Abstract
In contemporary times, the employment of vitrification freezing technology has led to the widespread adoption of frozen-thawed embryo transfer (FET) worldwide. Meanwhile, hormone replacement therapy (HRT) is a crucial protocol for priming the endometrium during FET cycles. Estrogen is required in HRT cycles for the induction of progesterone receptors and to promote endometrial thickness. However, there is no universal consensus on the treatment duration, dosage regimen, administration route, and target serum estrogen levels. Therefore, this study aimed to offer a comprehensive review of these topics. A shorter duration of estrogen exposure may elevate the risk of early miscarriage, while prolonged exposure to estrogen does not seem to confer advantages to general population and may be attempted in individuals with thin endometrium. Moreover, excessive estrogen levels on the day of progesterone administration may be associated with higher miscarriage rates and lower live birth rates (LBR). To offer more comprehensive guidance for clinical practice, extensive and prospective studies involving a large sample size are warranted to determine the optimal concentration and duration of estrogen exposure.
Introduction
As is well documented, the number of patients undergoing assisted reproductive technology (ART) is increasing as the incidence of infertility increases. Infertility has recently emerged as a global issue of concern. Frozen-thawed embryo transfer (FET) is an integral component of the In vitro fertilization and embryo transfer (IVF-ET) technique. In recent years, given the favorable efficacy and safety of vitrification [Citation1], the number of FET cycles has been progressively increasing and has exceeded that of fresh embryo transfer cycles in several countries [Citation2–4]. Earlier studies even inferred that a freeze-all policy may lead to superior IVF outcomes [Citation5,Citation6].
FET presents several advantages, such as a lower risk of developing ovarian hyperstimulation syndrome (OHSS) and adverse events of supraphysiologic levels of steroid hormones on the endometrium in fresh cycles, as well as reduced cycle cancelation rates [Citation7]. Thus, improving the success rate of FET cycles holds significant implications.
The preparation of a receptive endometrium is a prerequisite for a successful pregnancy. Several regimens are administered for endometrial preparation during FET cycles, encompassing natural, hormone replacement, and micro-stimulation cycles, but none has been validated to outperform the others [Citation8–11]. Hormone replacement therapy (HRT) has been extensively applied in clinical practice owing to its benefits, such as the relatively lower number of repeat visits and flexibility in scheduling the date of embryo transfer [Citation12].
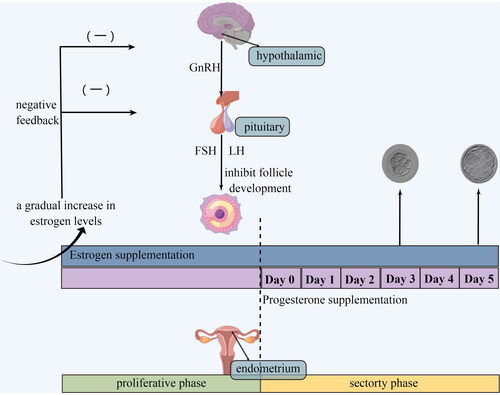
HRT refers to the method of preparing the endometrium by sequential use of estrogen and progesterone. Specifically, estrogen is continuously administered from the second to fifth days of menstruation, thereby mimicking hormonal fluctuations during natural cycles, inducing proliferation of endometrial cells, and eventually resulting in mucosal thickening. Meanwhile, increased estrogen levels inhibit follicular growth by negative feedback mechanisms on the hypothalamic-pituitary axis [Citation13]. Progesterone administration is typically initiated prior to transfer on Day 3 (cleavage-stage embryos) or Day 5 (blastocyst-stage embryos), inducing the endometrium to enter the secretory phase, thereby preparing it for the implantation of fertilized eggs (). Estrogen and progesterone treatment are maintained until the serum β-hCG level is measured 14 d after embryo transfer.
At present, many research efforts have been made toward optimizing the HRT protocol to improve pregnancy outcomes. However, there is no standardized recommendation on the ideal dose of exogenous estrogen and progestin, their route of administration, treatment duration, or the management of unexpectedly dominant follicles, and many other aspects, thus warranting future research [Citation14]. This article focused on the effect of estrogen exposure time, dose regimen, administration route, and serum estrogen concentration on pregnancy outcomes. The existing literature was reviewed to provide clinical guidance for the applications of HRT-FET as well as indicate directions for future research.
Duration of estrogen exposure before progesterone administration
Egg donation cycles
As previously reported, embryonic implantation can only occur within a specific time frame that is referred to as the ‘implantation window’. During the ‘implantation window’, synchronous development of both the embryo and the endometrium is also necessary. In order to guarantee synchronization, the time of progesterone administration is fixed and depends on the developmental stage of the embryos. However, prior to this, the timing of estrogen application maybe flexible due to the erratic growth rate of the endometrium or human factors (e.g. arranging a specific day for transfer). Therefore, the question of the impact of the duration of unopposed estrogen administration on pregnancy outcomes in HRT-FET is worth investigating.
Earlier studies principally focused on donor oocytes cycles. In the oocyte donation program, the recipient is administered estrogen until donor oocytes are available. This necessitates considerable flexibility in artificially manipulating the recipient’s endometrium and synchronize it with the developmental stage of the donor embryo. Navot et al. [Citation15] reported that 5 d of estrogen exposure provides sufficient stimulation for endometrial proliferation and induction of progesterone receptors, and the endometrium can normally mature with the addition of progesterone. Nevertheless, their study only included 12 samples. So, does this morphological maturity of the endometrium reflect functional integrity? Their subsequent studies answered the question. They documented that the early miscarriage rate was significantly higher in the group who underwent 5–10 d of estrogen exposure compared with the group subjected to 3–6 weeks of estrogen exposure and concluded that a short time exposure (5–10 d) of estrogen increased the rate of early abortion [Citation16].
This result was consistent with the finding of Borini et al. [Citation17], who also determined that the ideal duration of unopposed estrogen administration ranged between 11 and 40 d. Opinions regarding the optimal duration for unopposed estrogen administration are controversial. Michalas et al. [Citation18] demonstrated that superior pregnancy outcomes can be achieved when estrogen is administered 6–11 d before the initiation of progesterone supplementation, with a significant decrease in pregnancy rates beyond 11 d. Of 12–19 d of estrogen stimulation has also been proposed as the optimal option, given that cycles shorter than 12 d or longer than 19 d resulted in decreased pregnancy rates [Citation19]. Despite these controversial findings, they concluded that there is an optimal time frame for unopposed estrogen administration; outside of this window, it can cause deleterious effects on pregnancy.
What is the upper limit of this optimal range? Various studies have provided different answers. Several studies determined that prolonged maintenance of oocyte recipients with unopposed estrogen for 3–5 weeks did not exert adverse effect on endometrial morphology or pregnancy rates [Citation16,Citation17,Citation20,Citation21]. On the other hand, estrogen stimulation extending beyond 7 weeks led to a decrease in implantation and clinical pregnancy rates (CPRs) [Citation20]. In addition, studies evinced that prolonged unopposed estrogen administration was associated with a high rate of breakthrough bleeding in oocyte recipients. According to a previous study, bleeding was common in patients who continued to receive estrogen for more than 40 d [Citation17]. Another study reported an incidence of bleeding as high as 53.7% when estrogen was administered for more than 66 d. Notably, they found that the likelihood of bleeding during extended estrogen administration was proportional to the duration of estrogen administration [Citation22]. Surprisingly, successful embryo implantation was observed even after 100 d of unopposed estradiol administration in one isolated case [Citation23].
Autologous embryo transfer cycles
The aforementioned studies related to donor cycles have expanded our understanding of endometrial preparation and embryo-endometrial synchrony, providing knowledge that can be applied to FET [Citation24]. Nonetheless, studies on the duration of estrogen use in autologous FET cycles are limited. The relevant literature is summarized in .
Table 1. Literature on the effect of estrogen exposure time in HRT-FET cycles on pregnancy outcomes.
A retrospective analysis of data from 4142 HRT-FET cycles (D3/D5) revealed that estrogen administration for 7 or 14 d prior to progestin supplementation had no significant effect on cumulative live birth rates (LBR) or maternal and neonatal complications [Citation25]. Therefore, the study recommended only 7 d of estrogen stimulation in HRT-FET cycles for patients who wish to conceive as soon as possible. Despite this study’s large sample size and adjustment for potential confounders, it is worth emphasizing that some enrolled patients were pretreated with oral contraceptives for at least 21 d prior to their HRT cycles. However, the effect of pretreatment on pregnancy outcomes of HRT-FET remains elusive.
Several studies exclusively analyzed autologous single blastocyst embryo transfer cycles [Citation26–29]. For instance, an analysis of 1377 autologous single blastocyst transfer cycles by Bourdon et al. yielded that estrogen exposure exceeding 28 d prior to FET was associated with a significant decrease in LBR and a significant increase in early miscarriage rates beyond 35 d. In addition, a longer duration of estrogen exposure (36–48 d) resulted in lower birth weight and lower Z scores compared with E2 exposure of less than 22 d. Another similar study [Citation28] described that the number of days of estradiol administration was inversely associated with the gestational age at delivery. It is conceivable that prolonged exposure to estrogen may interfere with endometrial decidualization and affect the placenta, thereby influencing the birth weight and gestational age of the fetus. However, given that no statistical association was identified between prolonged estrogen administration and preterm birth [Citation28], its effect on the placenta is estimated to be negligible. Three studies on single blastocyst transfer uncovered that different durations of estrogen administration (10–36 d [Citation28],11–30 d [Citation27], and <23–>30 d [Citation29], respectively) did not affect the rate of implantation, clinical pregnancy, early miscarriage, or live birth (LB). However, a study on cleavage-stage embryo transfer cycles described that the days of E2 application were significantly lower in the ongoing pregnancy (OP)/LB group than in the non-OP/LB group (15.30 ± 2.50 vs.16.14 ± 3.36, p = .002). Further analyses using generalized estimating equations revealed that the days of E2 administration were independently correlated with OP/BL rates in cleavage-stage embryo transfer cycles (OR 0.912, 95% CI 0.854–0.973, p = .006) [Citation30].
Moreover, an unexpected phenomenon was observed in two studies [Citation27,Citation31]. That is, when estrogen was administered for extended durations and at higher doses, the endometrial thickness actually significantly shrank before the administration of progesterone. More specifically, the endometrial thickness on the day of progesterone supplementation was 9.5 (8.6, 10.9) vs. 8.3 (7.9, 9.0) (p<.01) when estrogen was given 11.0 (11.0, 12.0) vs.17.0 (15, 20.0) days, respectively, in the study undertaken by Hizkiyahu et al. Likewise, the Li et al. determined that the endometrial thickness was 10.818 ± 1.470 vs.10.344 ± 1.376 vs. 9.391 ± 1.383 (p < .001) when estrogen was administered for 11–14 vs.15–18 vs. ≥19 d, respectively. However, CPR was comparable between the different groups in both studies. Thus, further studies are necessitated to elucidate this phenomenon.
Additionally, Liu et al. [Citation32] pointed out that patients with thin endometrium required longer periods of estrogen therapy and that the effectiveness of treatment was contingent on the duration of estrogen therapy. In their study, patients who achieved their treatment goal (endometrial thickness ≥8 mm) were treated with a minimum of 30 d of estrogen therapy. Another study [Citation33] analyzing HRT-FET cycles in patients with thin endometrium demonstrated that extended estrogen therapy for 14–82 d significantly increased endometrial thickness (p = .031) and CPR (p = .016). However, there was a case of placenta accreta in a pregnant patient who received estrogen therapy for extended durations.
To sum up, results regarding the impact of the treatment duration of unopposed estrogen on pregnancy outcomes in HRT-FET cycles are conflicting, and the optimal exposure period remains inconclusive. However, that majority of centers allow estrogen exposure for at least 10 d before the administration of progestin [Citation27,Citation28,Citation34]. Additional studies are required to explore the impact of prolonged estrogen exposure on pregnancy outcomes.
Serum estrogen levels on the day of progesterone administration
Estrogen stimulates endometrial proliferation and up-regulates the expression of endometrial progesterone receptors, which are prerequisites for embryo implantation [Citation35]. Nevertheless, an excessively high estrogen level can exert detrimental effects. Prior studies have found that supraphysiological levels of estrogen are associated with a lower embryo implantation rate in fresh embryo transfer cycles [Citation36–38]. In addition, numerous studies have identified an increased risk of low birth weight, small for gestational age (SGA), and preeclampsia associated with superphysiological doses of estrogen in fresh embryo transfer cycles [Citation39–42]. However, whether estrogen is an independent risk factor for these adverse pregnancy outcomes is not certain, and it may be related to other factors, such as the use of GnRH and GnRH-a, which have been proven to exert anti-proliferative effects on the endometrium [Citation43]. HRT-FET cycles have markedly lower estrogen levels than fresh embryo transfer cycles but have a wider range of estrogen levels than natural cycles. In natural cycles, estrogen levels can typically peak at 250–300 pg/mL before ovulation. However, multiple studies did not demonstrate the superiority of natural cycles on pregnancy outcomes [Citation8,Citation44–47]. Hence, does the level of estrogen during HRT cycles influence pregnancy outcomes? Is there an optimal estrogen concentration range? The relevant literature is presented in .
Table 2. Literature on the effect of estrogen concentrations in HRT-FET cycles on pregnancy outcomes.
In an earlier study investigating donor cycles, recipients with estrogen levels < 100 pg/mL on the day of progesterone supplementation had comparable rates of embryo implantation and clinical pregnancy as those with estrogen levels within the range of 100–199 pg/mL, 200–299 pg/mL, 300–399 pg/mL, and ≥400 pg/mL. Regarding the estrogen < 100 pg/mL group, estrogen levels as low as < 50 pg/mL could also lead to normally implanted embryos [Citation48]. Despite the relatively small sample size of this study, involving only 26 cycles with estrogen < 100 pg/mL, their finding signaled that the estrogen requirement for embryo implantation may be very low. This is supported by the evidence from a recent study [Citation30] that demonstrated that approximately 40% of all pregnancies occurred at E2 < 100 pg/mL on progesterone initiation day in patients who underwent either cleavage-stage embryo transfer (95/233) or blastocyst-stage embryo transfer (65/165). In contrast, some infertility clinics stress that estrogen levels over 100 pg/mL [Citation49], or even 200 pg/mL [Citation50], are prerequisites for progesterone supplementation. Furthermore, some researchers recommended cancelation of the FET cycle if the estrogen level is lower than 75 pg/mL [Citation51]. Taken together, the minimum threshold of estrogen level remains highly controversial.
Some studies stratified patients according to estrogen levels on the day of endometrial transformation and observed no differences in pregnancy outcomes between the groups [Citation34,Citation52–56]. These were all retrospective studies involving cleavage-stage embryos [Citation52,Citation54], blastocysts [Citation34,Citation55,Citation56], or both [Citation53], and pregnancy outcomes comprising embryo implantation rates, CPR, miscarriage rates, and LBR. Another study failed to identify an association between mid-luteal estrogen levels (on day 8/9 of progesterone administration, 2–3 d after embryo transfer) and pregnancy outcomes [Citation57]. In three other studies, estrogen levels on the day of endometrial transformation were comparable between pregnant and non-pregnant women [Citation30,Citation58,Citation59]. Among them, two studies were retrospective [Citation30,Citation58], and the remaining one was prospective [Citation59]. Besides, two of the studies involved blastocysts [Citation30,Citation59], while the remaining study involved embryos in the cleavage stage [Citation58]. Collectively, these ten studies covered a wide range of estrogen levels ranging from 60 pg/mL to 3000 pg/mL. Based on the aforementioned findings, it appears that estrogen levels on the day of endometrial transformation do not impact pregnancy outcomes. Notably, none of these studies compared pregnancy complications, such as the incidence of SGA and low birth weight.
According to the results of a retrospective study, high levels of serum estradiol during HRT-FET negatively affected OP and LBR [Citation50]. In another study [Citation30], data from two different embryonic developmental stages were individually analyzed, and different results were obtained. The results of cleavage embryo transfer uncovered that the OP/LB group exhibited significantly lower E2 levels than the non-OP/LB group on the day of progesterone initiation. In addition, a downward trend was also observed in the implantation, clinical pregnancy, and OP/LB rates with increasing E2 levels. However, no difference or trend was found in blastocyst transfer cycles.
However, a study on blastocyst transfer cycles observed an association between E2 levels and pregnancy outcomes. Zhou et al. [Citation60] divided 3857 frozen-thawed blastocyst transfer cycles into three groups according to the serum E2 level before progesterone administration, which were <200 pg/mL, 200–399 pg/mL, and ≥400 pg/mL, and reported a significant reduction in LBR and a significant decrease in CPR in the ≥ 400 pg/mL group compared to the reference group (200–399 pg/mL), as well as a significant increase in miscarriage rate. Additionally, the neonatal birthweight, Z-score, and singleton SGA were comparable among the three groups. A unique strength of this study was the inclusion of E2 levels on the hCG trigger day of the controlled ovarian stimulation (COS) cycles in the regression model. High estrogen levels during the COS cycles have been hypothesized to affect pregnancy outcomes in subsequent FETs [Citation61]. This phenomenon may be ascribed to the low quality of oocytes following exposure to a hyperestrogenic environment.
Of note, several recent retrospective studies contributed to the search for a reasonable range of serum E2 levels before endometrial transformation in the HRT-FET cycles. Kong et al. [Citation62] proposed 300 pg/mL as the critical cutoff point for E2 levels before endometrial transformation by fitting a smoothed curve to data from 10,209 HRT-FET cycles and demonstrated that CPR was high when serum E2 levels were lower than 300 pg/mL, regardless of the actual serum E2 level. Contrastingly, Garimella et al. [Citation63] estimated that 100–500 pg/mL may be a more appropriate estrogen range, as the rate of miscarriage was significantly increased when estrogen was lower than 100 pg/mL or higher than 500 pg/mL.
To sum up, the required estrogen concentration before progesterone initiation in HRT-FET cycles is potentially not as high as anticipated and perhaps even lower than the estrogen level in natural cycles. Notwithstanding, the lower and upper limits of this threshold have not been established, mandating further research in this area.
Estrogen dose regimen
When preparing the endometrium, estradiol is commonly administered as two distinct regimens. The first regimen involves a fixed dose of 6 mg, aiming to effectively prevent the formation of unexpected dominant follicles. Conversely, the second regimen follows a stepwise incremental approach, commencing at 2 mg and gradually escalating to 6 mg. This approach aligns more closely with the natural progression of estrogen fluctuations. Therefore, it is necessary to investigate the impact of different dosage regimens on pregnancy outcomes.
A retrospective analysis of over 8000 cycles indicated that there was no statistically significant difference in LBR between fixed-dose regimens (FDR) and stepwise-escalation regimens (SER) in donor cycles, regardless of the mode of estrogen administration (oral or transdermal) [Citation64]. However, it is worthwhile emphasizing that a limitation of this study lies in the ambiguity surrounding the consistency between endometrial reactivity to donor oocytes and that of autologous oocytes. This conclusion is also supported by evidence from another retrospective study conducted by Ogawa et al. [Citation65]. The study revealed that the utilization of transdermal patches of estrogen in FDR and SER resulted in comparable rates of LBs and obstetric outcomes. However, it was also observed that the stepwise-escalation group exhibited a significantly higher incidence of miscarriages compared to the fixed-dose group. Importantly, the study did not exclude patients with abnormal uterine morphology, uterine fibroids, or adenomyosis, which could potentially serve as confounding factors contributing to the discrepancy in miscarriage rates between the two groups. Two additional studies [Citation31,Citation66] also examined the impact of FDR compared to SER on pregnancy outcomes; however, these studies employed SER with an initial dosage of 6 mg or higher. The findings revealed similar CPR between the two groups [Citation31]. Nevertheless, the stepwise-escalation group exhibited significantly lower average birth weight, a significantly higher occurrence of low-birth-weight infants, and a significantly higher prevalence of placental anomalies, including bilobated placentas, accessory lobes, and retroplacental hematomas [Citation66]. The poor obstetric outcomes and placental abnormalities are likely to be caused by the excessive administration of total estrogen doses. Furthermore, both studies have demonstrated a significant reduction in endometrial thickness within the stepwise-escalation group compared to the fixed-dose group. Nevertheless, due to the prolonged duration of estrogen administration and the substantial cumulative dose in the stepwise-escalation group, factors that primarily contributed to the disparity in endometrial thickness were not identifiable. In a recent retrospective cohort study [Citation67], the efficacy of FDR (6 mg) was compared to SER, consisting of a starting dose of 2 mg and a starting dose of 4 mg, respectively. The analysis revealed no statistically significant differences in LBR among the three groups, despite the 4 mg starting group displaying a higher rate. Furthermore, CPR and endometrial thickness were significantly higher in the 4 mg starting group compared to the remaining two groups. Upon conducting a more detailed analysis of blastocyst cycles, no statistically significant differences were detected in CPR and LBR among the three groups, although they were numerically higher in the 4 mg starting group. According to the aforementioned research, it is evident that both FDR and SER starting from 4 mg can yield favorable pregnancy outcomes.
Route of estrogen administration
In HRT-FET cycles, oral estradiol valerate tablets are currently the preferred option in clinical settings due to their cost-effectiveness and availability. However, compelling evidence indicates that oral estrogens undergo hepatic first-pass metabolism, resulting in reduced bioavailability and consequently requiring higher doses, which subsequently increases the risk of thrombotic events. Conversely, transdermal or transvaginal administration circumvents the adverse effects of enterohepatic circulation, exhibiting superior bioavailability and requiring lower dosages, thus theoretically presenting a more favorable alternative to the oral route.
Multiple prospective randomized controlled studies and retrospective cohort studies have provided evidence that both transdermal estrogens (both patch and gel) and oral estrogens achieve similar pregnancy outcomes, including embryo implantation rate, biochemical pregnancy rate, CPR, miscarriage rate, and LBR [Citation68–73]. Only a single study reported a significantly lower rate of miscarriage and a significantly higher rate of sustained pregnancies and LBs in the transdermal group compared to the oral group [Citation71]. However, it is crucial to recognize that this study was constrained by its small sample size, comprising only 20 clinical pregnancies and 15 LBs. Besides, two studies conducted a comparative analysis between the transdermal and vaginal routes, both reporting no significant difference in LBR [Citation74,Citation75]. Only one study evaluated differences in perinatal outcomes between the transdermal and transvaginal routes, demonstrating no significant differences in neonatal birth weight, gestational diabetes mellitus, preterm labor, or placenta previa [Citation74].
Two prospective randomized clinical trials demonstrated that the endometrial thickness on the day of progesterone administration was similar between the transdermal and oral routes [Citation69,Citation72]. Interestingly, the endometrial thickness was accessed at two specific time points in a prospective study [Citation70]. The findings indicated that the transdermal group displayed a thicker endometrium compared to the oral group on day 10 ± 1 of the treatment (7.6 mm vs. 7.0 mm, p = .026). However, this difference was non-significant on 15 ± 1 d, implying that the onset is faster via the transdermal route than the oral route but with a similar final effect on the endometrium. In addition, a prospective cohort study found that transdermal estrogen resulted in thicker endometrium compared to the vaginal route [Citation75]. Moreover, the treatment duration of the transdermal route was shorter than that of the oral [Citation70,Citation73] and vaginal [Citation75] routes.
With regard to the cycle cancelation rate, no significant differences were detected among the three modes of medication [Citation69,Citation73,Citation75]. However, studies have insinuated that the transdermal administration method is associated with a higher incidence of cycle cancelation resulting from spontaneous ovulation compared to both the oral [Citation69] and the vaginal administration routes [Citation75]. This phenomenon could potentially be attributed to the comparatively lower concentration of estrogen generated through transdermal administration compared to the oral [Citation69,Citation70,Citation73] and vaginal routes [Citation74,Citation75], thereby limiting its efficacy in suppressing follicular development. This issue can perhaps be addressed through the implementation of higher transdermal estrogen dosage or the administration of GnRH-a.
Another important aspect is the satisfaction degrees of patients. Generally, the transdermal group exhibited greater patient satisfaction compared to the oral or vaginal groups [Citation69,Citation75]. However, a study claimed that patients who were given estrogen orally were more satisfied than those who were given estrogen transdermally [Citation70]. The majority of patients who complained about the inconvenience of patch therapy cited issues related to patch detachment. The authors speculated that this could be ascribed to the high average annual humidity of approximately 70% in Valencia, where the majority of patients resided.
In short, transdermal estrogen can achieve pregnancy outcomes similar to those of oral or vaginal estrogen with shorter treatment duration, lower serum estrogen levels, fewer side effects, and higher patient satisfaction. It can be used effectively in HRT-FET cycles, especially in patients with chronic hepatic and renal dysfunction, high-risk factors for thrombosis, and dyslipidemia.
Conclusions and perspectives
To sum up, shorter periods of estrogen exposure may increase the risk of early miscarriage even though it may allow the endometrium to reach the desired thickness. Second, longer estrogen exposure durations do not appear to be beneficial for the average patient and increases the incidence of adverse events, such as vaginal bleeding. However, prolonged estrogen exposure may be attempted in patients with thin endometrium, but attention should concurrently be paid to the side effects of estrogen. Third, pregnancy can occur over a wide range of estrogen levels on the day of progesterone administration of the HRT-FET cycles, but clinicians should avoid exposing early embryos to excessively high levels of estrogen, which may lead to obstetric complications related to placental abnormalities. Fourth, studies on the effects of estrogen concentration and duration of exposure on pregnancy outcomes are almost always retrospective in nature. Therefore, large samples and prospective studies should be conducted to explore the optimal estrogen concentration range and exposure time to better guide clinical decision-making. In addition, studies should be separately conducted on embryos at the cleavage stages and blastocyst stages, as well as on HRT cycles with and without pretreatment. Fifth, transdermal estrogen can offer similar pregnancy outcomes to oral or vaginal estrogen. Finally, FDR and SER starting from 4 mg could yield better pregnancy outcomes.
Author contributions
FL and SX contributed to conception of the article. CW wrote the first draft of the manuscript. HW, YY, and YL reviewed the literature available on this topic. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Disclosure statement
The authors declare no competing interests.
Data availability statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Loutradi KE, Kolibianakis EM, Venetis CA, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):1–10. doi: 10.1016/j.fertnstert.2007.06.010.
- Calhaz-Jorge C, European IVF-monitoring Consortium (EIM), European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod Oxf Engl. 2017;32(10):1957–1973.
- Saito H, Jwa SC, Kuwahara A, et al. Assisted reproductive technology in Japan: a summary report for 2015 by the ethics committee of the Japan Society of Obstetrics and Gynecology. Reprod Med Biol. 2018;17(1):20–28. doi: 10.1002/rmb2.12074.
- Katagiri Y, Jwa SC, Kuwahara A, et al. Assisted reproductive technology in Japan: a summary report for 2019 by the ethics committee of the Japan Society of Obstetrics and Gynecology. Reprod. Med. Biol. 2022;21(1):E12434.
- Dieamant FC, Petersen CG, Mauri AL, et al. Fresh embryos versus freeze-all embryos - transfer strategies: nuances of a meta-analysis. JBRA Assist. Reprod. 2017;21(3):260–272.
- Roque M, Valle M, Guimarães F, et al. Freeze-all cycle for all normal responders? J Assist Reprod Genet. 2017;34(2):179–185. doi: 10.1007/s10815-016-0834-x.
- Shapiro BS, Daneshmand ST, Garner FC, et al. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102(1):3–9. doi: 10.1016/j.fertnstert.2014.04.018.
- Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2017;7(7):CD003414. doi: 10.1002/14651858.CD003414.pub3.
- Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2008;7(7):CD003414. doi: 10.1002/14651858.CD003414.pub2.
- Groenewoud ER, Cantineau AEP, Kollen BJ, et al. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. 2013;19(5):458–470. doi: 10.1093/humupd/dmt030.
- Groenewoud ER, Cohlen BJ, Al-Oraiby A, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod. 2016;31(7):1483–1492. doi: 10.1093/humrep/dew120.
- Mackens S, Santos-Ribeiro S, van de Vijver A, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. 2017;32(11):2234–2242. doi: 10.1093/humrep/dex285.
- Devroey P, Pados G. Preparation of endometrium for egg donation. Hum Reprod Update. 1998;4(6):856–861. doi: 10.1093/humupd/4.6.856.
- Groenewoud ER, Cohlen BJ, Macklon NS. Programming the endometrium for deferred transfer of cryopreserved embryos: hormone replacement versus modified natural cycles. Fertil Steril. 2018;109(5):768–774. doi: 10.1016/j.fertnstert.2018.02.135.
- Navot D, Anderson TL, Droesch K, et al. Hormonal manipulation of endometrial maturation. J Clin Endocrinol Metab. 1989;68(4):801–807. doi: 10.1210/jcem-68-4-801.
- Navot D, Bergh PA, Williams M, et al. An insight into early reproductive processes through the in vivo model of ovum donation. J Clin Endocrinol Metab. 1991;72(2):408–414. doi: 10.1210/jcem-72-2-408.
- Borini A, Dal Prato L, Bianchi L, et al. Effect of duration of estradiol replacement on the outcome of oocyte donation. J Assist Reprod Genet. 2001;18(4):185–190.
- Michalas S, Loutradis D, Drakakis P, et al. A flexible protocol for the induction of recipient endometrial cycles in an oocyte donation programme. Hum Reprod. 1996;11(5):1063–1066. doi: 10.1093/oxfordjournals.humrep.a019297.
- Younis JS, Mordel N, Lewin A, et al. Artificial endometrial preparation for oocyte donation: the effect of estrogen stimulation on clinical outcome. J Assist Reprod Genet. 1992;9(3):222–227. doi: 10.1007/BF01203817.
- Soares SR, Troncoso C, Bosch E, et al. Age and uterine receptiveness: predicting the outcome of oocyte donation cycles. J Clin Endocrinol Metab. 2005;90(7):4399–4404. doi: 10.1210/jc.2004-2252.
- Yaron Y, Amit A, Mani A, et al. Uterine preparation with estrogen for oocyte donation: assessing the effect of treatment duration on pregnancy rates. Fertil Steril. 1995;63(6):1284–1286. doi: 10.1016/s0015-0282(16)57612-2.
- Remohí J, Gallardo E, Guanes PP, et al. Donor-recipient synchronization and the use of gonadotrophin-releasing hormone agonists to avoid the premature luteinizing hormone surge in oocyte donation. Hum Reprod. 1995;10(2):84–90. doi: 10.1093/humrep/10.suppl_2.84.
- Remohí J, Gutiérrez A, Cano F, et al. Long oestradiol replacement in an oocyte donation programme. Hum Reprod. 1995;10(6):1387–1391. doi: 10.1093/humrep/10.6.1387.
- Steiner AZ, Paulson RJ. Oocyte donation. Clin Obstet Gynecol. 2006;49(1):44–54. doi: 10.1097/01.grf.0000197518.76553.c1.
- Jiang WJ, Song JY, Sun ZG. Short (seven days) versus standard (fourteen days) oestrogen administration in a programmed frozen embryo transfer cycle: a retrospective cohort study. J. Ovarian Res. 2022;15(1):36.
- Bourdon M, Santulli P, Kefelian F, et al. Prolonged estrogen (E2) treatment prior to frozen-blastocyst transfer decreases the live birth rate. Hum Reprod. 2018;33(5):905–913. doi: 10.1093/humrep/dey041.
- Li X, Shi W, Gao Y, et al. Is duration of estrogen supplementation associated with clinical outcomes in frozen-thawed autologous single-blastocyst transfer cycles? J Assist Reprod Genet. 2022;39(5):1087–1094. doi: 10.1007/s10815-022-02481-5.
- Sekhon L, Feuerstein J, Pan S, et al. Endometrial preparation before the transfer of single, vitrified-warmed, euploid blastocysts: does the duration of estradiol treatment influence clinical outcome? Fertil Steril. 2019;111(6):1177–1185.e3. doi: 10.1016/j.fertnstert.2019.02.024.
- Joly J, Goronflot T, Reignier A, et al. Impact of the duration of oestradiol treatment on live birth rate in hormonal replacement therapy cycle before frozen blastocyst transfer. Hum Fertil Camb Engl. 2023;26:1256–1263.
- Li Q, Ruan L, Zhu L, et al. Elevated estradiol levels in frozen embryo transfer have different effects on pregnancy outcomes depending on the stage of transferred embryos. Sci Rep. 2022;12(1):5592. doi: 10.1038/s41598-022-09545-7.
- Hizkiyahu R, Suarthana E, Kadour Peero E, et al. Does increasing estrogen dose during frozen embryo transfer affect pregnancy rate? J Assist Reprod Genet. 2022;39(5):1081–1085. doi: 10.1007/s10815-022-02470-8.
- Liu SM, Zhou YZ, Wang HB, et al. Factors associated with effectiveness of treatment and reproductive outcomes in patients with thin endometrium undergoing estrogen treatment. Chin Med J (Engl). 2015;128(23):3173–3177. doi: 10.4103/0366-6999.170258.
- Chen MJ, Yang JH, Peng FH, et al. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006;23(7-8):337–342. doi: 10.1007/s10815-006-9053-1.
- Celik C, Asoglu MR, Karakis LS, et al. The impact of serum oestradiol concentration prior to progesterone administration on live birth rate in single vitrified-warmed blastocyst transfer cycles. Reprod Biomed Online. 2019;39(6):1026–1033. doi: 10.1016/j.rbmo.2019.08.009.
- Szekeres-Bartho J, Balasch J. Progestagen therapy for recurrent miscarriage. Hum Reprod Update. 2008;14(1):27–35. doi: 10.1093/humupd/dmm035.
- Arslan M, Bocca S, Arslan EO, et al. Cumulative exposure to high estradiol levels during the follicular phase of IVF cycles negatively affects implantation. J Assist Reprod Genet. 2007;24(4):111–117. doi: 10.1007/s10815-006-9101-x.
- Joo BS, Park SH, An BM, et al. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93(2):442–446. doi: 10.1016/j.fertnstert.2009.02.066.
- Kutlu T, Ozkaya E, Ayvaci H, et al. Area under curve of temporal estradiol measurements for prediction of the detrimental effect of estrogen exposure on implantation. Int J Gynaecol Obstet. 2016;135(2):168–171. doi: 10.1016/j.ijgo.2016.04.015.
- Hu XL, Feng C, Lin XH, et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small-for-gestational-age birth. J Clin Endocrinol Metab. 2014;99(6):2217–2224. doi: 10.1210/jc.2013-3362.
- Imudia AN, Awonuga AO, Doyle JO, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97(6):1374–1379. doi: 10.1016/j.fertnstert.2012.03.028.
- Pereira N, Reichman DE, Goldschlag DE, et al. Impact of elevated peak serum estradiol levels during controlled ovarian hyperstimulation on the birth weight of term singletons from fresh IVF-ET cycles. J Assist Reprod Genet. 2015;32(4):527–532. doi: 10.1007/s10815-015-0434-1.
- Pereira N, Elias RT, Christos PJ, et al. Supraphysiologic estradiol is an independent predictor of low birth weight in full-term singletons born after fresh embryo transfer. Hum Reprod. 2017;32(7):1410–1417. doi: 10.1093/humrep/dex095.
- Montoya-Botero P, Polyzos NP. The endometrium during and after ovarian hyperstimulation and the role of segmentation of infertility treatment. Best Pract Res Clin Endocrinol Metab. 2019;33(1):61–75. doi: 10.1016/j.beem.2018.09.003.
- Fu Y, Chen D, Cai B, et al. Comparison of two mainstream endometrial preparation regimens in vitrified-warmed embryo transfers after PGT. Reprod Biomed Online. 2022;44(2):239–246. doi: 10.1016/j.rbmo.2021.09.009.
- Givens CR, Markun LC, Ryan IP, et al. Outcomes of natural cycles versus programmed cycles for 1677 frozen-thawed embryo transfers. Reprod Biomed Online. 2009;19(3):380–384. doi: 10.1016/s1472-6483(10)60172-1.
- Liu J, Zheng J, Lei YL, et al. Effects of endometrial preparations and transferred embryo types on pregnancy outcome from patients with advanced maternal age. Syst Biol Reprod Med. 2019;65(2):181–186. doi: 10.1080/19396368.2018.1501114.
- Mounce G, McVeigh E, Turner K, et al. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertil Steril. 2015;104(4):915–920.e1. doi: 10.1016/j.fertnstert.2015.07.1131.
- Remohí J, Ardiles G, García-Velasco JA, et al. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod. 1997;12(10):2271–2276. doi: 10.1093/humrep/12.10.2271.
- Labarta E, Mariani G, Holtmann N, et al. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod. 2017;32(12):2437–2442. doi: 10.1093/humrep/dex316.
- Fritz R, Jindal S, Feil H, et al. Elevated serum estradiol levels in artificial autologous frozen embryo transfer cycles negatively impact ongoing pregnancy and live birth rates. J Assist Reprod Genet. 2017;34(12):1633–1638. doi: 10.1007/s10815-017-1016-1.
- Gaggiotti-Marre S, Martinez F, Coll L, et al. Low serum progesterone the day prior to frozen embryo transfer of euploid embryos is associated with significant reduction in live birth rates. Gynecol Endocrinol. 2019;35(5):439–442. doi: 10.1080/09513590.2018.1534952.
- Banz C, Katalinic A, Al-Hasani S, et al. Preparation of cycles for cryopreservation transfers using estradiol patches and crinone 8% vaginal gel is effective and does not need any monitoring. Eur J Obstet Gynecol Reprod Biol. 2002;103(1):43–47. doi: 10.1016/s0301-2115(02)00004-0.
- Mackens S, Santos-Ribeiro S, Orinx E, et al. Impact of serum estradiol levels prior to progesterone administration in artificially prepared frozen embryo transfer cycles. Front Endocrinol (Lausanne). 2020;11:255. doi: 10.3389/fendo.2020.00255.
- Niu Z, Feng Y, Sun Y, et al. Estrogen level monitoring in artificial frozen-thawed embryo transfer cycles using step-up regime without pituitary suppression: is it necessary? J Exp Clin Assist Reprod. 2008;5(1):4. doi: 10.1186/1743-1050-5-4.
- Özdemir AZ, Karli P, Gülümser Ç. Does high estrogen level negatively affect pregnancy success in frozen embryo transfer? Arch Med Sci. 2022;18(3):647–651. doi: 10.5114/aoms.2020.92466.
- Du YR, Yang K, Liu J. Effects of serum estrogen levels before frozen-thawed blastocyst transfer on pregnancy outcomes in hormone replacement cycles. Sci Rep. 2023;13(1):1194. doi: 10.1038/s41598-023-27877-w.
- Yovich JL, Conceicao JL, Stanger JD, et al. Mid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacement. Reprod Biomed Online. 2015;31(2):180–191. doi: 10.1016/j.rbmo.2015.05.005.
- Bocca S, Real EB, Lynch S, et al. Impact of serum estradiol levels on the implantation rate of cleavage stage cryopreserved-thawed embryos transferred in programmed cycles with exogenous hormonal replacement. J Assist Reprod Genet. 2015;32(3):395–400. doi: 10.1007/s10815-014-0402-1.
- Boynukalin FK, Gultomruk M, Turgut E, et al. Measuring the serum progesterone level on the day of transfer can be an additional tool to maximize ongoing pregnancies in single euploid frozen blastocyst transfers. Reprod Biol Endocrinol. 2019;17(1):102. doi: 10.1186/s12958-019-0549-9.
- Zhou R, Zhang X, Huang L, et al. Association between serum estradiol levels prior to progesterone administration in artificial frozen-thawed blastocyst transfer cycles and live birth rate: a retrospective study. BJOG. 2021;128(13):2092–2100. doi: 10.1111/1471-0528.16777.
- Cai J, Liu L, Xu Y, et al. Supraphysiological estradiol level in ovarian stimulation cycles affects the birthweight of neonates conceived through subsequent frozen-thawed cycles: a retrospective study. BJOG. 2019;126(6):711–718. doi: 10.1111/1471-0528.15606.
- Kong N, Liu J, Zhang C, et al. The relationship between serum oestrogen levels and clinical outcomes of hormone replacement therapy-frozen embryo transfer: a retrospective clinical study. BMC Pregnancy Childbirth. 2022;22(1):265. doi: 10.1186/s12884-022-04605-2.
- Garimella S, Karunakaran S, Gedela DR. Does serum estrogen level have an impact on outcomes in hormonal replacement frozen-warmed embryo transfer cycles? Gynecol Endocrinol. 2021;37(10):891–894. doi: 10.1080/09513590.2021.1892631.
- Madero S, Rodriguez A, Vassena R, et al. Endometrial preparation: effect of estrogen dose and administration route on reproductive outcomes in oocyte donation cycles with fresh embryo transfer. Hum Reprod. 2016;31(8):1755–1764. doi: 10.1093/humrep/dew099.
- Ogawa T, Kasai T, Ogi M, et al. Effect of transdermal estrogen dose regimen for endometrial preparation of frozen-thawed embryo transfer on reproductive and obstetric outcomes. Reprod Med Biol. 2021;20(2):208–214. doi: 10.1002/rmb2.12370.
- Ganer Herman H, Volodarsky-Perel A, Nu TNT, et al. The effect of oestrogen dose and duration in programmed frozen cycles on obstetric outcomes and placental findings. Reprod Biomed Online. 2023;46(4):760–766. doi: 10.1016/j.rbmo.2023.01.003.
- Şükür YE, Aslan B, Özmen B, et al. Impact of an estrogen replacement regimen on live birth rate in frozen-thawed good-quality embryo transfer. Int J Gynaecol Obstet. 2023;160(3):829–835. doi: 10.1002/ijgo.14366.
- Scheffer JB, Scheffer BB, Aguiar AP, et al. A comparison of the effects of three different estrogen used for endometrium preparation on the outcome of day 5 frozen embryo transfer cycle. JBRA Assist Reprod. 2021;25(1):104–108.
- Garimella S, Karunakaran S, Gedela DR. A prospective study of oral estrogen versus transdermal estrogen (gel) for hormone replacement frozen embryo transfer cycles. Gynecol Endocrinol. 2021;37(6):515–518. doi: 10.1080/09513590.2020.1793941.
- Ferrer-Molina P, Calatayud-Lliso C, Carreras-Collado R, et al. Oral versus transdermal oestrogen delivery for endometrial preparation before embryo transfer: a prospective, comparative, randomized clinical trial. Reprod Biomed Online. 2018;37(6):693–702. doi: 10.1016/j.rbmo.2018.09.003.
- Tehraninejad ES ,Kabodmehri R ,Rashidi BH, et al. Trans dermal estrogen (oestrogel) for endometrial preparation in freeze embryo transfer cycle: an RCT. Int J Reprod Biomed. 2018;16(1):51–56.
- Kahraman S, Çetinkaya CP, Sahin Y, et al. Transdermal versus oral estrogen: clinical outcomes in patients undergoing frozen-thawed single blastocyst transfer cycles without GnRHa suppression, a prospective randomized clinical trial. J Assist Reprod Genet. 2019;36(3):453–459. doi: 10.1007/s10815-018-1380-5.
- Davar R, Janati S, Mohseni F, et al. A comparison of the effects of transdermal estradiol and estradiol valerate on endometrial receptivity in frozen-thawed embryo transfer cycles: a randomized clinical trial. J Reprod Infertil. 2016;17(2):97–103.
- Dubois E, Bouet PE, Descamps P, et al. Impact of the type of endometrial oestrogen preparation for frozen-thawed embryo (vaginal or transdermal) on perinatal outcomes in an artificial cycle. J Gynecol Obstet Hum Reprod. 2021;50(9):102187. doi: 10.1016/j.jogoh.2021.102187.
- Corroenne R, El Hachem H, Verhaeghe C, et al. Endometrial preparation for frozen-thawed embryo transfer in an artificial cycle: transdermal versus vaginal estrogen. Sci Rep. 2020;10(1):985. doi: 10.1038/s41598-020-57730-3.