Abstract
Objective
The aim of this study was to explore the impact of growth hormone (GH) therapy on the onset and progression of puberty in girls with idiopathic short stature.
Methods
This study included 541 girls aged between 4.5 and 10.6 years who were receiving GH treatment, monitored over a 22-year follow-up period. Of these, 126 girls have been followed up to the onset of menarche. The participants were divided into two groups: a ISS control group (n = 66) and a group receiving daily GH treatment at a dose of 0.15 iu/kg (n = 60). We assessed the pubertal development and GH usage of these girls every three months.
Results
(1) There was no significant difference in the onset of puberty between the growth hormone (GH) treatment group and the control group; however, the average duration of puberty was longer in the treatment group compared to the control group. (2) During puberty, there were no significant differences in height growth between the treated and untreated groups. (3) The duration of GH treatment showed a significant negative correlation with the age at onset of gonadal development and the age at menarche in females within the treatment group.
Conclusion
GH treatment does not seem to accelerate the onset of puberty but may extend its duration, without significantly impacting height growth during puberty. Additionally, longer GH treatment duration is linked to earlier gonadal development and menarche in females.
Introduction
Idiopathic short stature (ISS) is a general term used to describe short-stature diseases of unknown cause. In such cases, the individual’s height is significantly lower than 2 standard deviations or the 3rd percentile of the mean value of normal children of the same race, age and sex, excluding organic diseases, and there is no obvious family genetic history of short stature, a common cause of short stature in children [Citation1,Citation2]. The clinical manifestation of ISS in girls is mainly height below that of their peers, with otherwise normal physical development, such as head circumference, body proportion, and intelligence. Additionally, the growth rate of these girls is relatively slow, their growth rate is significantly lower than that of their normal peers, and their height is not easily affected by nutritional status or environmental factors. These girls usually enter puberty later than their peers, but the pubertal process is the same as that of normal girls [Citation3]. Girls with ISS often have a psychological burden, especially during childhood [Citation4,Citation5]. Growth hormone (GH) is a polypeptide hormone secreted by the pituitary gland that plays a vital role in bone growth, muscle development, fat metabolism, and overall growth and development. GH therapy was initially used only in children with growth hormone deficiency (GHD); however, with the advent of recombinant human growth hormone (rhGH) in the mid-1980s, GH began to be applied to children [Citation6–8], and the effect became obvious to all [Citation9]. However, there is controversy over whether accelerated growth due to GH also accelerates puberty. Some scholars believe that rhGH can improve the final height of a child with ISS [Citation10,Citation11] and that it has no significant effect on pubertal development [Citation12,Citation13]. Papadimitriou, A and other scholars have suggested that GH therapy will promote the onset of puberty and accelerate the process [Citation14]. In a five-year randomized controlled study, Kamp, GA and other scholars found that growth hormone treatment caused puberty in children with ISS to occur earlier and accelerated bone aging before puberty [Citation15]. Therefore, it is very important to study the effect of GH therapy on the pubertal development of girls with ISS.
To this end, we conducted a 22-year study, regularly followed up girls with ISS who received rhGH treatment from October 2001 to November 2023, observed the changes in their pubertal developmental status, and conducted research on the factors related to the influence of rhGH on sexual development. Analysis, research, and research results can provide important references for pubertal development in idiopathically short girls in the clinical treatment process and help doctors formulate more reasonable treatment plans to maximize the growth and development of patients.
Objectives and methods
Objectives
Girls aged 4.5–10.6 (6.7 ± 1.5) years, all without sexual development (Tanner stage 1) who were diagnosed with ISS by clinical features and laboratory tests, were examined. The inclusion criterion was a diagnosis of ISS. The diagnosis was made when the following conditions were met [Citation1,Citation16,Citation17]: ① The height was below the third percentile of the growth curve of normal healthy children of the same age and sex or below two standard deviations; ② The growth rate was slowed or normal (Children whose height growth rate is above the 25th percentile (based on bone age) are as follows: For children under 2 years old, a growth rate of ≥7 cm/year is required; for children aged 4.5 years to puberty, a growth rate of ≥5 cm/year is necessary, and during puberty, the growth rate should be ≥6 cm/year.); ③ Birth length was greater than −2.0 SD, and birth weight was normal (normal birth weight being between 2.5 kilograms and 4 kilograms); ④ arginine (0.5 g/kg) and clonidine (5 µg/kg) with two growth hormone stimulation tests with GH peak values greater than 10 μg/L; ⑤ normal karyotype; ⑥ normal brain MRI; ⑦ no endocrine-related diseases, chronic systemic diseases, nutritional diseases, or skeletal diseases; and ⑧ no serious emotional or eating disorders. This study initially screened 541 girls with ISS as potential subjects. In this research, 252 girls who exhibited breast development within one year after commencing growth hormone (GH) therapy were excluded. This exclusion is vital to accurately discern the effects of GH therapy on pubertal development, as premature onset of puberty could potentially confound these results [Citation10]. Additionally, 135 girls on developmental suppression medication, such as Leuprolide and Triptorelin, were also excluded to ensure the study’s precision and focus. Additionally, we excluded children who developed diseases that could affect growth during the treatment, those lost to follow-up, and those who had not yet experienced menarche. After this comprehensive screening, 60 girls with ISS were included in the GH treatment group. These patients began treatment at an average age of 8.21 ± 1.70 years, with an average treatment duration of 3.80 ± 1.41 years, and an average bone age of 6.74 ± 1.80 years at the start of treatment. For the control group, we randomly selected untreated girls with idiopathic short stature from outpatient follow-ups in approximately a 1:1 ratio for comparison.Currently, the 60 children receiving GH treatment and the 66 children in the control group have completed a 22-year long follow-up study ().
This study was conducted in accordance with the Declaration of Helsinki (2013 revision) and approved by the Ethics Committee of Wenzhou People’s Hospital and Wenzhou Maternal and Child Health Hospital (Ethics approval No.: KY-2022-086). Informed consent was obtained from all patients.
Methods
Treatment and follow-up: In the GH treatment group, 60 ISS girls were treated with subcutaneous injection of rhGH (Jintropin AQ, GeneScience Pharmaceuticals) at a dose of 0.15 U/kg/day, 6 nights per week before going to bed (0.9 U/kg/w). The treatment time continued until the child reached near final height, which was considered to have been reached when the growth rate was <1.5 cm/year or the female bone age was 15 years old. During treatment, investigators performed assessments of pubertal development and monitored GH use every 3 months. During GH therapy, regular testing of height, weight, bone age, fasting blood glucose, insulin-like growth factor-1 (IGF-1) levels and other indicators was needed. If adverse reactions occurred during treatment, it was necessary to adjust the treatment dose or discontinue treatment in time; after the treatment period was over, long-term follow-up was needed to evaluate the durability of the treatment effect and any possible long-term effects. The safety and efficacy of GH therapy were ensured.
Assessment methods for pubertal development: In this study, the researchers conducted pubertal developmental assessments for all enrolled children every 3 months. In the evaluation of pubertal development, determinations of whether puberty had started in girls were made by examining signs and performing clinical evaluations. Commonly used assessments included breast development, in which breast size, shape, and symmetry for signs of initial growth were evaluated. In instances of breast development, subsequent examinations will be conducted, such as uterine evaluation and serum sex hormone level assessment. The uterine examination consisted of evaluations of the size and shape of the uterus, the length and thickness of the lining of the cervix, and the size of the follicles. The serum hormone levels of estradiol, follicle-stimulating hormone, and luteinizing hormone were detected to evaluate ovarian function and gonadal hormone levels. By evaluating these indicators, it was possible to determine whether a girl had entered puberty. This helped researchers assess the impact of GH treatment on pubertal development.
Control group: Clinical examinations, including height and weight measurements, pubertal development assessment, relevant blood tests and bone age assessment, were performed every 3 months. In addition, the researchers recorded and analyzed factors such as menstruation, fertility and genetic family history. The follow-up doctor and the interpretation of the bone age film were performed by the same doctor in the growth and development department to avoid different interpretations of the appearance time of secondary sexual characteristics and bone age due to different personal experiences. Bone age was evaluated by the Greulich-Pyle wrist bone age atlas method (GP atlas method) to evaluate the bone age of the child’s left hand.
Statistical method: SPSS 23.0 statistical software was used for data analysis and sorting. Count data are presented as the number (n), and measurement data are presented as the mean ± standard deviation (x ± s). Between-group comparisons were analyzed by means of independent sample t tests. Correlations were evaluated by Pearson analysis. Statistical significance was defined as P < 0.05.
Results
Does growth hormone therapy accelerate the onset of puberty? Does growth hormone therapy affect the duration of puberty development?
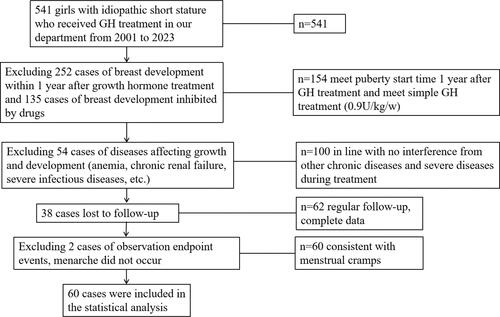
According to our research findings, GH treatment does not appear to affect the age at which puberty begins. Specifically, the duration of pubertal development in the GH treatment group seems to be longer than that of the control group. In the GH treatment group, the average age of onset of secondary sexual characteristics was 9.89 ± 1.10 years, compared to 9.58 ± 1.07 years in the control group, with no significant difference between the two (see and ), indicating that GH treatment does not affect the initiation time of development. However, regarding the average age of menarche onset, the treatment group had an average age of 12.71 ± 0.93 years, while the control group had an average of 11.91 ± 0.88 years, showing a significant difference (see and ). Furthermore, the average duration of puberty in the treatment group was 2.82 ± 0.87 years, compared to 2.32 ± 0.73 years in the control group (), suggesting that the duration of puberty in the treatment group was significantly longer, implying that GH treatment might extend the time of gonadal development. Therefore, our conclusion is that GH treatment does not accelerate the onset of puberty and may potentially extend the duration of puberty.
Figure 2. (A) Comparison of age at puberty; (B) comparison of age at menarche; (C) comparison of the period of sexual maturation; (D) comparison of height at puberty; (E) comparison of height at menarche; (F) comparison of height growth from puberty to menarche.
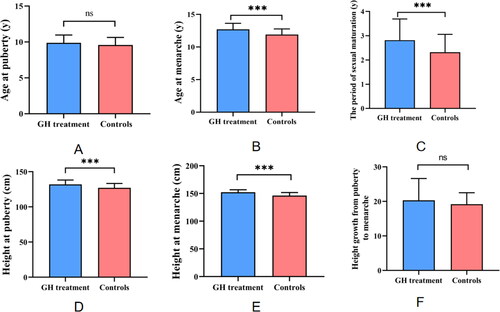
Table 1. Comparison of developmental indicators between the treatment group and the control group.
During the pubertal growth period, does growth hormone treatment accelerate height increase?
As illustrated in and parts D, E, and F of , children undergoing growth hormone (GH) therapy exhibited significantly higher stature and height standard deviation scores (SDS) at the onset of puberty and the arrival of menarche compared to the untreated control group. However, when comparing the height growth differences between the two groups from the onset of puberty to menarche, no statistically significant differences were observed. These findings suggest that the primary impact of growth hormone therapy on enhancing final height predominantly occurs before the initiation of pubertal development. During the growth phase of puberty, growth hormone therapy does not appear to accelerate the rate of height increase.
Demonstrate that the growth hormone (GH) treatment group had significantly higher measurements in height at the onset of development, height SDS (Standard Deviation Score) at the onset of development, age at menarche, height at menarche, height SDS at menarche, and the time span from development onset to menarche, compared to the control group. Furthermore, the bone age of the treatment group at the onset of development was notably younger than that of the control group. However, there were no significant differences observed between the two groups in terms of bone age at the onset of development and at the time of menarche. The target height of the GH treatment group was significantly lower than that of the control group.
Does the duration of GH treatment affect pubertal development?
As depicted in and , there is a significant negative correlation between the duration of growth hormone (GH) therapy in the treatment group and the age at onset of gonadal development, as well as the age at menarche in females. This finding suggests that the longer the duration of growth hormone therapy, the earlier the onset of gonadal development and the occurrence of menarche in females. This result underscores the potential influence of growth hormone on the maturation process of children’s gonads, indicating that the length of therapy might accelerate the onset of pubertal physiological events.
Figure 3. (A) The correlation between GH usage time and age at onset of development; (B) the correlation between GH usage time and age at menarche.
Table 2. Correlation analysis of GH usage duration and development indicators in the treatment group.
shows that in the treatment group, the duration of GH use is significantly negatively correlated with both the age at onset of development and the age at menarche (r < 0, p < 0.05). There is no significant correlation between GH usage duration and other measured indicators
Discussion
Puberty is a critical phase that significantly influences adult height, as the age at the onset of puberty, its duration, and the rate of growth during puberty all have a profound impact on final adult stature. Height growth during puberty accounts for approximately 17% to 18% of adult height. GH, a peptide secreted by the anterior pituitary gland, plays a key role in regulating cell growth, development, and the metabolism of various target tissues [Citation18]. The GH/IGF-1 axis (IGF-1 is induced by the activity of GH) is a crucial endocrine system that profoundly affects the human body by acting on various peripheral tissues and the brain [Citation19,Citation20].
To minimize the influence of hormonal levels during puberty on the outcomes, this study excluded cases exhibiting breast development within one year of initiating Growth Hormone (GH) therapy [Citation3]. This approach was critical for several reasons: (1) Such exclusion facilitates distinguishing the impact of GH treatment on idiopathic short stature from natural pubertal development processes. (2) Early pubertal changes independently affecting growth patterns and hormone levels might confound results. Excluding these cases enhances the credibility of the research in assessing GH treatment. (3) By excluding these individuals, the study provides a more accurate interpretation of GH treatment’s efficacy and safety in the targeted population, as it eliminates variables associated with early puberty. However, this exclusion criterion might limit the study’s applicability to a broader population, as it does not encompass girls who exhibit early pubertal symptoms post-GH treatment initiation.
In this study, we compared the GH treatment group with a control group of untreated girls with ISS and found that GH treatment does not appear to affect the age at onset of puberty (as seen in and ). Moreover, the duration of pubertal development in the GH treatment group seems to be longer than that in the control group (as indicated in ). This outcome suggests that GH treatment may extend the period of gonadal development. This could be linked to the exogenous administration of recombinant human GH, which may induce negative feedback suppression of the body’s own anterior pituitary secretion of GH. This closely ties the hypothalamic-pituitary-somatotropic (HPS) axis with the hypothalamic-pituitary-gonadal (HPG) axis, mutually regulating each other’s activities [Citation21]. This interaction may indirectly affect the anterior pituitary’s secretion of gonadotropins, thereby influencing the developmental process. Therefore, we believe that GH treatment does not accelerate the onset of puberty but may have a potential effect on extending the duration of puberty.
During puberty, the secretion of GH proportionally increases with the rise in sex hormones, while the increase in GH also promotes the secretion of sex hormones, demonstrating a mutual regulatory interaction between the two. According to the results presented in and , there is a significant negative correlation between the duration of GH treatment in the girls in the treatment group and both the age at the onset of development and the age at menarche. This suggests that the longer the duration of GH use is, the earlier the age at onset of development and menarche in girls will be. This indicates that prolonged use of growth hormone may gradually alter the hormonal levels and developmental state of the body, thereby accelerating the process of gonadal development. This might be related to the following mechanisms: GH and IGF-1 receptors are expressed [Citation13] and can regulate the activity of sex hormones [Citation22–29]. Additionally, GH has a gonadotropin-like effect is capable of enhancing the secretion of gonadotropin-releasing hormone (GnRH) and gonadotropins (FSH, LH) from the hypothalamus and pituitary and upregulating the expression of gonadotropin receptors (FSH and LH receptors), thus enhancing the reactivity of granulosa cells to gonadotropins [Citation29–32]. GH can also activate primordial follicles and stimulate granulosa and oocyte synthesis of estrogen [Citation33] through the PI3K-PTEN-AKT-FOX3 signaling pathway and AMH-mediated stimulation [Citation34–36], promoting the growth and development of follicles, by regulating the expression and secretion of various growth factors such as IGF and epidermal growth factor (EGF) [Citation37], thereby promoting the growth and development of gonads [Citation21,Citation38].
Growth hormone may exert both direct and indirect effects on gonadal development. The direct effect could involve the impact of growth hormone on gonadal tissues, whereas the indirect effect might pertain to the influence of growth hormone on overall growth and maturation. The duration of growth hormone therapy appears to be a critical factor. Prolonged use may lead to the body’s adaptation to growth hormone, thereby affecting gonadal development. Conversely, short-term use may not be sufficient to elicit significant biological changes. A negative correlation between the duration of growth hormone therapy and the age of gonadal activation or menarche might suggest that growth hormone accelerates the overall maturation process, including the development of the gonads. However, this acceleration might initially manifest as a relative delay, as growth hormone may first impact other aspects of bodily growth.
As a subset of the female subjects in the treatment group have not yet experienced menarche, and the sample size of the growth hormone (GH) therapy group is relatively small, the possibility of sample bias or random error cannot be excluded. Therefore, to more accurately assess the impact of growth hormone levels on gonadal development, future research needs to be both more extensive and in-depth to validate this hypothesis.
In the research data presented in and , it was observed that children undergoing growth hormone (GH) therapy exhibited significantly greater height and height standard deviation scores (SDS) at the onset of puberty and the arrival of menarche compared to their counterparts in the untreated control group. Notably, during the peak growth period of puberty, girls receiving GH treatment showed a similar pattern of height growth to those in the untreated control group. This finding supports the efficacy of growth hormone therapy in accelerating height growth, particularly before the onset of puberty, as indicated in references [Citation39,Citation40].
Limitations of this study: (1) It is difficult to control for all variables. For example, there are large individual differences in pubertal growth and development, and large-sample research would be conducive to narrowing this difference. Therefore, our research is continuing, and follow-up of GH treatment for short girls is ongoing. (2) The sample size of this study is relatively small, and there may be sample bias. The study used a comparison method between the treatment group and the control group and did not adjust for other potential factors, such as genetic factors and nutritional status, which may have an impact on the research results.
Conclusion
Recent research indicates that growth hormone therapy does not expedite the onset of puberty, yet it appears to extend the total duration of the pubertal phase, influencing specific stages of maturation. The therapy has a significant effect on promoting final height in children with ISS, particularly before the onset of puberty. However, once puberty begins, GH treatment does not seem to accelerate the rate of height growth. These findings suggest that treatment plans should prioritize initiating therapy before puberty to maximize its effectiveness. Additionally, longer GH treatment duration is linked to earlier gonadal development and menarche in females. Although these observations are enlightening, due to the limited sample size, they require further validation through larger-scale studies.
Authors’ contributions
The first author, Feng Zhu, developed the original concept of this study, collected the data, analyzed the data, and wrote the article. Qi Xu, Lingxiao Huang, Jieqian Zhu and Lina Huang helped to collect data. The corresponding author, Yu Zhang, interpreted the data and critically approved the final article. All authors had full access to all the data in the study and approved the final manuscript.
Ethical approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki (2013 revision) and was approved by the Ethics Committee of Wenzhou People’s Hospital and Wenzhou Maternal and Child Health Hospital. Informed consent was obtained from the participants and their families.
| Abbreviations | ||
| GH | = | Growth hormone |
| GHD | = | Growth hormone deficiency |
| RhGH | = | Recombinant human growth hormone |
| ISS | = | Idiopathic short stature |
| IGF-1 | = | Insulin-like growth factor-1 |
| PSM | = | Propensity score matching |
| MA | = | Menarche age |
| DA | = | Developmental onset age |
| FSH | = | Follicle-stimulating hormone |
| LH | = | Luteinizing hormone |
| HPG | = | Hypothalamic-pituitary-gonadal axis |
| HPS | = | Hypothalamic-pituitary-growth axis |
Acknowledgments
We thank the doctors, nurses, laboratory staff, and study participants for the work that has been done, and we also thank all patients who were involved in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality agreements and the need to adhere to ethical guidelines but are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Cohen P, Rogol AD, Deal CL, R.A.D.C., 2007 ISS Consensus Workshop participants. et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93(11):1–8. doi: 10.1210/jc.2008-0509.
- Ranke MB. Towards a consensus on the definition of idiopathic short stature. Horm Res. 1996;45(Suppl 2):64–66. doi: 10.1159/000184851.
- Maghnie M, Ranke MB, Geffner ME, et al. Safety and efficacy of pediatric growth hormone therapy: results from the full KIGS cohort. J Clin Endocrinol Metab. 2022;107(12):3287–3301. doi: 10.1210/clinem/dgac517.
- Chen SK. Is growth hormone treatment associated with the psychological status in children with short stature? Am J Ther. 2021;28(3):368–370. doi: 10.1097/MJT.0000000000001275.
- Shemesh-Iron M, et al. Growth hormone therapy and short stature-related distress: a randomized placebo-controlled trial. Clin Endocrinol (Oxf). 2019;90(5):690–701.
- Bamba V, Kanakatti SR. Approach to the patient: safety of growth hormone replacement in children and adolescents. J Clin Endocrinol Metab. 2022;107(3):847–861. doi: 10.1210/clinem/dgab746.
- Graber E, Reiter EO, Rogol AD. Human growth and growth hormone: from antiquity to the recominant age to the future. Front Endocrinol (Lausanne). 2021;12:709936. doi: 10.3389/fendo.2021.709936.
- Tidblad A. The history, physiology and treatment safety of growth hormone. Acta Paediatr. 2022;111(2):215–224.
- Kim J, Suh B-K, Ko CW, et al. Recombinant growth hormone therapy for prepubertal children with idiopathic short stature in Korea: a phase III randomized trial. J Endocrinol Invest. 2018;41(4):475–483. doi: 10.1007/s40618-017-0786-8.
- Abucham J, Boguszewski M. From the full KIGS cohort: on safety and efficacy of growth hormone treatment. J Clin Endocrinol Metab. 2022;108(1):e1–e2. doi: 10.1210/clinem/dgac625.
- Lonero A, Giotta M, Guerrini G, et al. Isolated childhood growth hormone deficiency: a 30-year experience on final height and a new prediction model. J Endocrinol Invest. 2022;45(9):1709–1717. doi: 10.1007/s40618-022-01808-4.
- Albin A-K, Ankarberg-Lindgren C, Tuvemo T, et al. Does growth hormone treatment influence pubertal development in short children? Horm Res Paediatr. 2011;76(4):262–272. doi: 10.1159/000329743.
- Han L, Tian H, Guo X, et al. Regulation of ovarian function by growth hormone: potential intervention of ovarian aging. Front Endocrinol (Lausanne). 2022;13:1072313. doi: 10.3389/fendo.2022.1072313.
- Papadimitriou A, Marakaki C, Papadimitriou DT. Growth variations with opposite clinical outcomes and the emerging role of IGF-1. Trends Endocrinol Metab. 2022;33(5):359–370.
- Kamp GA, Waelkens JJJ, de Muinck Keizer-Schrama SMPF, et al. High dose growth hormone treatment induces acceleration of skeletal maturation and an earlier onset of puberty in children with idiopathic short stature. Arch Dis Child. 2002;87(3):215–220. doi: 10.1136/adc.87.3.215.
- Shen Y. Guidelines for diagnosis and treatment of children with short stature. Zhonghua Er Ke Za Zhi. 2008;46(6):428–430.
- Shenkin SD, Starr JM, Deary IJ. Birth weight and cognitive ability in childhood: a systematic review. Psychol Bull. 2004;130(6):989–1013.
- Halmos T, Suba I. The physiological role of growth hormone and insulin-like growth factors. Orv Hetil. 2019;160(45):1774–1783. doi: 10.1556/650.2019.31507.
- Al-Massadi O, Parini P, Fernø J, et al. Metabolic actions of the growth hormone-insulin growth factor-1 axis and its interaction with the Central nervous system. Rev Endocr Metab Disord. 2022;23(5):919–930. doi: 10.1007/s11154-022-09732-x.
- Gusmao DO, de Sousa ME, Tavares MR, et al. Increased GH secretion and body growth in mice carrying ablation of IGF-1 receptor in GH-releasing hormone cells. Endocrinology. 2022;163(11):151.doi:10.1210/endocr/bqac15.
- Xu Y, Han CY, Park MJ, et al. Increased testicular insulin-like growth factor 1 is associated with gonadal activation by recombinant growth hormone in immature rats. Reprod Biol Endocrinol. 2022;20(1):72. doi: 10.1186/s12958-022-00944-z.
- Devoto L, Christenson LK, McAllister JM, et al. Insulin and insulin-like growth factor-I and -II modulate human granulosa-lutein cell steroidogenesis: enhancement of steroidogenic acute regulatory protein (StAR) expression. Mol Hum Reprod. 1999;5(11):1003–1010. doi: 10.1093/molehr/5.11.1003.
- Zhao J, Taverne MAM, van der Weijden GC, et al. Immunohistochemical localisation of growth hormone (GH), GH receptor (GHR), insulin-like growth factor I (IGF-I) and type I IGF-I receptor, and gene expression of GH and GHR in rat pre-antral follicles. Zygote. 2002;10(1):85–94. doi: 10.1017/s0967199402002125.
- Kajimura S, Kawaguchi N, Kaneko T, et al. Identification of the growth hormone receptor in an advanced teleost, the tilapia (Oreochromis mossambicus) with special reference to its distinct expression pattern in the ovary. J Endocrinol. 2004;181(1):65–76. doi: 10.1677/joe.0.1810065.
- Ahumada-Solórzano SM, et al. Local expression and distribution of growth hormone and growth hormone receptor in the chicken ovary: effects of GH on steroidogenesis in cultured follicular granulosa cells. Gen Comp Endocrinol. 2012;175(2):297–310.
- Marchal R, Caillaud M, Martoriati A, et al. Effect of growth hormone (GH) on in vitro nuclear and cytoplasmic oocyte maturation, cumulus expansion, hyaluronan synthases, and connexins 32 and 43 expression, and GH receptor messenger RNA expression in equine and porcine species. Biol Reprod. 2003;69(3):1013–1022. doi: 10.1095/biolreprod.103.015602.
- Steffl M, Schweiger M, Mayer J, et al. Expression and localization of growth hormone receptor in the oviduct of cyclic and pregnant pigs and mid-implantation conceptuses. Histochem Cell Biol. 2009;131(6):773–779. doi: 10.1007/s00418-009-0573-5.
- de Prada JK, VandeVoort CA. Growth hormone and in vitro maturation of rhesus macaque oocytes and subsequent embryo development. J Assist Reprod Genet. 2008;25(4):145–158. doi: 10.1007/s10815-008-9208-3.
- Chang C-W, Sung Y-W, Hsueh Y-W, et al. Growth hormone in fertility and infertility: mechanisms of action and clinical applications. Front Endocrinol (Lausanne). 2022;13:1040503. doi: 10.3389/fendo.2022.1040503.
- Zhao Y, et al. Circadian transcription factor Dbp promotes rat calvarial osteoprogenitors osteogenic differentiation through Kiss1/GnRH/E2 signaling pathway loop. J Cell Biochem. 2021;122(2):166–179.
- Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485–500. doi: 10.1093/humupd/dmu009.
- Hull KL, Harvey S. GH as a co-gonadotropin: the relevance of correlative changes in GH secretion and reproductive state. J Endocrinol. 2002;172(1):1–19. doi: 10.1677/joe.0.1720001.
- Pan P, Huang X. The clinical application of growth hormone and its biological and molecular mechanisms in assisted reproduction. Int J Mol Sci. 2022;23(18):10768. doi: 10.3390/ijms231810768
- Cannarella R, et al. Anti-Mullerian hormone, growth hormone, and insulin-like growth factor 1 modulate the migratory and secretory patterns of GnRH neurons. Int J Mol Sci. 2021;22(5):2445. doi: 10.3390/ijms22052445.
- Gong Y, Luo S, Fan P, et al. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod Biol Endocrinol. 2020;18(1):121. doi: 10.1186/s12958-020-00677-x.
- Schneider A, Zhi X, Bartke A, et al. Effect of growth hormone receptor gene disruption and PMA treatment on the expression of genes involved in primordial follicle activation in mice ovaries. Age (Dordr). 2014;36(4):9701. doi: 10.1007/s11357-014-9701-9.
- Wu WB, et al. VEGF concentration in a preovulatory leading follicle relates to ovarian reserve and oocyte maturation during ovarian stimulation with GnRH antagonist protocol in in vitro fertilization cycle. J Clin Med. 2021;10(21):5032. doi: 10.3390/jcm10215032.
- Tenuta M, Carlomagno F, Cangiano B, et al. Somatotropic-Testicular axis: a crosstalk between GH/IGF-I and gonadal hormones during development, transition, and adult age. Andrology. 2021;9(1):168–184. doi: 10.1111/andr.12918.
- Crowe BJ, et al. Effect of growth hormone dose on bone maturation and puberty in children with idiopathic short stature. J Clin Endocrinol Metab. 2006;91(1):169–175.
- Chae HW, Hwang I-T, Lee J-E, et al. Height outcomes in Korean children with idiopathic short stature receiving growth hormone treatment. Front Endocrinol (Lausanne). 2022;13:925102. doi: 10.3389/fendo.2022.925102.