Abstract
Objectives
Polycystic ovary syndrome (PCOS) and subclinical hypothyroidism (SCH) are prevalent gynecological conditions. However, the interrelationship between the two remains elusive. This study aims to elucidate the association between these conditions and determine the potential impact of SCH on the physiological and metabolic characteristics of patients with PCOS.
Methods
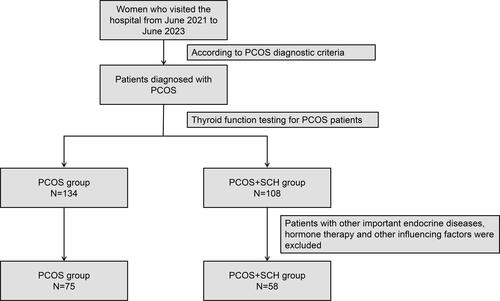
This cross-sectional study enrolled 133 patients with PCOS from our Hospital. Participants were categorized into two groups: those with PCOS + SCH (n = 58) and those with PCOS (n = 75). Serum hormonal levels, metabolic markers, ovarian volume, and follicle count were compared between the groups.
Results
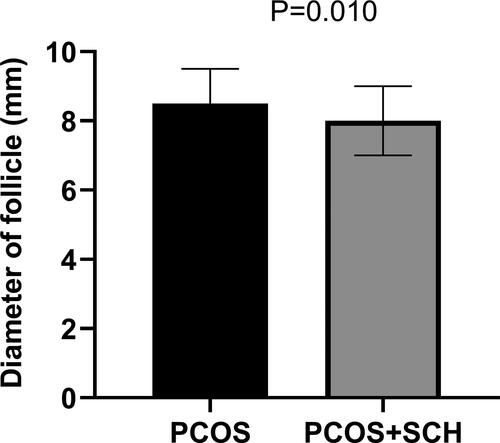
There was a significant difference in BMI between the two groups, with a higher prevalence of obesity in the PCOS + SCH group (p = .014). Compared to the PCOS group, patients with PCOS + SCH had significantly higher levels of TSH (p < .001), triglycerides (p = .025), and HOMA-IR (p < .001), while LH levels were significantly lower (p = .048). However, multivariate linear regression analysis revealed that TSH, triglycerides, LH, and HOMA-IR were not determinants for the occurrence of SCH in patients with PCOS. Additionally, there was a notable reduction in follicle count in the left ovary for the PCOS + SCH group compared to the PCOS group (p = .003), and the overall follicle diameter of the PCOS + SCH group was also smaller (p = .010).
Conclusion
SCH may exert effects on the physiological and metabolic profiles of patients with PCOS. Further investigation into the relationship between these disorders is warranted to delineate their clinical implications.
Introduction
Polycystic ovary syndrome (PCOS) is a complex endocrine disorder that affects approximately 9–13% of women of reproductive age, characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology [Citation1]. It is often accompanied by metabolic abnormalities such as insulin resistance, obesity, and dyslipidemia, leading to a heightened risk for type-2 diabetes, cardiovascular diseases, and infertility [Citation2]. Meta-analysis results showed that PCOS also had an adverse impact on patients’ mental health [Citation3]. Subclinical hypothyroidism (SCH) is a state of mildly underactive thyroid that affects more than 10% of adults and is associated with cardiovascular risk, dyslipidemia, and metabolic syndrome [Citation4].
From the perspective of pathogenesis, the two diseases have certain similarities. PCOS patients are usually accompanied by insulin resistance, which leads to disorders of blood sugar and lipid metabolism, which in turn affects the synthesis and secretion of thyroid hormones [Citation5]. Patients with hypothyroidism also often have insulin resistance, which leads to elevated serum gonadotropin (LH) levels and stimulates the ovaries to produce excessive androgens, thereby inducing or exacerbating PCOS. Ding et al. found that women with PCOS have a significantly higher chance of suffering from SCH than normal women [Citation6]. Another study found that 22.5% of PCOS patients also had SCH, compared with only 8.3% of normal controls [Citation7]. The clinical manifestations of the two also have certain overlap. PCOS patients often have symptoms such as irregular menstruation, infertility, hirsutism, and obesity, and these symptoms may also be manifestations of hypothyroidism [Citation8]. However, de-Medeiros et al. believed that SCH should not exclude the diagnosis of PCOS [Citation9]. Some studies also believe that SCH is not a risk factor for PCOS [Citation10]. Currently, it is unclear whether such comorbid conditions affect the severity of metabolic and hormonal levels in PCOS patients [Citation11].
Therefore, this study aimed to investigate the effects of SCH on hormonal and metabolic profiles and ovarian morphology in PCOS patients. By examining differences in these parameters between PCOS patients with and without SCH, as assessed by homeostasis model assessment of insulin resistance (HOMA-IR), this study sought to elucidate the impact of SCH on the complex pathophysiology of PCOS.
Methods
Study design and patients
A retrospective analysis of 133 patients who visited the Reproductive Medicine Center of Yinchuan Women and Children’s Healthcare Hospital from June 2021 to June 2023, including 58 patients with PCOS and 75 patients with PCOS combined with SCH. The detailed inclusion process of participants was shown in . The diagnostic criteria for PCOS adopt the diagnostic criteria recommended by the Rotterdam Expert Meeting of the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) in 2003 [Citation12], in which there are at least two of the following characteristics: oligoovulation or anovulation, clinical and/or Biochemical hyperandrogenism and polycystic ovaries on ultrasound. Exclusion criteria were as follows: pregnancy, breastfeeding, use of hormonal contraceptives or other drugs that may affect thyroid function or glucose metabolism in the past 3 months, history of thyroid disease or autoimmune disease, and the presence of obvious hypothyroidism or hyperthyroidism. SCH is defined as elevated serum TSH (>4.5 mIU/L) with normal free thyroxine (FT4) levels (0.8–1.8 ng/dL). Patients with thyroid hormone changes caused by pituitary and hypothalamic lesions were also excluded.
The study protocol was approved by the Ethics Committee of Yinchuan Women and Children’s Healthcare Hospital, and written informed consent was obtained from all subjects. The study was conducted in accordance with the guidelines of the Declaration of Helsinki.
Data
All participants underwent a comprehensive clinical and biochemical evaluation, including medical history, physical examination, anthropometric measurements, blood pressure, and transvaginal ultrasound. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood samples were collected in the early follicular phase (days 2–5) of the menstrual cycle or on any day in case of amenorrhea, after an overnight fast of at least 10 h. Serum levels of TSH, FT4, FT3, luteinizing hormone (LH), follicle-stimulating hormone (FSH), insulin, glucose, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides were measured using standard methods. HOMA-IR measures fasting plasma glucose and fasting insulin levels and uses the relationship between them to estimate insulin sensitivity and beta cell function. The HOMA-IR was calculated as fasting insulin (mU/L) × fasting plasma glucose (mmol/L)/22.5. Ovarian volume and follicle count were assessed by transvaginal ultrasound using a 7.5-MHz probe. The ovarian volume was calculated as the product of the three orthogonal diameters multiplied by 0.523. The follicle count was defined as the number of antral follicles with a diameter of 2–9 mm in both ovaries.
Statistical analysis
Statistical analysis was performed using SPSS software version 22.0 (SPSS Inc., Chicago, IL). Data were expressed as mean ± standard deviation (SD) for normally distributed variables, and number (percentage) for categorical variables. Normality of distribution was tested by the Kolmogorov–Smirnov test. Differences between the two groups were analyzed by the independent t-test or the Mann–Whitney U test for continuous variables and by the chi-square test or the Fisher’s exact test for categorical variables, as appropriate. Multivariate linear regression analysis was used to identify the factors associated with SCH in patients with PCOS, adjusting for potential confounders. A p value of less than .05 was considered statistically significant.
Results
Clinical and biochemical characteristics
The clinical characteristics of the study population were summarized in . The average age of PCOS patients was 27.11 ± 4.75 years old, and the average age of patients in the PCOS + SCH group was 28.03 ± 5.53 years old. There was no significant difference between the groups. At the same time, there was no statistically significant difference in marital status, irregular menstruation, and acne between the two groups of patients. However, there was a significant difference in BMI between the two groups, and the PCOS + SCH group had a higher prevalence of obesity (p = .014). Among hormone levels, compared with the PCOS group, PCOS + SCH patients had significantly higher TSH (p < .001), triglyceride (p = .025), and HOMA-IR (p < .001) levels, while LH levels were significantly decreased (p = .048)). In addition, the levels of FT3, FT4, total cholesterol, HDL-C, LDL-C, glucose, insulin, and FSH were similar in both groups ().
Table 1. Table of patient baseline characteristics.
Table 2. Metabolic and hormonal related parameters between groups.
Ovarian morphology
There was no significant difference in ovarian volume between the two groups. However, there were significant differences in follicle counts between the two groups. Although there was no significant difference in the number of follicles in the right ovary, the number of follicles in the left ovary was significantly reduced in the PCOS + SCH group compared with the PCOS group (p = .003) (). In addition, we found that the follicle diameter of the PCOS + SCH group was also significantly lower than that of the PCOS group (p = .010) ().
Table 3. The differences in overall ovarian volume and the number of follicles on each side between the groups.
Factors associated with SCH in patients with PCOS
However, we did not find the influencing factors of SCH on PCOS patients after using multiple regression linear model analysis. The negative coefficient suggested that the PCOS + SCH group was associated with a reduction in outcome variables, but the effect was not statistically significant. The positive coefficient for TSH indicated a potential increase in the outcome variable as TSH levels increased, although this was not statistically significant. Likewise, the positive coefficient on HOMA-IR indicates a potential increase in the outcome variable as the level of HOMA-IR increases, but this was also not statistically significant ().
Table 4. Multiple linear regression analysis.
Discussion
In our study, the prevalence of SCH in PCOS patients was found to be 43.6%, which was significantly higher than the reported prevalence of SCH in the general adult population of China (12.93%, as of 2017) [Citation13] and in Chinese women with PCOS (19.6%, as of 2018) [Citation6]. Notably, the prevalence in our study aligns with the findings of Xing et al.) [Citation14], who, in their meta-analysis, reported an increased prevalence of SCH in PCOS patients at 26.97%. While our study’s elevated prevalence rate could be attributed to the relatively small sample size, it resonated with findings reported by Farhadi-Azar et al. [Citation15]. It further reinforces the previously established correlation between the severity of PCOS and the presence of SCH [Citation6]. Past research suggests that PCOS can induce or exacerbate SCH by increasing androgen production, potentially interfering with thyroid hormone synthesis or metabolism. Conversely, SCH may exacerbate PCOS by increasing TSH secretion, stimulating ovarian theca cells to produce more androgens [Citation16]. This is consistent with our findings, where the PCOS + SCH group exhibited higher TSH levels compared to the PCOS alone group. However, the optimal cutoff value for TSH in PCOS patients remains controversial, with some studies suggesting that the upper normal limit of TSH should be lower in the PCOS population. Therefore, the reported prevalence and impact of SCH in PCOS patients may vary depending on the TSH standards applied.
Furthermore, our study observed that SCH in PCOS patients correlates with higher levels of triglycerides and HOMA-IR, and lower levels of LH. This association between SCH and elevated triglycerides aligns with previous studies, which have reported higher triglyceride levels in patients with both PCOS and SCH compared to those with PCOS alone [Citation17]. This may be due to the potential role of SCH in impairing lipoprotein lipase activity, increasing hepatic lipid production, or reducing the clearance of very low-density lipoproteins (VLDL) and chylomicrons. HOMA-IR, an index used to assess individual insulin resistance levels, has also been shown to be influenced by SCH [Citation11]. The association between SCH and LH levels is somewhat surprising, as previous reports have indicated that LH is not affected by SCH in PCOS patients [Citation11]. This discrepancy might be attributed to thyroid hormone levels interfering with the normal feedback mechanism for pituitary secretion of luteinizing hormone. However, our multivariate linear regression analysis revealed that none of the aforementioned variables were determinants for the occurrence of SCH in PCOS patients. This suggests that the relationship between SCH and these variables may be influenced by other factors, such as age, duration of PCOS, and genetic or environmental factors. Hence, further research is necessary to identify the risk factors and establish a causal relationship for SCH in patients with PCOS.
Additionally, our study found a correlation between SCH and higher BMI and obesity in PCOS patients, consistent with previous studies that reported a positive correlation between SCH and obesity/visceral obesity in PCOS patients [Citation18,Citation19]. However, other studies have not found a significant association between SCH and BMI or obesity in PCOS patients, which could be attributed to differences in sample size, definitions of SCH, or confounding factors [Citation20]. It is also important to consider that PCOS patients inherently tend to have higher BMIs [Citation21].
Moreover, our findings indicated that SCH does not significantly affect ovarian volume, but it does reduce follicle count and diameter in the left ovary of PCOS patients. This aligns with the findings of Trakakis et al. who reported no significant differences in ovarian volume and endometrial thickness between PCOS patients with and without SCH [Citation22]. As for follicle count, there is limited research available, but we hypothesize that SCH may affect follicular development, maturation, or atresia. Yue et al. found that in PCOS women with normal FT4 levels, the ratio of follicle-stimulating hormone was higher [Citation18]. The reason for the asymmetric impact of SCH on the left and right ovaries remains unclear, but it could be related to anatomical or functional differences between the two ovaries.
This study still has some limitations. First, because this was a cross-sectional study, a causal relationship or temporal sequence between SCH and PCOS cannot be established. Second, the sample size of this study was relatively small, which may have limited the statistical power and generalizability of the results. Finally, thyroid autoantibody levels and clinical outcomes in patients with PCOS and SCH, such as ovulation rate, pregnancy rate, or miscarriage rate, have not been fully discussed.
Conclusion
This cross-sectional study showed that SCH was prevalent in patients with PCOS and was associated with higher BMI and obesity, higher levels of TSH, triglycerides, and HOMA-IR, and lower levels of LH. However, SCH was not a determinant for the occurrence of PCOS. SCH had no significant effect on ovarian volume, but reduced the follicle count and diameter in the left ovary of patients with PCOS. Further investigation into the relationship between these disorders is warranted to delineate their clinical implications.
Authors’ contribution
Dongmei Shi: Conceptualization, Methodology, Writing – Original Draft; Juan Du: Data Curation; Huixian Kang: Formal Analysis; Liping Feng: Visualization; Fang Liu: Writing – Review & Editing. All authors have approved the final manuscript.
Acknowledgments
Not application.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author.
Additional information
Funding
References
- Tay CT, Garrad R, Mousa A, et al. Polycystic ovary syndrome (PCOS): international collaboration to translate evidence and guide future research. J Endocrinol. 2023;257(3):1.
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–6. doi: 10.1038/nrendo.2018.24.
- Dybciak P, Raczkiewicz D, Humeniuk E, et al. Depression in polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Med. 2023;12(20):6446.
- Urgatz B, Razvi S. Subclinical hypothyroidism, outcomes and management guidelines: a narrative review and update of recent literature. Curr Med Res Opin. 2023;39(3):3C–365. doi: 10.1080/03007995.2023.2165811.
- Lee MS, Nguyen H, Philipson D, et al. Single-operator" technique for advancing the orbital atherectomy device. J Invasive Cardiol. 2017;29(3):92–95.
- Ding X, Yang L, Wang J, et al. Subclinical hypothyroidism in polycystic ovary syndrome: a systematic review and Meta-Analysis. Front Endocrinol (Lausanne). 2018;9:700. doi: 10.3389/fendo.2018.00700.
- Fatima M, Amjad S, Sharaf Ali H, et al. Correlation of subclinical hypothyroidism with polycystic ovary syndrome (PCOS). Cureus. 2020;12(5):e8142. doi: 10.7759/cureus.8142.
- Couto RA, Waltzman JT, Tadisina KK, et al. Objective assessment of facial rejuvenation after massive weight loss. Aesthetic Plast Surg. 2015;39(6):847–855.
- de-Medeiros SF, Yamamoto MMW, de-Medeiros MAS, et al. Should subclinical hypothyroidism be an exclusion criterion for the diagnosis of polycystic ovary syndrome? J Reprod Infertil. 2017;18(2):242–250.
- Zhang B, Wang J, Shen S, et al. Subclinical hypothyroidism is not a risk factor for polycystic ovary syndrome in obese women of reproductive age. Gynecol Endocrinol. 2018;34(10):875–879.
- Pergialiotis V, Konstantopoulos P, Prodromidou A, et al. MANAGEMENT OF ENDOCRINE DISEASE: the impact of subclinical hypothyroidism on anthropometric characteristics, lipid, glucose and hormonal profile of PCOS patients: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(3):R159–R166. doi: 10.1530/EJE-16-0611.
- Geisthovel F. A comment on the European society of human reproduction and embryology/American society for reproductive medicine consensus of the polycystic ovarian syndrome. Reprod Biomed Online. 2003;7(6):602–605.
- Li Y, Teng D, Ba J, et al. Efficacy and safety of Long-Term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of mainland China. Thyroid. 2020;30(4):568–579. doi: 10.1089/thy.2019.0067.
- Xing Y, Chen J, Liu J, et al. The impact of subclinical hypothyroidism on patients with polycystic ovary syndrome: a meta-analysis. Horm Metab Res. 2021;53(6):382–390. doi: 10.1055/a-1463-3198.
- Farhadi-Azar M, Behboudi-Gandevani S, Rahmati M, et al. The prevalence of polycystic ovary syndrome, its phenotypes and Cardio-Metabolic features in a community sample of Iranian population: tehran lipid and glucose study. Front Endocrinol (Lausanne). 2022;13:825528. doi: 10.3389/fendo.2022.825528.
- Rojhani E, Rahmati M, Firouzi F, et al. Polycystic ovary syndrome, subclinical hypothyroidism, the Cut-Off value of thyroid stimulating hormone; is there a link? Findings of a population-Based study. Diagnostics (Basel). 2023;13(2):316. doi: 10.3390/diagnostics13020316.
- de Medeiros SF, de Medeiros MAS, Ormond CM, et al. Subclinical hypothyroidism impact on the characteristics of patients with polycystic ovary syndrome. A meta-analysis of observational studies. Gynecol Obstet Invest. 2018;83(2):105–115. doi: 10.1159/000485619.
- Yue F, Zhang D, Gong F, et al. [Subclinical hypothyroidism and endocrine metabolic characteristics in women with polycystic ovary syndrome]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42(8):940–946. doi: 10.11817/j.issn.1672-7347.2017.08.011.
- Platt AM. Insulin resistance, metabolic syndrome, and polycystic ovary syndrome in obese youth. NASN Sch Nurse. 2015;30(4):207–213. doi: 10.1177/1942602X15575355.
- Bedaiwy MA, Abdel-Rahman MY, Tan J, et al. Clinical, hormonal, and metabolic parameters in women with subclinical hypothyroidism and polycystic ovary syndrome: a cross-sectional study. J Womens Health (Larchmt). 2018;27(5):659–664.
- Raj D, Pooja F, Chhabria P, et al. Frequency of subclinical hypothyroidism in women with polycystic ovary syndrome. Cureus. 2021;13(9):e17722. doi: 10.7759/cureus.17722.
- Trakakis E, Pergialiotis V, Hatziagelaki E, et al. Subclinical hypothyroidism does not influence the metabolic and hormonal profile of women with PCOS. Horm Mol Biol Clin Investig. 2017;31(3):20160058. doi: 10.1515/hmbci-2016-0058.