 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Menopausal transition in women involves complex neurobiochemical changes linked to ovarian dysfunction, resulting in symptoms like vasomotor symptoms (VMS), sleep disturbances, anxiety, and cognitive impairments. Hormone replacement therapy is the first-line treatment. However, many women are reluctant to use HRT or have contraindications toward HRT and seek for alternatives. Non-hormonal therapies with extracts of Cimicifuga racemosa rhizomes like the isopropanolic extract (iCR, black cohosh) offer a promising alternative. A preclinical pilot study exploring iCR’s effects on gene expression in the hippocampus and hypothalamus of ovarectomized (OVX) rats mimicking menopausal conditions identified important signaling pathways and CNS-based contributions to the multitargeted modes of action of iCR. Especially in the hippocampus, iCR compensated effects of OVX on gene expression profiles. These changes are reflected by the genes AVPR1A, GAL, CALCA, HCRT, PNOC, ESR1, ESR2 and TAC3 contributing to the formation of hot flushes or thermoregulation as well as to secondary effects such as blood pressure, metabolism, hormonal regulation, homeostasis, mood regulation, neuroendocrine modulation, regulation of sleep and arousal, and in learning, memory and cognition. To understand the mechanisms in the brain of estrogen-depressed animals (OVX) and subsequent iCR treatment we combined the results of the pilot study with those of up-to-date literature and tried to transfer the current knowledge to humans during menopausal transition and adaptation. Focus was laid on changes in the hippocampal function, that is disturbed by hormonal fluctuations, but can also be brought back into balance by iCR.
Introduction
During menopause women experience a variety of changes in their body and mind as a consequence of the neurobiochemical changes, associated with ovarian dysfunction, i.e. vasomotor symptoms (VMS) as well as sleep disturbances, anxiety, depressive moods and changes in cognitive performance [Citation1]. Hormone replacement therapy (HRT) is considered first choice for treating menopausal symptoms [Citation2] as it artificially compensates for the hormone deficiency. This approach seems very simple, but until now it is known that circulating peripheral estrogen levels are not correlated with VMS. Instead, the pace of estrogen decrease and fluctuations (among other sex hormones) causes an imbalance of serotonin- and noradrenalin levels (and other) in the thermoregulatory center, leading to VMS and cognitive impairments [Citation1, Citation3,Citation4].
Especially for women with an increased risk of thrombosis or hormone-dependent cancer, non-hormonal therapies such as isopropanolic extract of Cimicifuga racemosa rhizomes (iCR) can be used instead of HRT due to its confirmed positive risk-benefit profile [Citation5,Citation6]. Its use is well established in the therapy of climacteric complaints such as hot flashes, night sweats and associated sleep disturbances [Citation5,Citation7]. The knowledge of the etiology and mechanisms of hot flashes on the one hand and the mode(s) of action of CR on the other hand is limited.
Decades ago, it was presumed that the mode of action of CR involves non-organ-selectively hormonal signaling through estrogen receptors (ER). But CR does not have an estrogenic effect mediated through ERs and its mode of action is even more complex [Citation8]. The mechanism(s) by which iCR reduces neurovegetative climacteric complaints has partially been clarified. CR exerts a multifaceted and complex set of effects by combining various pathways [Citation9,Citation10]. As previously summed up [Citation9] iCR binds to serotonin, dopamine, GABA and μ-opioid brain receptors leading to receptor-mediated functional recovery [Citation11–13]. In addition, iCR ameliorates OVX-induced changes of the serotonergic and noradrenergic brainstem-preoptic anterior hypothalamus pathways [Citation14].
One aspect which contributes to the multifaceted mode(s) of iCR’s action is shown for that it can compensate ovariectomy (OVX)-induced changes of gene expression in hypothalamus and especially in the hippocampus in rats [Citation10]. Exploratory qPCR and microarray analyses revealed that some signaling paths are changed due to OVX and subsequent estrogen decline, and several of these alterations are, at least partially, restored by iCR in the hippocampus [Citation10]. The results of this in vivo study together with an up-to-date literature search should bring us closer to an explanation of menopausal processes in the hippocampus and therapy options for menopausal complaints.
Overview of the preclinical gene expression study results
We critically reviewed the results already described in the pilot study and supplementary data [Citation10] and combined them with those of up-to-date literature, additional data from the full study report and with focus on which conclusions are relevant for clinic and understanding VMS development as well as the mode of action of CR. The underlying methods used in the pilot study were: Estrogen decline in menopausal women is mimicked in the pilot study approach by the established animal model of OVX. Gene expression profiles from hippocampal and hypothalamic tissues from 20 female Sprague Dawley rats with or without treatment with iCR (OVX + iCR) were compared to those of intact rats (PRAE) using a 44K RNA-microarray-chip. An overlap analysis filtered features for an exclusive iCR effect and the subsequent compensation analysis for a compensatory effect where the treatment with CR counteracted the effect of ovariectomy. Gene ontology and pathway analyses were performed using the Broad Institute Gene Set Enrichment Analysis (GSEA) platform. Subsequently, verification of such changes of the expression of target genes selected by the DNA-chip based analyses was done by qPCR. For more detail see [Citation10].
In the pilot study, the primary analysis of differentially expressed genes filtered 4,812 genes out of a 44K microarray chip [Citation10]. Thereupon, 1759 genes were differentially expressed in the hypothalamus and 2210 in the hippocampus representing the effect of iCR after OVX treatment. The subsequent GSEA analysis pointed on possible interactions and treatment-induced processes in these brain areas. As shown in Stute, von Wolff [Citation15], there was a strong correlation between the binding/activity of receptors in hippocampus, especially between the binding of odorants and olfactory receptor (OR) activity. In the hypothalamus, pathways mainly regarding metabolic processes could be filtered out.
CR is able to alleviate OVX-induced thermoregulatory impairments [Citation16]. Also further climacteric symptoms like anxiety, depressive mood and sleep problems are reduced by iCR treatment as shown in a recent clinical trial [Citation17]. To find out what mechanism underlies this compensatory effect at the molecular level, overlap analysis with criteria for compensation was performed. The group of iCR-treated compensated features represented 213 genes in the hippocampus and 349 genes in the hypothalamus. This approach led to a more specific picture of the processes initiated by iCR [Citation10]. In the hypothalamus, the molecular functional analysis was condensed to a single pathway in which insulin-like growth factor binding was found to be significant (p = .007). In the hippocampus, differentially expressed genes are overrepresented in gene ontology terms such as neuropeptide hormone activity (p = 754E-11) and positive regulation of blood pressure (p = 563E-06) ( and supplementary table 2 in Stute, Ehrentraut [Citation10]).
Figure 1. Word cloudFootnote1 of results from GSEA analysis of samples whose OVX-induced change in expression was compensated for by iCR treatment in the hippocampus (213). Terms are highlighted analogous to its significance (for raw values see Supplementary Data of the original publication [Citation10]).
![Figure 1. Word cloudFootnote1 of results from GSEA analysis of samples whose OVX-induced change in expression was compensated for by iCR treatment in the hippocampus (213). Terms are highlighted analogous to its significance (for raw values see Supplementary Data of the original publication [Citation10]).](/cms/asset/875421ad-254c-46a3-b4f7-7ae86cb8a6ff/igye_a_2360066_f0001_c.jpg)
To verify the microarray analysis in principle and to look more closely at possible target genes for iCR, qPCR analyses were carried out. Representative genes were selected to answer the questions about a compensatory, exclusive iCR effect, and about genes commonly discussed in science. The results showed that qPCR did not confirm the microarray results for all target genes, but for essential parts. In particular, compensatory effects in the hippocampus, for example in GAL, CALCA and HCRT. In some cases, an exclusive iCR effect in the hippocampus was observed (AVPR1A, PNOC, KISS1). Furthermore, a compensatory effect at AVPR1A in the hypothalamus was detected. shows the main results of the examined qPCR analyses. illustrates the counteracting effect of iCR treatment in the hippocampus. This effect was not pronounced in the hypothalamus (). Here, the OVX-induced increase in gene expression of KISS1 was evident.
Figure 2. Gene expression of selected target genes in the ahippocampus and bhypothalamus and determined by qPCR, effects of OVX and OVX + iCR treatment compared to untreated (PRAE) animals.
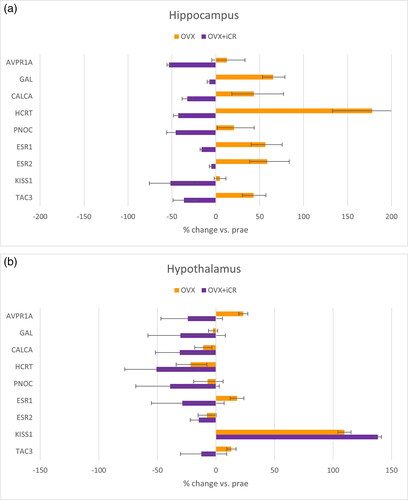
Table 1. Effect of OVX and OVX + iCR treatment on target genes in hippocampus and hypothalamus determined in microarray and qPCR. The arrows indicate increased or decreased gene expression of the groups (OVX vs. PRAE/OVX + iCR vs. OVX), the asterisk indicates a significant effect.
Peer-reviewed literature was searched for new scientific insights into the pathophysiology of menopause and the multifaceted mode of action of black cohosh. We focused the search on the selected target genes that were compensated by iCR and the association of the hippocampus with estrogen decline, OVX, vasomotor symptoms and cognition. This search was not carried out systematically, but served to find out how the hippocampus is involved in the transition to menopause and in thermoregulation, and how the identified compensated genes are involved in menopausal transformation and targeted by iCR.
Transfer of preclinical study data on clinical importance
Gene expression profiling may identify a few genes as ‘key players’ in observed biological phenomena, as well as to look at the ‘big picture’ and uncover important interactions between multiple genes and understand changes at the level of molecular pathways and networks [Citation18]. Challenges with this DNA-chip-technique include interpretation of results and reproducibility [Citation18], but it allows the clinician to understand pathophysiology and to get closer to the mode of action of a multi-component medicine.
The high quality-defined herbal extract iCR contains a multitude of substances. Therefore, it is not a matter of ‘one substance modulates one gene’ but a story of ‘a multitude of substances modulates a set of genes’, i.e. a multi-target approach typical of modern systems biology. An important tool to uncover the ‘big picture’ for iCR′s mode(s) of action(s) was the GSEA together with the overlap analyses including the filtering for iCR-compensated OVX-induced changes of gene expressions. This led to a set of genes already more or less known to be involved in menopausal transformation and estrogen decline.
The focus was on two important brain regions involved in functions known to be affected during menopause, namely the hypothalamus and the hippocampus. The hypothalamus is the most important control center of the autonomic nervous system. It connects between the nervous system and the endocrine system and has the control over the pituitary gland, which releases hormones that regulate various autonomous body functions like body temperature, sleep, circadian rhythm, water and electrolyte balance, energy balance, sexual function, emotional functions. Therefore, research on thermoregulatory disorders during menopause focuses mainly on this area of the brain, as it is the control point for it (Wang, Yang [Citation19] for example). But the pilot study [Citation10] and others [Citation20–24] also indicated hippocampal involvement. iCR led to changes in the spectral frequencies of rat brain electrical activity, with the strongest effects in the frontal cortex and hippocampus [Citation24]. The hippocampus is the switching point between short and long-term memory, involved in information selection, memory consolidation, learning, evaluation of odors, spatial orientation, control and processing of emotions, emotional memory [Citation15].
Hippocampal changes are observed in women with the bilateral salpingo-oophorectomy, a female-specific risk factor for dementia [Citation25]. The ventral hippocampus, rich in ER-β-receptors and therefore influenced by estrogens [reviewed by 23], is a functionally relevant region that mediates anxiety, stress, and emotional responses that are often dysregulated during the menopausal transition [Citation21]. It is sensitive to changing estrogen levels, resulting in verbal memory lapses and hot flashes [Citation20,Citation23]. Thurston, Maki [Citation20] concluded that the default mode network connectivity in the hippocampus is associated with menopausal hot flashes. This brain region is responsible for modulation of the body physiology, including the activity of the hypothalamus–pituitary–adrenal axis, blood pressure, immunity, and reproductive function [Citation26].
The GSEA of hippocampal samples resulted in some iCR-compensated OVX-induced changes of pathways/terms of major interest [Citation10]. These were positive regulation of blood pressure, hormones, feeding behavior, cytokine-cytokine receptor interaction, inflammatory response, response to insulin, orexigenic neuropeptide QRFP/P518, opioid receptor binding, cytokine activity, positive regulation of vasoconstriction. illustrates the strength of the enrichment of the respective processes. The target genes AVPR1A, GAL, CALCA, HCRT and PNOC were chosen for qPCR to represent these pathways. All these target genes display a compensation or overcompensation of the OVX effect by iCR treatment especially in the hippocampus; i.e. elevated expression values of OVX samples in comparison to PRAE samples were reversed or diminished by iCR in comparison to PRAE levels (for detail see [Citation10]). Literature research on these five target genes revealed several overlaps with known associations with menopause:
AVPR1A serves as receptor for arginine vasopressin (AVP) and oxytocin. AVP acts as the major regulator of body water homeostasis and is one the target genes suggested to play potential role in the pathogenesis of climacteric syndrome [Citation19,Citation27]. Oxytocin contracts smooth muscle during parturition and lactation. It is also involved in cognition, tolerance, adaptation and complex sexual and maternal behavior, as well as in the regulation of water excretion and cardiovascular functions [Citation28]. Neuronal metabolic activity in the supraoptic nucleus, a major source of plasma AVP as well as in the hippocampus was markedly enhanced in women after menopause [Citation22].
A recently published study [Citation19] elucidated the possible molecular mechanisms underlying the menopausal syndrome in the hypothalamus of OVX mice. They identified differential expression of genes involved in thermoregulation, eating, sleeping, homeostasis, and endocrine regulation 8 weeks after OVX. Also in that study, the endocrine-related gene AVP was upregulated in OVX mice.
Estrogen receptor beta (ESR2) was found to co-localize in the hypothalamus with cells expressing oxytocin and AVP-receptors in rodents [Citation29]. Therefore, it is not surprising that ovarian steroids can modulate vasopressin release [Citation30]. ER-β-selective estrogen receptor modulator (SERMs) in the hippocampus, decreased anxiety and depressive behavior [Citation31]. CR extracts actions were partially attributed to SERMs [Citation32], so the compensatory effect on this neuronal axis by iCR seems quite conclusive.
Estrogen stimulates AVP gene expression in the bed nucleus of the stria terminalis (BNST) in rodents, the BNST-AVP system enhances and/or maintains ‘social’ or ‘olfactory’ memory [Citation33]. iCR influenced olfactory receptor linked gene expression profiles and pathways [Citation10].
GAL: Serum galanin concentration in post-menopausal women is related to severity of climacteric syndrome [Citation34], as it can be seen in GAL-knock-out mice, that show hyperalgesia to mechanical and thermal stimuli [Citation35]. Galanin acts predominantly as an inhibitory neuromodulator on glucose-induced insulin release and stimulates growth hormone and prolactin secretion [Citation35]. It is involved in in several functions including cognition, feeding, nociception, mood regulation, and neuroendocrine modulation. Expression of the galanin gene shows a 50-fold increase in plasma immunoreactivity after stimulation with the estrogen [reviewed by 35]. The observed decrease of GAL by iCR [Citation10] implicate that the herbal does not act like estrogen, at least in this context. However, the OVX effect on Galanin expression in hypothalamus and hippocampus is counteracted by iCR, reconstituting the GAL status like ovar-bearing animals [Citation10].
CALCA, also named Calcitonin gene-related peptide (CGRP), is a very potent vasodilator maintaining cardiovascular homeostasis and thermoregulation by altering blood flow at the cutaneous microvascular level [Citation36]. The acute release of CGRP has been associated with the occurrence of menopausal hot flashes reviewed by [Citation36,Citation37], that is reconstituted by HRT [Citation38]. GRP receptor antagonists may be beneficial for vasomotor symptoms, cardiovascular risk, obesity, and major depressive disorders [Citation39]. iCR compensated OVX-induced changes of CALCA gene expression in hippocampus [Citation10].
HCRT encodes a neuropeptide precursor protein that gives rise to two mature neuropeptides, orexin A and orexin B, which regulate sleep and arousal and may play a role in feeding behavior, metabolism and homeostasis [Citation40]. Sakakibara, Uenoyama [Citation41] hypothesized that HCRT is a possible candidate gene, which is responsible for brain sexual differentiation via neonatal estrogen. HRT downregulates HCRT expression and reverses hypoestrogenism, that is linked to hypoestrogenism and higher orexin A values [Citation41,Citation42]. Also, iCR returned the OVX-induced change of expression level of HCRT to normal levels [Citation10].
PNOC encodes for multiple protein products including nociceptin, nocistatin, and orphanin FQ2. Nociceptin induce increased pain sensitivity, and may additionally regulate body temperature, learning and memory, and hunger [Citation43]. Steroids regulate PNOC expression and so the onset and termination of reproductive behavior and reproductive physiology of the hypothalamic-pituitary-gonadal axis via integrating hormonal, olfactory, and mating stimuli [Citation44]. Interestingly, the preliminary analyses preceding our study revealed gene expression changes in the olfactory signaling pathway due to OVX, which were compensated by iCR [Citation15]. PNOC may be a key link to this set of functions.
Although microarray analyses revealed compensation by iCR in the hippocampus and qPCR revealed even overcompensation of some genes (AVPR1A, PNOC), this result should not be overestimated as the number of samples was too small to come to a final conclusion.
Anyhow, the good accordance of the target genes analyses by PCR with microarray results at the hippocampal compensation approach leads to the conclusion that the pathways/terms covered by the target genes are confirmed, too. The iCR actions are not mediated via a single receptor. It remains unclear in which order the downstream processes happen, only their involvement is shown.
Also, studies in hypothalamic regions showed counteracting properties of CR, that ameliorated metabolic disorders in OVX rats, including reducing weight gain, serum triglycerides, and liver steatosis without inducing hepatotoxicity, independently of estrogen receptor activation [Citation45,Citation46]. Hui, Xiaoyan [Citation3] suggested that estrogen and iCR ‘can improve the function of the hypothalamic nuclei’ and ‘may act on the hypothalamic nuclei and have therapeutic effects on menopausal symptoms’.
But there are also distinct differences in the mode of action between estrogen and iCR, as the herbal extract has no effects on serum estradiol, uterus weight and body weight like estrogen [Citation47]. Several clinical studies independently investigated whether CR extracts affect critical estrogen-sensitive tissues [Citation48–53]. None of these studies detected any safety concerns regarding breast tissue or endometrium. Therefore, it exerts central activity rather than a hormonal effect [Citation47]. It is widely discussed, if CR acts via any estrogen receptor (ESR1 and ESR2). Microarray analyses with both genes showed no differentially expression, but using qPCR, ESR1 showed a compensatory effect of iCR in both brain regions. And ESR2 showed also a compensatory effect in the hippocampus. A direct binding of CR was previously excluded [Citation11,Citation54]. Nevertheless, it is conceivable, that the expression of ESR1 and ESR2 are secondarily regulated, albeit there is no direct interaction of CR with these receptors.
The here obtained results confirm the known involvements of KISS1 and TAC3 in menopausal transformations in the brain. Hypothalamic expression of KISS1 and TAC3 (encoding kisspeptin and neurokinin B, respectively) is upregulated in postmenopausal compared to premenopausal women [Citation55] as well as in ovarectomized young cynomolgus monkeys, which could be reversed by HRT [reviewed by Citation56]. iCR compensated the effect of OVX at TAC3 but effect on KISS1 remains unclear due to the very low signal intensity in that experiment. The receptor of TAC3, i.e. TACR3, was previously linked to hot flashes in menopause [Citation57] and is a target for selective antagonists like NK3R-antagonists that are effective treatments for hot flashes [Citation57,Citation58].
qPCR validation analyses in a set of genes preceding the current analyses also revealed, that iCR partially reversed the influence of OVX on expression of the identified OR genes in the hippocampus and hypothalamus and compensated them, respectively [Citation15]. Olfactory processes are linked to serotonin and GABAB receptors [Citation59] and it is known, that CR extract contains GABAA receptor modulating constituents [Citation60] with selective agonistic serotonin receptor activity [Citation61], these receptors are modulated by estrogens as well [Citation62]. Wang, Cui [Citation14] summarized, that ‘serotonin pathway is changed after ovariectomy, including the serotonin synthesis, both estrogen and iCR have an equivalent therapeutic effect on it’. However, the role of centrally expressed ORs in the context of menopause is not investigated very well. But ectopical human ORs are becoming increasingly important, as OR activation has been shown to influence cancer cell growth and progression [Citation63]. HRT has a beneficial effect on some olfactory and cognitive measures in the menopause [Citation64]. Therefore, reconstitution of the olfactory gene expression profiles may be associated with positive effects on cognition. This was successfully shown in mice by restoration of active ovarian function in aged female mice [Citation65]. Especially in the case of the olfactory gene family, it is unclear, whether studies in the animal model could be transferred to humans, because human olfactory receptor genes mostly match to pseudogenes in rodents, but these interspecies homologs have likely similar functional relationship [Citation66].
Of course, OVX also results in more widespread effects and is not completely similar to the processes that occur during menopausal transformation. Nevertheless, these in vivo studies at the animal model yield in hints toward better understanding of the CNS procedures due to estrogen decline and the multi-faceted mode(s) of action of CR.
From all this information about gene expression analyses and findings from the scientific literature, we would have liked to paint a picture of menopause in which a distinct causal chain becomes apparent. Unfortunately, because many processes occur at the same time, this picture is still blurred, even though some of the connections have now become clearer. The drop in estrogen throws the learning and memory center hippocampus into turmoil that is indicated by changed expression profiles of several genes. The ability to deal with a temperature stimulus no longer functions in the usual way as the learned reaction to regulate temperature overshoots and hot flushes occur. Menopausal symptoms can be reduced either by compensating for the hormone deficiency or by adapting more quickly to the new hormonal conditions. Like a person whose legs are shortened and as a result stumbles. A gradual shortening would not lead to stumbling as much as an amputation. The affected person learns to walk again with the help of prostheses (equivalent to HRT) or by adjusting the step length (equivalent to Black cohosh). We know from the clinic that some women have difficulties with temperature adjustment not only in menopause [Citation67,Citation68]. Others have learned to adjust more quickly their physical and emotional symptoms experienced during menopause, for example through exercise and cognitive therapies [Citation69]. In the course of the adaptation process, the body adjusts new reaction procedures, but this sometimes takes more and sometimes less time, and therapies such as black cohosh help to overcome these difficulties.
Conclusion
Estrogen fluctuation as it occurs upon OVX or menopause in women is associated with changes in central processes especially in the hippocampus, leading to symptoms such as VMS, depression and anxiety. iCR is able to reverse processes thereof. Genes involved in blood pressure, metabolism, hormonal regulation, homeostasis, mood regulation, neuroendocrine modulation, thermoregulation, regulation of sleep and arousal, and in learning, memory and cognition are dysregulated by OVX and normalized by iCR in the hippocampus, that is a key organ in menopausal transition and adaptation processes.
Authors’ contributions
PS was responsible for the management and implementation of the project. PS, HHHvZ, and PN contributed to the study conception and design. The analysis and presentation of the material was done by PN. HHHvZ prepared the manuscript-outline. The first draft of the manuscript was written by PN; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank Stefan Ehrentraut for providing the extensive analysis and material, and Jennifer-Christin Kuchernig for lively discussions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors declare that the data and materials that support the results or analyses presented in their paper will be freely available upon request.
Additional information
Funding
Notes
1 Created with the help of WorldCloud.com.
References
- Monteleone P, Mascagni G, Giannini A, et al. Symptoms of menopause - global prevalence, physiology and implications. Nat Rev Endocrinol. 2018;14(4):1–9. Apr doi: 10.1038/nrendo.2017.180.
- The Hormone Therapy Position Statement of The North American Menopause Society" Advisory P. The 2022 hormone therapy position statement of the North American menopause society. Menopause. 2022; 29(7):767–794. doi: 10.1097/GME.0000000000002028.
- Hui Z, Xiaoyan M, Mukun Y, et al. Effects of black cohosh and estrogen on the hypothalamic nuclei of ovariectomized rats at different temperatures. J Ethnopharmacol. 2012;142(3):769–775.
- Kundakovic M, Rocks D. Sex hormone fluctuation and increased female risk for depression and anxiety disorders: from clinical evidence to molecular mechanisms. Front Neuroendocrinol. 2022; 66:101010. doi: 10.1016/j.yfrne.2022.101010.
- European Union herbal monograph on Cimicifuga racemosa (L.) Nutt. rhizoma. EMA/HMPC/48745/2017 Internet]. 2018. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Herbal_monograph/2017/08/WC500233056.pdf.
- Ruan X, Mueck AO, Beer AM, et al. Benefit-risk profile of black cohosh (isopropanolic cimicifuga racemosa extract) with and without St John’s wort in breast cancer patients. Climacteric. 2019;22(4):339–347. Aug doi: 10.1080/13697137.2018.1551346.
- Castelo-Branco C, Gambacciani M, Cano A, et al. Review & meta-analysis: isopropanolic black cohosh extract iCR for menopausal symptoms - an update on the evidence [review. Climacteric. 2021;24(2):109–119. doi: 10.1080/13697137.2020.1820477.
- Assessment report on Cimicifuga racemosa (L.) Nutt., rhizoma [Internet]. 2018. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2018/07/WC500251366.pdf.
- Nicken P, Henneicke-von Zepelin HH, Kuchernig JC, et al. Neuromodulating action of cimicifuga racemosa for alleviation of vasomotor symptoms. Z Phytother. 2019 ;40(S 01):26–27. doi: 10.1055/s-0039-1697296.
- Stute P, Ehrentraut S, Henneicke-von Zepelin HH, et al. Gene expression analyses on multi-target mode of action of black cohosh in menopausal complaints - a pilot study in rodents. Arch Gynecol Obstet. 2022;305(1):275–286. doi: 10.1007/s00404-021-06105-8.
- Burdette JE, Liu J, Chen SN, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem. 2003;51(19):5661–5670. doi: 10.1021/jf034264r.
- Nisslein T, Koetter U, Freudenstein J. In vitro binding of an isopropanolic extract of black cohosh to selected central nervous receptors. Maturitas. 2006;54: S65.
- Rhyu MR, Lu J, Webster DE, et al. Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem. 2006;54(26):9852–9857. Dec 27 doi: 10.1021/jf062808u.
- Wang W, Cui G, Jin B, et al. Estradiol valerate and remifemin ameliorate ovariectomy-induced decrease in a serotonin dorsal raphe-preoptic hypothalamus pathway in rats. Ann Anat. 2016;208:31–39. Nov
- Einfluss von Cimicifuga racemosa auf die olfaktorische Genexpression im Hippokampus und Hypothalamus von Ratten nach Ovarektomie [Influence of Cimicifuga racemosa on olfactory gene expression in the hippocampus and hypothalamus of rats after ovariectomy. Lecture at DMG: 2016.
- Ma X, Zhang H, Wang K, et al. Effects of an isopropanolic-aqueous black cohosh extract on Central body temperature of ovariectomized rats. J Ethnopharmacol. 2011;138(1):156–161.
- Guida M, Raffone A, Travaglino A, et al. Cimicifuga racemosa isopropanolic extract for menopausal symptoms: an observational prospective case-control study. Gynecol Endocrinol. 2021;37(12):1132–1137.
- Kim K, Zakharkin SO, Allison DB. Expectations, validity, and reality in gene expression profiling. J Clin Epidemiol. 2010; Sep63(9):950–959. doi: 10.1016/j.jclinepi.2010.02.018.
- Wang W, Yang Q, Zhou C, et al. Transcriptomic changes in the hypothalamus of ovariectomized mice: data from RNA-seq analysis. Ann Anat. 2022;241:151886
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103(6):1572–1578.e1.
- Zhang M, Flury S, Kim CK, et al. Absolute quantification of phosphorylated ERβ amino acids in the hippocampus of women and in a rat model of menopause. Endocrinology. 2021;162(9):bqab122. doi: 10.1210/endocr/bqab122.
- Ishunina TA, Swaab DF. Alterations in the human brain in menopause. Maturitas. 2007; 57(1):20–22. doi: 10.1016/j.maturitas.2007.02.009.
- Greendale GA, Derby CA, Maki PM. Perimenopause and cognition. Obstet Gynecol Clin North Am. 2011; 38(3):519–535. doi: 10.1016/j.ogc.2011.05.007.
- Garcia de Arriba S, Henneicke-von Zepelin H.-H., Dimpfel W., Nolte K.-U. Isopropanolic Cimicifuga extract modulates brain activity: Electropharmacogram from various brain areas in freely moving rats (Tele-Stereo-EEG), Annual Conference of the European Menopause Association, Madrid, 21 May 2015, Maturitas 82:318, 2015.
- Gervais NJ, Gravelsins L, Brown A, et al. Scene memory and hippocampal volume in Middle-aged women with early hormone loss. Neurobiol Aging. 2022;117:97–106. Sep
- Lathe R. Hormones and the hippocampus. J Endocrinol. 2001; 169(2):205–231. doi: 10.1677/joe.0.1690205.
- Verbalis J, et al. Cellular and molecular mechanisms of hormone actions on behavior. In: Pfaff D, Arnold A, Etgen A. editors. Hormones, brain and behavior. (Second Edition)2009. Elsevier, Academic Press. https://shop.elsevier.com/books/molecular-mechanisms-of-hormone-actions-on-behavior/etgen/978-0-12-374939-0
- NCBI. OXT oxytocin/neurophysin I prepropeptide [Homo sapiens (human)] 2023. Available from: https://www.ncbi.nlm.nih.gov/gene/5020.
- Alves SE, Lopez V, McEwen BS, et al. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc Natl Acad Sci U S A. 1998;95(6):3281–3286.
- Forsling ML, Strömberg P, Akerlund M. Effect of ovarian steroids on vasopressin secretion. J Endocrinol. 1982; 95(1):147–151. doi: 10.1677/joe.0.0950147.
- Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007; 86(2):407–414.
- Cobin RH, Goodman NF, Committee ARES. American association of clinical endocrinologists and American college of endocrinology position statement on menopause-2017 update. Endocr Pract. 2017; 23(7):869–880.
- Fink G, Sumner BE, Rosie R, et al. Estrogen control of Central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325–344. doi: 10.1007/BF02088099.
- Słopien R, Meczekalski B, Warenik-Szymankiewicz A. Relationship between climacteric symptoms and serum galanin levels in post-menopausal women. J Endocrinol Invest. 2004; 27(9):RC21–3. doi: 10.1007/BF03346271.
- Lundström L, Elmquist A, Bartfai T, et al. Galanin and its receptors in neurological disorders. Neuromolecular Med. 2005;7(1–2):157–180. doi: 10.1385/NMM:7:1-2:157.
- Sturdee DW, Hunter MS, Maki PM, et al. The menopausal hot flush: a review. Climacteric. 2017;20(4):296–305. doi: 10.1080/13697137.2017.1306507.
- Chen JT, Hirai Y, Seimiya Y, et al. Menopausal flushes and calcitonin-gene-related peptide. Lancet. 1993;342(8862):49.
- Valentini A, Petraglia F, De Vita D, et al. Changes of plasma calcitonin gene-related peptide levels in postmenopausal women. Am J Obstet Gynecol. 1996;175(3 Pt 1):638–642. doi: 10.1053/ob.1996.v175.a74287.
- Sharma S, Mahajan A, Tandon VR. Calcitonin gene-related peptide and menopause. J Midlife Health. 2010; 1(1):5–8. doi: 10.4103/0976-7800.66985.
- HCRT hypocretin neuropeptide precursor [Homo sapiens (human)] [Internet]. 2018 cited 01 August 2018]. Available from: https://www.ncbi.nlm.nih.gov/gene/3060.
- Sakakibara M, Uenoyama Y, Minabe S, et al. Microarray analysis of perinatal-estrogen-induced changes in gene expression related to brain sexual differentiation in mice. PLOS One. 2013;8(11):e79437. doi: 10.1371/journal.pone.0079437.
- El-Sedeek M, Korish AA, Deef MM. Plasma orexin-A levels in postmenopausal women: possible interaction with estrogen and correlation with cardiovascular risk status. BJOG. 2010; 117(4):488–492. doi: 10.1111/j.1471-0528.2009.02474.x.
- PNOC prepronociceptin [Homo sapiens (human)] [Internet]. 2018 cited 01 August 2018]. Available from: https://www.ncbi.nlm.nih.gov/gene/5368.
- Sinchak K, Romeo HE, Micevych PE. Site-specific estrogen and progestin regulation of orphanin FQ/nociceptin and nociceptin opioid receptor mRNA expression in the female rat limbic hypothalamic system. J Comp Neurol. 2006; 496(2):252–268.
- Rabenau M, Dillberger B, Günther M, et al. Cimicifuga racemosa extract Ze 450 re-balances energy metabolism and promotes longevity. Antioxidants . 2021;10(9):1432. doi: 10.3390/antiox10091432.
- Sun Y, Yu Q, Shen Q, et al. Black cohosh ameliorates metabolic disorders in female ovariectomized rats. Rejuvenation Res. 2016;19(3):204–214. doi: 10.1089/rej.2015.1724.
- Zhang J, Bai W, Wang W, et al. Mechanisms underlying alterations in norepinephrine levels in the locus coeruleus of ovariectomized rats: modulation by estradiol valerate and black cohosh. Neuroscience. 2017;354:110–121. Jun 23 doi: 10.1016/j.neuroscience.2017.04.029.
- Bai W, Henneicke-von Zepelin HH, Wang S, et al. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: a randomized, double blind, parallel-controlled study versus tibolone. Maturitas. 2007;58(1):31–41. doi: 10.1016/j.maturitas.2007.04.009.
- Nappi RE, Malavasi B, Brundu B, et al. Efficacy of cimicifuga racemosa on climacteric complaints: a randomized study versus low-dose transdermal estradiol. Gynecol Endocrinol. 2005;20(1):30–35. Jan
- Raus K, Brucker C, Gorkow C, et al. First-time proof of endometrial safety of the special black cohosh extract (actaea or cimicifuga racemosa extract) CR BNO 1055. Menopause. 2006;13(4):678–691. doi: 10.1097/01.gme.0000196813.34247.e2.
- Ruhlen RL, Haubner J, Tracy JK, et al. Black cohosh does not exert an estrogenic effect on the breast. Nutr Cancer. 2007;59(2):269–277.
- Reed SD, Newton KM, LaCroix AZ, et al. Vaginal, endometrial, and reproductive hormone findings: randomized, placebo-controlled trial of black cohosh, multibotanical herbs, and dietary soy for vasomotor symptoms: the herbal alternatives for menopause (HALT) study. Menopause. 2008;15(1):51–58. doi: 10.1097/gme.0b013e318057787f.
- Lundström E, Hirschberg AL, Söderqvist G. Digitized assessment of mammographic breast density–effects of continuous combined hormone therapy, tibolone and black cohosh compared to placebo. Maturitas. 2011; Dec70(4):361–364. doi: 10.1016/j.maturitas.2011.08.009.
- Wuttke W, Jarry H, Haunschild J, et al. The non-estrogenic alternative for the treatment of climacteric complaints: black cohosh (Cimicifuga or actaea racemosa). J Steroid Biochem Mol Biol. 2014;139:302–310.
- Skorupskaite K, George JT, Veldhuis JD, et al. Neurokinin 3 receptor antagonism reveals roles for neurokinin B in the regulation of gonadotropin secretion and hot flashes in postmenopausal women. Neuroendocrinology. 2018;106(2):148–157. Apr 05 doi: 10.1159/000473893.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–227.
- Meijsen JJ, Shen H, Vemuri M, et al. Shared genetic influences on depression and menopause symptoms. Psychol Med. 2023;53(6):2241–2251.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate to severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;108(8):1981–1997. doi: 10.1210/clinem/dgad058.
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009; 12(6):784–791. doi: 10.1038/nn.2335.
- Cicek SS, Khom S, Taferner B, et al. Bioactivity-guided isolation of GABA(A) receptor modulating constituents from the rhizomes of actaea racemosa. J Nat Prod. 2010;73(12):2024–2028. doi: 10.1021/np100479w.
- Röhrl J, Künstle G. Serotonin receptor targeting activities for cimicifuga racemosa dry extract (BNO 1055) as active component of klimadynon®. Maturitas. 2017;100:143. doi: 10.1016/j.maturitas.2017.03.101.
- Bossé R, DiPaolo T. The modulation of brain dopamine and GABAA receptors by estradiol: a clue for CNS changes occurring at menopause. Cell Mol Neurobiol. 1996;16(2):199–212. doi: 10.1007/BF02088176.
- Weber L, Al-Refae K, Ebbert J, et al. Activation of odorant receptor in colorectal cancer cells leads to inhibition of cell proliferation and apoptosis. PLOS One. 2017;12(3):e0172491. doi: 10.1371/journal.pone.0172491.
- Doty RL, Tourbier I, Ng V, et al. Influences of hormone replacement therapy on olfactory and cognitive function in postmenopausal women. Neurobiol Aging. 2015;36(6):2053–2059.
- Parkinson KC, Peterson RL, Mason JB. Cognitive behavior and sensory function were significantly influenced by restoration of active ovarian function in postreproductive mice. Exp Gerontol. 2017; 92:28–33. doi: 10.1016/j.exger.2017.03.002.
- Malnic B, Godfrey PA, Buck LB. The human olfactory receptor gene family. Proc Natl Acad Sci U S A. 2004; 101(8):2584–2589. doi: 10.1073/pnas.0307882100.
- University of Iowa. Acclimatization (adjusting to the temperature) 2016 24.05.2023]. Available from: https://uihc.org/educational-resources/acclimatization-adjusting-temperature#:∼:text=Body%20fat,cool%20the%20body%20through%20evaporation.
- Yu J, Ouyang Q, Zhu Y, et al. A comparison of the thermal adaptability of people accustomed to air-conditioned environments and naturally ventilated environments. Indoor Air. 2012;22(2):110–118. doi: 10.1111/j.1600-0668.2011.00746.x.
- Green SM, Haber E, McCabe RE, et al. Cognitive-behavioral group treatment for menopausal symptoms: a pilot study. Arch Womens Ment Health. 2013;16(4):325–332. doi: 10.1007/s00737-013-0339-x.
