagnosis, guided treatment, and disease prevention in women with POI. Researchers can choose different technologies to decipher the genetic code according to their needs.
The keyword ‘consanguinity’ is not obvious, which may be because the research results are not generalizable. As direct relatives generally lead to mutations at the locus, these studies may make it easier to pinpoint the genetic factors contributing to POI. Qin Y et al. [Citation20] identified novel variants in the SOHLH2 gene in both Chinese and Serbian-origin women with premature ovarian failure. However, some studies have shown differences in gene regulatory pathways and gene causes between ethnic groups. Qin Y et al. [Citation21] found no association with the four tested genes 8q22.3, HK3, ESR1, and BRSK1 in Serbian women with premature ovarian failure compared to Chinese women. The team of Rah H and Kim NK has been working on genetic polymorphisms in premature ovarian failure in Korean women. Since 2011, they have continued to explore vascular endothelial growth factor (VEGF) [Citation22], kinase insertion domain-containing receptor (KDR) gene, methylenetetrahydrofolate reductase (MTHFR) gene variants (MTHFR 677 C > T), thymidylate synthase (TS) gene variants [Citation23,Citation24], microRNA (miRNA) [Citation25], and Hox transcript antisense RNA (HOTAIR) polymorphisms [Citation26] whether they confer a risk of premature ovarian failure in Korean women. In 2020, genome-wide association analysis of 564 women of Korean descent was performed to search for novel candidate genes predictive of influencing the development of premature ovarian failure [Citation27]. Kim NK’s team has interpreted the genetic landscapes of Korean patients with premature ovarian failure over a long period and has accumulated and systematically managed gene polymorphisms for a premature failure-specific population by contributing to a range of genetic data. However, the team’s article citation is only 208, which may be due to the population-specific study limiting its reference application. Focusing on a specific population of women with POI has also helped us to target the treatment of women with POI and develop improved treatment plans.
Analyzing the keyword outbreak, the X chromosome was the focus of research in the genetic startup phase of POI females, with the main outbreak occurring from 1997 to 2006. The overall prevalence of chromosomal abnormalities in POI females was 12.1% and was dominated by the prevalence of X structural abnormalities, X autosomal translocations, and X aneuploidy [Citation28]. Fu YH et al. [Citation29] discussed in Cell that the variation of CGG repeat sequences at the fragile X site leads to genetic instability, which has been widely recognized. In 2004, the latest keyword FOXL2 gene, encoding a forkhead transcription factor and one of the earliest ovarian markers, emerged as a citation outbreak. In 2011, Crisponi L et al. [Citation30] reported that FOXL2 is mainly present in adult ovaries, and this article is highly cited. In addition, the Veitia RA team has provided many pieces of evidence on the impact of FOXL2 on ovarian function [Citation31,Citation32]. The bone morphogenetic protein-15 (BMP-15) keyword has been a highly regarded oocyte-derived growth factor since its continuous outbreak in 2007 until 2015. Margulis et al. [Citation33] showed that BMP-15 was expressed in the primordial follicles of fetuses between 21 and 33 weeks of age and in women between 5 and 39 years of age and that the secretion of sufficient BMP-15 in the follicular fluid is essential for the maintenance of normal fertility in women. The defective production of biologically active proteins as a result of heterozygous mutations in BMP-15 has been suggested as a possible mechanism for the development of POI. The most frequent outbreaks in the last five years have been about fertility and treatments for women with POI, namely ‘conservation’, ‘transplantation’, and ‘DNA repair’, which may be due to the severe population loss and the increasing number of women with POI worldwide through a series of fertility treatments. The world is adopting a series of fertility support policies. Transplantation is also currently the most effective technique to address the fertility needs of women with POI. In addition, transplantation of mesenchymal stem cells (MSCs) has been shown to functionally restore ovarian reserve in mouse models of POI [Citation34]. ‘DNA repair’ is the different repair responses of cells to various causes of DNA damage. MCM8, MCM9, and CSB-PGBD3-related mutations causing impaired chromosome strand break repair are the main cause of POI pathogenesis [Citation5], and Veitia RA [Citation35] has also demonstrated that pathogenic variants in ‘DNA repair and meiotic genes’ underlie the pathogenesis of POI.
Most of the research themes in recent years have centered on the prevention and diagnosis of POI, and deciphering the genes of women with POI has become a major avenue for managing women’s health and taking preventive measures. The article published by Webber L et al. [Citation3] is the strongest citation outbreak and discusses health management programs for women with POI. It was shown that the loci leading to POI involve a wide range of DNA damage response (DDR) processes and include loss-of-function variants in key DDR-related genes [Citation36]. Numerous experimental results suggest that these DDR processes play a role throughout the life course, including shaping ovarian reserve, bone health, and diabetes [Citation37,Citation38]. FOXO3 not only contributes to premature ovarian failure in women but is also thought to affect insulin/insulin growth factor signaling and induce diabetes [Citation39]. Extending reproductive life in women with POI improves bone health and reduces the risk of suffering from complications such as diabetes, and cardiovascular disease. In addition, the second most cited outbreak was published by Qin Y et al. [Citation40], which argued that analyzing candidate genes is slow and that family-based whole-exome and whole-genome sequencing is the most promising approach for detecting potential genes that contribute to POI. It is now generally accepted that the use of exome sequencing for well-validated definitive selection of causative variants maximizes the chances of a POI diagnosis and suggests sibling or offspring risk. As early as 1998, Conway GS et al. [Citation41] screened for the genetic marker Fragile X Chromosome (FRAXA) pre-mutation and showed that FRAXA screening was particularly valuable in predicting premature ovarian failure. The data suggest that FOXO3 [Citation42], miR-22-3p [Citation43], and miR-4516 [Citation44] are diagnostic markers for premature ovarian failure. Increasingly, genetic testing and genetic counseling are particularly important for first- or second-degree relatives with POI. Expanding and identifying the key regions affecting the POI phenotype could improve their reproductive and genetic counseling and relieve psychological stress. However, POI, as a highly heterogeneous and fluctuating disease with a wide range of complex etiologies, setting prediction criteria remains limited.
Notably, researchers are often inspired by randomized controlled trials to explore the mechanisms by which genetic abnormalities cause POI and to treat POI through gene therapy. However, animal models have been lagging behind human studies, and investigating the mechanisms by which genes are involved in the complex crosstalk of POI requires enhanced basic research. A large number of genes have been shown to be associated with the development of POI, and most of the studies are from clinical randomized controlled trials with large samples and are reliable. Some of these studies have reported only patient-specific genetic problems in POI. Although a number of important locus mutations have been identified that are thought to be associated with the development of POI, any of the mutations currently thought to be associated with POI have not been confirmed in multiple populations, and previous studies on the relationship between a particular mutation and POI have often yielded different conclusions from different studies. Therefore, a large-sample, multi-population study is necessary not only for the etiologic study of POI, but also for prenatal screening, and to lay the foundation for gene therapy. In the future, we need to explore the relationship between genes and women with POI at the level of molecular mechanisms, and how to utilize these known causative genes to prevent the premature decline of ovarian function.
POI is widely recognized as a polygenic genetic disease, and deciphering the genetic code and genetic information is the first step to elucidate its pathological mechanisms. The literature-based visualization analysis shows the current research status of the field of POI genetics more clearly, providing researchers with the complete system, research hotspots and development trajectory of the field. This can provide a reference for researchers who are looking for the research direction of POI genetics. In addition, the systematic summarization of genetic defects in POI patients can also help researchers to fully or partially explain the mechanism of action of POI prevention and treatment drugs. Currently, POI treatments are mainly focused on improving symptoms and preserving fertility, and treatment ideas need to be broadened. Through this study, researchers can quickly understand the genes and patterns of POI genetic pathogenesis and expand clinical thinking and treatment options. With the continuous advancement of precision medicine, research in the field of POI genetics is bound to become more in-depth, and the trend of multidisciplinary cooperation will also promote the development of the field. This study establishes a clear framework based on the existing research in the field of POI genetics is favorable to the implementation of precision medicine, provides information value, and makes scientific guidance for clinical practice.
This study has some limitations. In this study, only articles from the WoSCC database were searched. Although the use of the WoSCC database for high-quality bibliometric analyses has been widely accepted by researchers, there are still some studies that may be genetically relevant to women with POI that were not included, which may have altered the results of the study. Secondly, CiteSpace and VOSviewer can only analyze a limited number of nodes such as authors, organizations, keywords, etc., and cannot capture the content of the article, which reduces the comprehensiveness of the findings and may result in a certain amount of analysis bias. Finally, this study analyzed the overall publication trends and research hotspots of genes in POI from 1990 to 2022, and did not provide a systematic and precise analysis of publications. Future research could cover a wider range of databases (e.g. PubMed, Google Scholar, and Scope) for the purpose of more comprehensively analyzing the dynamics of research in the field. Inclusion of papers published in other languages in other databases may increase the rigor of the study. In addition, a variety of visualization tools can also be used comprehensively to ensure the objectivity of its research results, to carry out a systematic and precise analysis.
Emerging trends and outlook
Taken together, we hypothesize that more and more researchers are interested in the role of genes in POI, which remains a research hotspot, and that the literature related to POI genes will continue to increase over the next decade. We recommend more active collaboration between countries, institutions, and authors for large-sample clinical studies and basic research related to gene POI. Whole genome sequencing is an effective and reliable technology option. Ongoing research focusing on summarizing patterns in terms of country, race, and lineage is the main way to gradually improve the genetic map of patients with POI. In addition, this study has found that ‘DNA repair’ and ‘copy number reduction’ are emerging and active themes in recent years, and further exploration of them may bring surprising results. Currently, research on the genetic etiologic mechanisms of POI focuses on the link between gene mutations and deletions, X chromosome abnormalities, DNA damage repair, oxidative stress, apoptosis, and autophagy. The research hotspots in this field are mainly focused on fertility preservation, gene therapy, and the mining of genes related to ovarian aging. Future research trends in this area are predicted to be the pathogenic mechanisms of cell death, DNA damage and repair pathways, as well as the application of MSCs transplantation in POI treatment.
Conclusions
This bibliometric study is the first to analyze publications on POI genes from around the world. Based on these literatures, the research bases, research hotspots and future development trends of POI genes were analyzed. The number of documents has increased every year. With the gradual expansion of the scope of research and the depth of research, the diagnosis and treatment of POI are developing in the direction of precision medicine. This study reveals trends and characteristics of genes in women with POI, which can help to develop effective prevention strategies and treatment trials targeting genetic inheritance and provides a useful bibliometric analysis for researchers to conduct further studies.
Author contributions
XZ collected and processed data, wrote and revised manuscripts. WYS provided comments and expertise on the manuscript, XJL checked the material data, and WZ revised and approved the final manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The dataset provided in this study can be found in the https://www.webofscience.com/wos/alldb/basic-search. The article/supplement contains the original research. For further requests, please contact the corresponding author directly.
Additional information
Funding
References
- Chon SJ, Umair Z, Yoon MS. Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biol. 2021;9:1. doi: 10.3389/fcell.2021.672890.
- Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68(4):499–12. doi: 10.1111/j.1365-2265.2007.03073.x.
- Webber L, Davies M, Anderson R, et al. Eshre guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937.
- Jiao X, Ke H, Qin Y, et al. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29(11):795–807. doi: 10.1016/j.tem.2018.07.002.
- Yang Q, Mumusoglu S, Qin Y, et al. A kaleidoscopic view of ovarian genes associated with premature ovarian insufficiency and senescence. Faseb J. 2021;35(8):e21753. doi: 10.1096/fj.202100756R.
- Turkyilmaz A, Alavanda C, Ates EA, et al. Whole-exome sequencing reveals new potential genes and variants in patients with premature ovarian insufficiency. J Assist Reprod Genet. 2022;39(3):695–710. doi: 10.1007/s10815-022-02408-0.
- Huang C, Guo T, Qin Y. Meiotic recombination defects and premature ovarian insufficiency. Front Cell Dev Biol. 2021;9:652407. doi: 10.3389/fcell.2021.652407.
- Jaillard S, Bell K, Akloul L, et al. New insights into the genetic basis of premature ovarian insufficiency: novel causative variants and candidate genes revealed by genomic sequencing. Maturitas. 2020;141:9–19. doi: 10.1016/j.maturitas.2020.06.004.
- Ke H, Tang S, Guo T, et al. Landscape of pathogenic mutations in premature ovarian insufficiency. Nat Med. 2023;29(2):483–492. doi: 10.1038/s41591-022-02194-3.
- Ma C, Su H, Li H. Global research trends on prostate diseases and erectile dysfunction: a bibliometric and visualized study. Front Oncol. 2020;10:627891. doi: 10.3389/fonc.2020.627891.
- Wu H, Li Y, Tong L, et al. Worldwide research tendency and hotspots on hip fracture: a 20-year bibliometric analysis. Arch Osteoporos. 2021;16(1):73. doi: 10.1007/s11657-021-00929-2.
- Auffray C, Caulfield T, Griffin JL, et al. From genomic medicine to precision medicine: highlights of 2015. Genome Med. 2016;8(1):12. doi: 10.1186/s13073-016-0265-4.
- Qin Y, Shi Y, Zhao Y, et al. Mutation analysis of nobox homeodomain in chinese women with premature ovarian failure. Fertil Steril. 2009;91(4 Suppl):1507–1509. doi: 10.1016/j.fertnstert.2008.08.020.
- Qin Y, Sun M, You L, et al. Esr1, hk3 and brsk1 gene variants are associated with both age at natural menopause and premature ovarian failure. Orphanet J Rare Dis. 2012;7(1):5. doi: 10.1186/1750-1172-7-5.
- Zhao H, Chen ZJ, Qin Y, et al. Transcription factor figla is mutated in patients with premature ovarian failure. Am J Hum Genet. 2008;82(6):1342–1348. doi: 10.1016/j.ajhg.2008.04.018.
- Chen X, Mu Y, Li C, et al. Mutation screening of hoxa7 and hoxa9 genes in chinese women with müllerian duct abnormalities. Reprod Biomed Online. 2014;29(5):595–599. doi: 10.1016/j.rbmo.2014.07.012.
- Qin Y, Zhao H, Kovanci E, et al. Mutation analysis of nanos3 in 80 chinese and 88 caucasian women with premature ovarian failure. Fertil Steril. 2007;88(5):1465–1467. doi: 10.1016/j.fertnstert.2007.01.020.
- Tassone F, Hagerman PJ, Hagerman RJ. Fragile x premutation. J Neurodev Disord. 2014;6(1):22.
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile x premutation is a significant risk factor for premature ovarian failure: the international collaborative pof in fragile x study–preliminary data. Am. J. Med. Genet. 1999;83(4):322–325. doi: 10.1002/(SICI)1096-8628(19990402)83:4<322::AID-AJMG17>3.0.CO;2-B.
- Qin Y, Jiao X, Dalgleish R, et al. Novel variants in the sohlh2 gene are implicated in human premature ovarian failure. Fertil Steril. 2014;101(4):1104–1109.e6. e1106. doi: 10.1016/j.fertnstert.2014.01.001.
- Qin Y, Vujovic S, Li G, et al. Ethnic specificity of variants of the esr1, hk3, brsk1 genes and the 8q22.3 locus: no association with premature ovarian failure (pof) in serbian women. Maturitas. 2014;77(1):64–67. doi: 10.1016/j.maturitas.2013.09.006.
- Jeon YJ, Choi Y, Shim SH, et al. Vascular endothelial growth factor gene polymorphisms in korean patients with premature ovarian failure. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):138–142. doi: 10.1016/j.ejogrb.2011.07.007.
- Rah H, Jeon YJ, Choi Y, et al. Association between kinase insert domain-containing receptor polymorphisms (-604t > c, 1192g > a, 1719a > t) and premature ovarian failure in korean women. Menopause. 2012;19(9):1037–1042. doi: 10.1097/gme.0b013e318248f2e8.
- Rah H, Jeon YJ, Choi Y, et al. Association of methylenetetrahydrofolate reductase (mthfr 677c > t) and thymidylate synthase (tser and ts 1494del6) polymorphisms with premature ovarian failure in korean women. Menopause. 2012;19(11):1260–1266. doi: 10.1097/gme.0b013e3182556b08.
- Rah H, Jeon YJ, Shim SH, et al. Association of mir-146ac > g, mir-196a2t > c, and mir-499a > g polymorphisms with risk of premature ovarian failure in korean women. Reprod Sci. 2013;20(1):60–68. doi: 10.1177/1933719112450341.
- Cho SH, Kim JH, Park HW, et al. Associations between hotair polymorphisms rs4759314, rs920778, rs1899663, and rs7958904 and risk of primary ovarian insufficiency in korean women. Maturitas. 2021;144:74–80. doi: 10.1016/j.maturitas.2020.10.023.
- Park J, Park Y, Koh I, et al. Association of an apba3 missense variant with risk of premature ovarian failure in the korean female population. J Pers Med. 2020;10(4):193. doi: 10.3390/jpm10040193.
- Jiao X, Qin C, Li J, et al. Cytogenetic analysis of 531 chinese women with premature ovarian failure. Hum Reprod. 2012;27(7):2201–2207. doi: 10.1093/humrep/des104.
- Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the cgg repeat at the fragile x site results in genetic instability: resolution of the sherman paradox. Cell. 1991;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5.
- Crisponi L, Deiana M, Loi A, et al. The putative forkhead transcription factor foxl2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27(2):159–166. doi: 10.1038/84781.
- Nallathambi J, Moumné L, De Baere E, et al. A novel polyalanine expansion in foxl2: the first evidence for a recessive form of the blepharophimosis syndrome (bpes) associated with ovarian dysfunction. Hum Genet. 2007;121(1):107–112. doi: 10.1007/s00439-006-0276-0.
- Benayoun BA, Dipietromaria A, Bazin C, et al. Foxl2: at the crossroads of female sex determination and ovarian function. Adv Exp Med Biol. 2009;665:207–226. doi: 10.1007/978-1-4419-1599-3_16.
- Margulis S, Abir R, Felz C, et al. Bone morphogenetic protein 15 expression in human ovaries from fetuses, girls, and women. Fertil Steril. 2009;92(5):1666–1673. doi: 10.1016/j.fertnstert.2008.08.119.
- Yoon SY, Yoon JA, Park M, et al. Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice. Stem Cell Res Ther. 2020;11(1):255. doi: 10.1186/s13287-020-01769-6.
- Veitia RA. Primary ovarian insufficiency, meiosis and DNA repair. Biomed J. 2020;43(2):115–123. doi: 10.1016/j.bj.2020.03.005.
- Ruth KS, Day FR, Hussain J, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596(7872):393–397. doi: 10.1038/s41586-021-03779-7.
- Chen Q, Liu K, Robinson AR, et al. DNA damage drives accelerated bone aging via an nf-κb-dependent mechanism. J Bone Miner Res. 2013;28(5):1214–1228. doi: 10.1002/jbmr.1851.
- Lee SC, Chan JC. Evidence for DNA damage as a biological link between diabetes and cancer. Chin Med J (Engl). 2015;128(11):1543–1548. doi: 10.4103/0366-6999.157693.
- Lee S, Dong HH. Foxo integration of insulin signaling with glucose and lipid metabolism. J Endocrinol. 2017;233(2):R67–r79. doi: 10.1530/JOE-17-0002.
- Qin Y, Jiao X, Simpson JL, et al. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808. doi: 10.1093/humupd/dmv036.
- Conway GS, Payne NN, Webb J, et al. Fragile x premutation screening in women with premature ovarian failure. Hum Reprod. 1998;13(5):1184–1187. doi: 10.1093/humrep/13.5.1184.
- Watkins WJ, Umbers AJ, Woad KJ, et al. Mutational screening of foxo3a and foxo1a in women with premature ovarian failure. Fertil Steril. 2006;86(5):1518–1521. doi: 10.1016/j.fertnstert.2006.03.054.
- Dang Y, Zhao S, Qin Y, et al. Microrna-22-3p is down-regulated in the plasma of han chinese patients with premature ovarian failure. Fertil Steril. 2015;103(3):802–807.e1. e801. doi: 10.1016/j.fertnstert.2014.12.106.
- Umair Z, Baek MO, Song J, et al. Microrna-4516 in urinary exosomes as a biomarker of premature ovarian insufficiency. Cells. 2022;11(18):2797. doi: 10.3390/cells11182797.