Abstract
Objective
The main purpose of this systematic review and meta-analysis was to investigate the diagnostic value of ultrasound elastography in the evaluation of polycystic ovary syndrome (PCOS).
Methods
A comprehensive and methodical investigation was carried out in the databases of PubMed, EMBASE, Cochrane, Scopus, Web of Science, and China National Knowledge Infrastructure, covering the entire duration of these databases until October 18, 2023. The primary purpose of this research was to evaluate and contrast ovarian tissue elasticity in people with and without PCOS. The elasticity of ovarian tissue was quantified using standardized mean difference (SMD).
Results
A total of eight studies were ultimately selected for systematic evaluation and meta-analysis. Five studies used shear wave elastography (SWE) as a diagnostic tool, and it was discovered that women with PCOS had higher levels of ovarian shear wave elasticity than their healthy counterparts. The SMD was determined to be 1.86 kilopascal (95% CI: 1.27 to 2.44). Three studies were conducted using strain elastography (SE) to compare the ovarian strain ratio of patients with PCOS to that of a healthy control group. The SMD for the PCOS group was 2.07 (95% CI: 1.79 to 2.34), which indicated that the ovarian strain ratio was significantly higher in that group.
Conclusion
This systematic review and meta-analysis found that women with PCOS had stiffer ovarian tissue than women without the disorder. Ultrasound elastography may provide clinicians with value beyond 2D ultrasound in the diagnosis of PCOS.
Introduction
PCOS is a complex endocrine and metabolic disorder that affects women during reproductive years and beyond. It is characterized by irregular menstrual cycles, hirsutism, infrequent ovulation, and the presence of multiple cysts on the ovaries. PCOS often occurs alongside insulin resistance, dyslipidemia, and obesity, which can have detrimental effects on women’s metabolic, reproductive, and cardiovascular well-being [Citation1–3]. Ultrasonography is a significant diagnostic imaging modality utilized for the detection of PCOS, characterized by the presence of enlarged ovaries and polycystic ovarian morphology [Citation4].
SWE and SE are commonly used ultrasound elastography techniques to assess ovarian stiffness [Citation5]. Elasticity scores and elastic strain ratio are two methods of assessing tissue stiffness using SE. Strain rate, a semi-quantitative measurement tool that reveals the relative difference between tissue afflicted by a lesion and nearby normal tissue, was used in this investigation. The SWE technique employs an ultrasonic probe to generate transverse waves by emitting acoustic radiation pulses that vibrate the tissue. Color coding technology is utilized to display the elasticity of the tissue in real-time and determine the value of Young’s modulus, where the higher the value of Young’s modulus, the harder the tissue [Citation6–9]. This method obviates the need for tissue pressure and enables the quantitative measurement of tissue elasticity in units of kilopascal or Meter per second.
However, when scanning for polycystic ovary syndrome using two-dimensional ultrasound, the number of follicles is susceptible to error when judged visually, depending on the patient’s body size and ovarian section. In contrast, ultrasound elastography can provide semi-quantitative and quantitative parameters to judge the degree of ovarian stiffness, and more objectively evaluate the ovarian changes in patients with polycystic ovary syndrome. The physiologic mechanism of altered ovarian elasticity in patients with PCOS may be due to cyst extrusion and interstitial fibrosis, resulting in highly compressed and stiffened ovarian tissue.
To enhance the accuracy and precision of PCOS diagnosis, it is necessary to supplement the evaluation of ovarian morphology with other quantitative markers. The ovarian elasticity index has emerged as a dependable metric for distinguishing between solid lesions and fibrotic/cystic tissue [Citation10]. Nevertheless, the current understanding regarding the assessment of ovarian tissue stiffness by the utilization of ultrasound elastography remains inconclusive due to inconsistent findings in the existing literature [Citation11,Citation12].
The current literature on ultrasound elastography to assess changes in ovarian elasticity in patients with polycystic ovary syndrome does not yield consistent conclusions, and no systematic review or meta-analysis on this topic has been performed before this time.
The purpose of this systematic review and meta-analysis is to provide a comprehensive summary of the existing data to examine the role of ultrasound elastography in the evaluation of PCOS.
Material and methods
Protocol registration
This systematic review and meta-analysis was filed in PROSPERO (protocol number CRD42023473452) and was done according to the “PRISMA” Statement’ [Citation13]. The supplementary files provide the data and diagrams utilized to substantiate the research findings.
Search strategy
A comprehensive search was conducted across six databases, namely PubMed, Embase, Cochrane, Scopus, Web of Science, and China National Knowledge Infrastructure, to identify relevant papers from their respective start dates up until October 18, 2023. The “Population/Patient, Intervention, Comparison, Outcome, and Study design” strategy was formulated in the following manner: “What changes in the ovary’s elasticity factors measured by ultrasound elastography between people with PCOS and people who don’t have any symptoms or are healthy?” [Citation14,Citation15]
The utilization of Pubmed serves as an illustrative instance, wherein the integration of medical topic headings is coupled with free-text headings and Boolean operators, specifically "AND," "OR," and "NOT." There were no limitations placed on language or publication date in the course of the search. Additionally, the reference lists of the papers that were included in the study were examined to identify any new relevant studies. The complete search strategy is presented in the Supplemental files.
Inclusion and exclusion criteria
Inclusion criteria:
Patients who have been clinically diagnosed with PCOS according to the Rotterdam (ESHRE/ASRM) criteria amended in 2003 exhibit symptoms such as irregular or absent ovulation, clinical or biochemical hyperandrogenemia, ultrasonography (Either one or both of the ovaries have a minimum of twelve follicles with diameters ranging from 2 to 9 millimeters, and/or an ovarian volume that exceeds ten milliliters);
Detection of patients with PCOS, using ultrasound elastography;
Control participants in the included studies consist of medically evaluated women who are in good health, exhibit regular ovulation, and have no previous medical records indicating hyperandrogenemia;
The outcome indicators are the ultrasound elasticity parameters, such as strain ratio, shear wave elasticity, or shear wave velocity.
Exclusion criteria:
Literature types are Conference Abstract, Letter, Review, Synthesis, Conference Paper;
Studies for which full text is not available and data are incomplete;
The literature which Newcastle-Ottawa Scale (NOS) scores <6;
Selection of studies and extraction of data
First, two independent reviewers (Zhongtan Ruan and Zhen Yu) reviewed the title, abstract, and keywords. In addition, two reviewers thoroughly assessed the full text of the literature identified during the initial screening process to determine eligibility based on predetermined inclusion and exclusion criteria. In case of disagreement between two independent reviewers, the corresponding author acted as the senior reviewer for screening. Finally, the reference lists of papers included in the study were carefully checked to identify possible omissions.
Two independent reviewers extracted the required data from the literature used in this study using a pre-designed data extraction form. The extracted information included several elements such as the author, year of publication, study design used, inclusion criteria for participants, measurement methods used in the ultrasound, indicators used to evaluate the results, number of cases studied, and average age of participants. The data were inputted by the primary author and subsequently validated by the secondary author.
Quality assessment
Two separate reviewers used the Newcastle-Ottawa quality scale to evaluate the listed studies. Three factors were considered by the NOS [Citation1]: the selection of study groups (which included PCOS patients and healthy controls) [Citation2]; the comparability of the study groups; and [Citation3] the exposure/outcome. The cross-sectional study got a score of 7, while the case-control and cohort studies both got a score of 9. Discrepancies between the results of an independent review by two reviewers were resolved by the corresponding author through deliberation or discernment.
Statistical analysis
The meta-analyses were conducted with STATA version 17. The researchers employed a random-effects model to aggregate effect sizes, utilizing the SMD as a measure of the relative disparity in stiffness between diseased and healthy ovaries. This approach accounted for variations in the units and absolute values of the outcome indicators across the studies included in the analysis [Citation16,Citation17]. The SMD values of 0.2, 0.5, and 0.8 are used to denote small, moderate, and substantial differences, correspondingly. The researchers conducted sensitivity analyses employing the one-by-one exclusion approach to examine modifications in the aggregated findings and evaluate the potential impact of specific studies on the meta-analysis outcomes. The degree of variability among the studies that were included was assessed using I2 and H2. If the value of H2 is greater than 1.5 or the value of I2 is equal to or greater than 50%, it indicates a higher level of heterogeneity among the studies. Subgroup analyses were performed to investigate the potential sources of heterogeneity, which were categorized based on the kind of study [Citation18]. Publication bias was detected qualitatively and quantitatively using funnel plots and Egger’s test, respectively [Citation19].
Results
Screening literature process
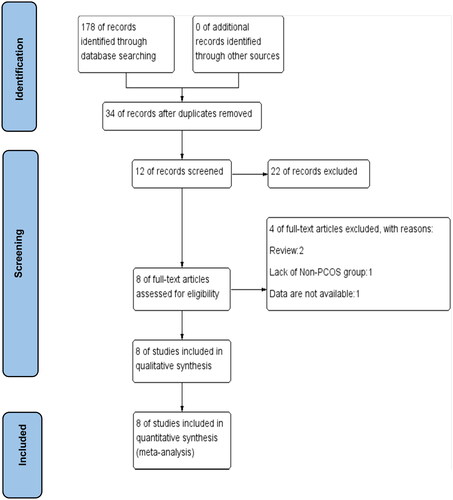
A comprehensive search of 6 databases initially yielded a total of 178 studies. After excluding 144 duplicate studies, the remaining 34 studies were screened for the first time. A total of 22 studies were subsequently excluded from consideration due to various factors, such as the inclusion of subjects with a single ovary. These exclusions were determined based on an evaluation of study titles, abstracts, and keywords. Finally, a comprehensive review of 12 studies was conducted and 4 articles were excluded. Excluded articles were identified as review type (n = 2), without a non-PCOS control group (n = 1), and with incomplete data (n = 1). Eight pieces of literature were incorporated into the final systematic review and meta-analysis [Citation11,Citation12,Citation20–25]. The PRISMA flowchart is depicted in .
Characteristics of the studies included
The literature comprised retrospective research, consisting of seven case-control studies and one cross-sectional study. The meta-analysis had a sample size of 1029 people, with their average age spanning from 21.50 years to 30.36 years. Three studies involved the use of the SE technique, while shear wave elastography was used in five studies. The studies that were incorporated in the analysis were classified into three distinct subgroups, which were determined based on three specific outcome measures: strain ratio, shear wave elasticity, and shear wave velocity.
Noteworthy, within the subgroup of strain ratio, two research studies [Citation21,Citation23] employed the participation of two sonographers for measurement purposes, resulting in two sets of outcome measures. Within the Shear wave elasticity subgroup, two investigations [Citation11,Citation12] assessed the rigidity of the left and right ovaries, while another study [Citation21,Citation23] evaluated the stiffness of both ovaries individually, as well as their collective mean stiffness. The data mentioned earlier is presented in
Table 1. Basic characteristics of the included studies.
Methodological quality of included studies
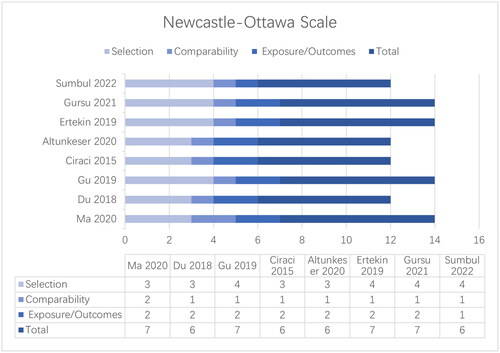
The results of the quality assessment using the Newcastle-Ottawa Scale are shown in . The area of greatest noncompliance was ‘comparability of cohorts’, as most studies controlled only for age and sex, ignoring other important confounders such as weight and disease severity. Other items, including representativeness of patients with polycystic ovary syndrome, selection of controls, and exposure/outcome of interest, were met in all of the included studies.
Strain ratio and shear wave elasticity
Three studies [Citation20,Citation21,Citation23] employed the SE technique to assess the stiffness of ovarian tissue in individuals diagnosed with PCOS. The ovarian strain ratio is a comparison of the elasticity of the ovarian mesenchyme with that of the adjacent soft tissue, such as the Fallopian tubes, which were not associated with the intestine or arteries, values from three measurements were then averaged. According to the forest plot presented in , the SMD of the ovarian strain ratio between the PCOS group and healthy controls was found to be 2.07 (95% [CI] of 1.79 to 2.34). Since the SMD was greater than 0.8, there was a big difference in how stiff the ovaries were in the PCOS group compared to the healthy controls.
Figure 3. Forest plot of ovarian strain ratio and shear wave elasticity in the PCOS and non-PCOS groups.
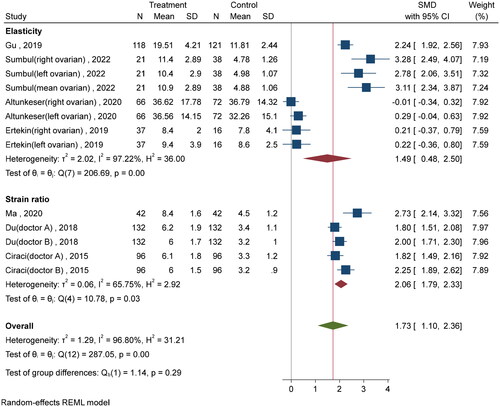
Five studies [Citation11,Citation12,Citation22,Citation24,Citation25] used the SWE technique to detect ovarian stiffness. Shear wave elasticity was higher in the PCOS group compared to the non-PCOS group, and an SMD of 1.51 (95% CI: 0.49 to 2.53) ().
The effect sizes between groups for both the strain ratio and shear wave elasticity subgroups demonstrated a higher level of ovarian stiffness in the group with PCOS. By aggregating the effect sizes of the two groups, it was seen that the SMD for ovarian stiffness was 1.86 kilopascal (95% [CI], 1.27 to 2.44). This finding further supports the notion that individuals with PCOS exhibit higher levels of ovarian stiffness when compared to healthy control subjects.
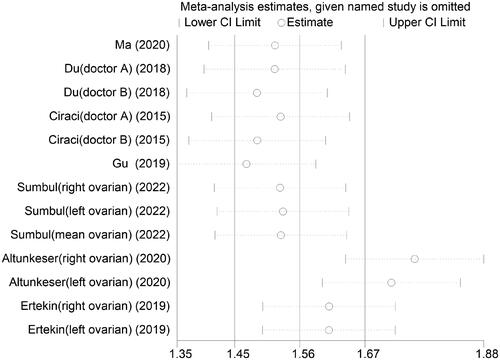
Additionally, sensitivity analyses were conducted on the studies that were included in the analysis. By systematically removing each study one at a time, the combined effect sizes of the remaining studies were found to be statistically significant (95% CI that included 1). These findings were consistent with the initial combined effect size values (). Egger’s regression tests and funnel plots are shown in the Supplemental files.
Shear wave velocity
Only one study [Citation24] examined shear wave velocity. Transvaginal probes were inserted bilaterally into the ovaries, and the examination was conducted inside the avascular region. The average shear wave velocity (SWV) values of each ovary were individually assessed, and subsequently, the overall mean SWV was calculated as the average of the SWV values obtained from the left ovary and the right ovary. The SMD was found to be 1.6, with a 95% CI ranging from 1.03 to 2.16. This result suggests a statistically positive association between shear wave velocity and hardness.
Discussion
In individuals diagnosed with PCOS, the utilization of two-dimensional ultrasound typically reveals the presence of a minimum of 12 cysts, each measuring less than 10 mm, in each ovary, and/or an ovarian volume exceeding 10 ml. Nevertheless, relying solely on the quantification of follicles and the assessment of ovarian size may result in diagnostic inaccuracies caused by inadequate imaging quality or inaccurate visual enumerations [Citation26,Citation27]. Hence, the utilization of advanced imaging modalities has the potential to broaden the clinician’s perspective when it comes to the diagnosis of polycystic ovarian syndrome. The utilization of ultrasound elastography has facilitated the assessment of ovarian fibrosis in patients with PCOS through the ultrasound elasticity index. In this systematic review and meta-analysis, both strain ratio and shear wave elasticity were higher in the ovaries of the PCOS group compared to healthy controls.
Shear wave elasticity, as a non-pressure-dependent elastic metric, serves to quantify alterations in ovarian stiffness. The results reported by Altunkeser et al. [Citation11] and Ertekin et al. [Citation12] indicate that there was no statistically significant disparity in ovarian shear wave elasticity values between the groups with PCOS and those without PCOS. The findings of our analysis contradict the results presented in the aforementioned studies. There are several potential explanations for this discrepancy. Firstly, the patients included in the above two papers were in the early stages of PCOS due to their young age, and histopathologic changes had not yet manifested [Citation28,Citation29]. Additionally, SWE was conducted transabdominal, and in certain patients, the deep location of the ovaries hindered a comprehensive evaluation.
SE offers a distinct advantage over SWE in terms of providing a more immediate evaluation of PCOS. This is achieved by swiftly assessing the existence of irregular elasticity patterns by the application of color mapping on the elastogram. Nevertheless, SE is dependent on the external force applied by the operator, making SE data vulnerable to inconsistencies caused by either intra- or inter-operator variability [Citation30]. Hence, all three articles incorporated the utilization of the strain ratio as an elastic measure, which serves as a semi-quantitative indicator of the comparative stiffness of ovarian tissue. The findings of Ma et al. opted for a strain ratio of 5.7, but Du et al. [Citation21] and Ciraci et al. opted for a strain ratio of 3.8 as the cutoff value for differentiating the PCOS group from the control group. Given the quantitative and qualitative limitations of the included studies, further research is needed to determine appropriate cutoff values for strain ratios. This requires large-scale, multicenter, and methodologically complex studies.
The results of Gursu et al. [Citation24] showed a significant acceleration of SWV due to stiffness in the ovaries of patients with PCOS. This is because the motion becomes heterophasic and the responsiveness of the transition time decreases as the acoustic frequency increases. Thus, shear wave velocity may be a useful indirect indicator of ovarian elasticity.
The present study has the following limitations: the number of included studies was small; polycystic ovarian appearance can also be seen in many healthy women in some cases, such as when taking a combination of contraceptive pills, but there were no healthy patients with the polycystic ovarian appearance in the control group of the included study; These studies did not compare the changes in ovarian stromal strain ratios before and after PCOS treatment; the age of the patients was not tightly controlled, and histopathologic changes can vary at different stages of the PCOS process.
The application of ultrasound elastography to the ovary is not significantly different from its application to other organs such as the liver and breast, notably the ovarian strain ratio is calculated as the ratio between the ovarian mesenchyme and adjacent soft tissues (e.g. fallopian tubes), measured three times and then averaged. In daily clinical practice, ultrasound elastography can be additionally performed in patients in whom the clinician suggests the possibility of polycystic ovary syndrome or ultrasound observation of a follicle number >12, and appropriately explained in the report.
Conclusion
This systematic review and meta-analysis showed increased ovarian tissue stiffness in the PCOS group compared to healthy controls. Ultrasound elastography to assess ovarian tissue elasticity for PCOS has some diagnostic value. However, given the quantity and caliber of the included studies, more investigation is necessary.
Supplemental Material
Download MS Word (58.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary files.
Additional information
Funding
References
- Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the amsterdam ESHRE/ASRM-Sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97(1):1–8. doi:10.1016/j.fertnstert.2011.09.024.
- Meier RK. Polycystic ovary syndrome. Nurs Clin North Am. 2018;53(3):407–420. doi:10.1016/j.cnur.2018.04.008.
- Taylor AE. Polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1998;27(4):877–902, ix. doi:10.1016/s0889-8529(05)70045-1.
- Lujan ME, Chizen DR, Pierson RA. Diagnostic criteria for polycystic ovary syndrome: pitfalls and controversies. J Obstet Gynaecol Can. 2008;30(8):671–679. doi:10.1016/s1701-2163(16)32915-2.
- Snoj Ž, Wu CH, Taljanovic MS, et al. Ultrasound elastography in musculoskeletal radiology: past, present, and future. Semin Musculoskelet Radiol. 2020;24(2):156–166. doi:10.1055/s-0039-3402746.
- Wu CH, Chiu YH, Chang KV, et al. Ultrasound elastography for the evaluation of plantar fasciitis: a systematic review and meta-analysis. Eur J Radiol. 2022;155:110495. doi:10.1016/j.ejrad.2022.110495.
- Wu CH, Lin CY, Hsiao MY, et al. Altered stiffness of microchamber and macrochamber layers in the aged heel pad: shear wave ultrasound elastography evaluation. J Formos Med Assoc. 2018;117(5):434–439. doi:10.1016/j.jfma.2017.05.006.
- Săftoiu A, Gilja OH, Sidhu PS, et al. The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-Hepatic applications: update 2018. Ultraschall Med. 2019;40(4):425–453. doi:10.1055/a-0838-9937.
- Xu X, He XL, Guo LL. [The diagnostic value of the maximum value of young’s modulus of shear-wave elastography and ACR TI-RADS for thyroid nodules]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;33(8):764–767. doi:10.13201/j.issn.1001-1781.2019.08.020.
- Dewall RJ. Ultrasound elastography: principles, techniques, and clinical applications. Crit Rev Biomed Eng. 2013;41(1):1–19. doi:10.1615/critrevbiomedeng.2013006991.
- Altunkeser A, Inal ZO, Baran N. Evaluation of ovaries in patients with polycystic ovary syndrome using shear wave elastography. Curr Med Imaging. 2020;16(5):578–583. doi:10.2174/1573405615666190114150538.
- Ertekin E, Turan OD, Tuncyurek O. Is shear wave elastography relevant in the diagnosis of polycystic ovarian syndrome? Med Ultrason. 2019;21(2):158–162. doi:10.11152/mu-1849.
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi:10.1136/bmj.g7647.
- Methley AM, Campbell S, Chew-Graham C, et al. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14(1):579. doi:10.1186/s12913-014-0579-0.
- Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7(1):16. doi:10.1186/1472-6947-7-16.
- Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry. 2020;81(5):20f13681. doi:10.4088/JCP.20f13681.
- Kanters S. Fixed- and Random-Effects models. Methods Mol Biol. 2022;2345:41–65. doi:10.1007/978-1-0716-1566-9_3.
- Barraclough H, Govindan R. Biostatistics primer: what a clinician ought to know: subgroup analyses. J Thorac Oncol. 2010;5(5):741–746. doi:10.1097/JTO.0b013e3181d9009e.
- Andrade C. Understanding the basics of meta-analysis and how to read a Forest plot: as simple as it gets. J Clin Psychiatry. 2020;81(5):20f13698. doi:10.4088/JCP.20f13698.
- Ma W, Jia W, Liu Y, et al. Diagnostic value of ultrasound elastography in polycystic ovary syndrome. J Clin Ultrasound in Med. 2020;22:1. (in Chinese) doi:10.16245/j.cnki.issn1008-6978.2020.01.011.
- Du Q, Mi X, Xu H, et al. Application of Real-Time ultrasonic elastic imaging in the diagnosis of polycystic ovary syndrome. Chinese J Ultrasound Med. 2018;34:8. (in Chinese)
- Gu M, Lu C. Value of real-time shear wave elastography in the diagnosis of polycystic ovary syndrome. Jiangsu Med J. 2019;45:10. (in Chinese) doi:10.19460/j.cnki.0253-3685.2019.10.018.
- Çıracı S, Tan S, Özcan AŞ, et al. Contribution of real-time elastography in diagnosis of polycystic ovary syndrome. Diagn Interv Radiol. 2015;21(2):118–122. doi:10.5152/dir.2014.14094.
- Gursu T, Cevik H, Desteli GA, et al. Diagnostic value of shear wave velocity in polycystic ovarian syndrome. J Ultrason. 2021;21(87):e277–e81. doi:10.15557/JoU.2021.0047.
- Sumbul HE, Avci BS, Bankir M, et al. Ovarian stiffness is significantly increased in polycystic ovary syndrome and related with anti-Mullerian hormone: a point shear wave elastography study. Ultrasound Q. 2022;38(1):83–88. doi:10.1097/RUQ.0000000000000592.
- Lauritsen MP, Svendsen PF, Andersen AN. Diagnostic criteria for polycystic ovary syndrome. Ugeskr Laeger. 2019;181(15).
- Lujan ME, Jarrett BY, Brooks ED, et al. Updated ultrasound criteria for polycystic ovary syndrome: reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28(5):1361–1368. doi:10.1093/humrep/det062.
- Witchel SF, Burghard AC, Tao RH, et al. The diagnosis and treatment of PCOS in adolescents: an update. Curr Opin Pediatr. 2019;31(4):562–569. doi:10.1097/mop.0000000000000778.
- Peña AS, Witchel SF, Hoeger KM, et al. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med. 2020;18(1):72. doi:10.1186/s12916-020-01516-x.
- Wu H, Zhang S, Wang C, et al. Comparing the accuracy between shear wave elastography and strain elastography in the diagnosis of breast tumors: systematic review and meta-analysis. Medicine (Baltimore). 2022;101(18):e29139. doi:10.1097/md.0000000000029139.