Abstract
Objective
To analyze differences in the menstrual pattern, age at menarche, and body mass index (BMI) in adolescents with Hypothalamic-Pituitary-Ovarian (HPO) axis immaturity and Polycystic Ovary Syndrome (PCOS) through a systematic review and meta-analysis.
Methods
The PubMed, EMBASE, Web of Science, Virtual Health Library, Scopus databases were searched using combinations of descriptors. Study quality was assessed using the Newcastle-Ottawa Scale. For data analysis, the results were grouped into PCOS group and NPCOS group (HPO axis immaturity). We performed a meta-analysis of raw data and the inverse variance method, employing the standardized mean difference, of the age at menarche and BMI of adolescents.
Results
Participants totaled 1,718 from nine selected studies. The meta-analysis showed that the PCOS group had a higher BMI than the NPCOS group (SMD 0.334; CI95% 0.073 − 0.595; p = .012). The degree of heterogeneity of the studies was approximately 40%. No significant difference in age at menarche (SMD − 0.027; CI95% −0.227 − 0.172; p = 0.790) and menstrual patterns was found, but amenorrhea was described only in adolescents with PCOS.
Conclusions
The main characteristic in menstrual pattern that differentiated PCOS patients from girls with HPO axis immaturity was amenorrhea. Also, the BMI of PCOS patients was nearly one third higher than that of adolescents with HPO axis immaturity.
Introduction
Abnormal uterine bleeding (AUB) is defined as an alteration of the physiological pattern of bleeding from endometrial desquamation. Such a change interferes with duration and flow volume, as well as frequency (cyclicity) and/or regularity [Citation1,Citation2].
Menstruation results from a complex interaction between the hypothalamus, the anterior pituitary, the ovary, and the endometrium. During adolescence, menstrual cycles are irregular, especially in the first two years on average after menarche due to hypothalamic-pituitary-ovarian (HPO) axis immaturity [Citation3,Citation4]. This occurs due to an anomalous response from the luteinizing hormone (LH) secretion to estradiol. In a typical menstrual cycle, as a follicle matures in the ovary, estradiol levels rise and stimulate a surge in LH. However, in adolescents, this LH response can be atypical. Rather than a totally positive feedback to a rising estradiol level, what is observed is a tenuous production of LH, which prevents the formation of a peak necessary for ovulation [Citation5,Citation6]. Besides, inadequate inhibition of follicle-stimulating hormone (FSH) production by inhibin (resulting in more ovarian follicles starting to grow than normal) also contributes to HPO axis immaturity and the ensuing irregularity in the menstrual cycle [Citation7].
Anovulatory cycles, which can manifest as amenorrhea, oligomenorrhea, or heavy menstrual bleeding due to HPO axis immaturity, are the most common causes of AUB among adolescents [Citation1,Citation3,Citation4]. Adolescence is a transitory stage of physical and psychological development, time when functional variations in the HPO axis during normal puberty leads to changes in reproductive hormones and menstrual patterns that mimic some of the characteristics of polycystic ovary syndrome (PCOS), hindering its diagnosis in adolescents [Citation8–10].
PCOS is prevalent during the reproductive years, affecting 8% to 18% of women [Citation11] and representing the predominant endocrine disorder during this stage. The diagnosis of PCOS is essentially clinical and based on signs and symptoms, especially menstrual irregularity. It manifests along a broad and heterogeneous spectrum as menstrual alterations, hyperandrogenism, hirsutism, metabolic manifestations, and infertility. This can lead to diagnostic difficulties, particularly in cases of adolescents with HPO axis immaturity, thus, differential diagnosis is challenging.
It assumes different forms and phenotypes associated with peripheral insulin resistance, obesity, and risk of type II diabetes [Citation12]. In recent years, the diagnosis of PCOS has expanded to allow for several potential phenotypes. The European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine have established criteria, often referred to as the Rotterdam consensus, which include polycystic ovary ultrasound findings and allow for multiple phenotypes based on a combination of 2 of the following 3 findings: hyperandrogenism, menstrual irregularity/ovulatory dysfunction (OD), and polycystic ovarian morphology (PCOM) as observed with ultrasound. Thus, there are four phenotypes based on Rotterdam criteria [Citation13]): A (hyperandrogenism + OD + PCOM), B (hyperandrogenism + OD), C (hyperandrogenism + PCOM) and D (OD + PCOM) [Citation14]. However, the new 2023 Evidence-Based International Guideline Recommendations for the Assessment and Treatment of Polycystic Ovary Syndrome show that diagnosis among adolescents remains difficult and controversial, and some investigators advise against using ultrasound findings as part of the diagnostic criteria (Practice points). And for hyperandrogenism, there is a consensus recommendation for a comprehensive investigation of the clinical history and a physical examination that includes severe acne and hirsutism in adolescents [Citation13].
Knowledge gaps exist regarding several clinical aspects of PCOS in adolescents, due to either the absence of longitudinal studies or the absence of specific diagnostic criteria for the identification of PCOS during adolescence, including the absence of normative values for several biochemicals markers as anti-mullerian hormone (AMH), testosterone, LH, glycemia, and insulin. Thus, a description of the differential characteristics between physiological alterations resulting from HPO axis immaturity and PCOS and involving aspects of menstrual pattern, age at menarche, and body mass index (BMI), might facilitate the management of these conditions in adolescents and help lay down criteria for the diagnosis of PCOS in this population. Hence, the objective of this study was to analyze the differences in the menstrual pattern, age at menarche, and BMI of adolescents with HPO axis immaturity and PCOS through a systematic review and meta-analysis.
Methods
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) recommendations, and it was registered in PROSPERO (CRD 42020163429, https://www.crd.york.ac.uk/prospero).
The PubMed (https://www.ncbi.nlm.nih.gov/pubmed), EMBASE (https://www.embase.com), Web of Science (https://isiknowledge.com), Virtual Health Library (http://bvsalud.org), and Scopus (https://www.scopus.com) databases were searched electronically using combinations of relevant MESH terms, keywords, and word variants based on the PI(E)COS strategy ().
Table 1. Modified PI(E)COS strategy for the selection of descriptors used in the search for articles in the databases.
The search was restricted to the English, Italian, French, Portuguese, and Spanish languages, without using filters or delimiters. To broaden the scope of our search strategy, we included gray literature articles, complete thesis documents, and reference lists of other articles and related reviews.
The age group of 10 to19 years was established in accordance with the definitions of adolescence provided by the World Health Organization [Citation15].
Duplicate articles were excluded, and the others were screened by title and abstract using the following exclusion criteria: 1) Adolescents with comorbidities other than those presumably interconnected with the pathophysiology of PCOS, such as type 1 diabetes mellitus [Citation16–20], autoimmune diseases [Citation21–23], obesity, type 2 diabetes mellitus, metabolic syndrome [Citation24–26], thyroid dysfunction, congenital adrenal hyperplasia, androgen secreting adrenal tumor, hyperprolactinemia, and hypothalamic disorders; 2) Studies with pharmacological or surgical intervention in cases of PCOS; and 3) Studies unrelated to the subject.
After this step, the remaining articles were read in their entirety and evaluated according to the following inclusion criteria: 1) presence of a description of the menstrual pattern of adolescents with HPO axis immaturity or PCOS; 2) free access to the full text.
The selected articles were analyzed using a standardized form to extract the data relevant to this review. Relevant information consisted of population, number of participants, age, age at menarche, and menstrual pattern.
The quality of the studies was assessed using the Newcastle-Ottawa Scale (NOS) [Citation27,Citation28] adapted for cross-sectional studies. The NOS has a “star system” in which a study is judged in terms of three dimensions: selection (five stars), comparability (two stars), and results (three stars). The NOS stars range from 0 to 10 stars.
To minimize bias, all steps of identification, selection, quality assessment, and data extraction were carried out independently by two authors. In case of disagreement, a third reviewer was consulted.
For data analysis, the results were grouped into the PCOS group, which comprised adolescents with a diagnosis of PCOS, or into the NPCOS group, which comprised adolescents without the diagnosis of PCOS, therefore with immaturity in the HPO axis.
Statistical heterogeneity between studies was examined using the I2 statistical test. The meta-analysis of age at menarche and BMI of the studies were performed through the Mantel-Haenszel method, utilizing the mean and standard deviation of the PCOS and NPCOS groups evaluated in each study and executed using the metan command. The meta-analysis of raw data and the inverse variance method, employing the standardized mean difference, were also applied. The meta-analysis was performed under the random effects model.
The statistical analysis of the meta-analysis was performed with the STATA-SE 64 software, version 15.
Results
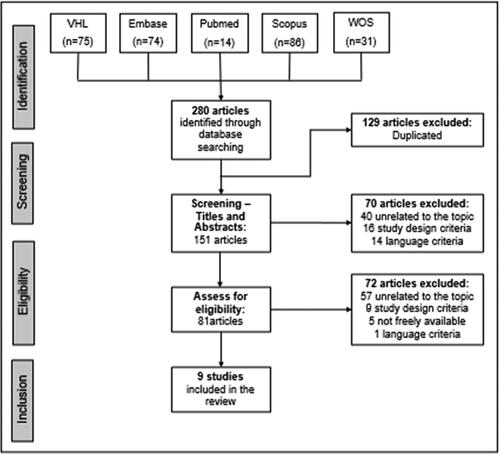
The initial search was carried out in five databases and resulted in 280 articles. Duplicate articles were manually removed, and selection was performed by title and abstract. After these steps, 70 more articles were excluded as follows: 40 were unrelated to the theme, 16 had a study design that did not meet our criteria, and 14 fell outside our language range (7 in Chinese, 5 in Russian, and 2 in Arabic). The 81 remaining articles were fully read. Of these, only nine studies were selected for the final analysis. The article selection flowchart is shown in .
Population
The nine studies were cross-sectional studies, published between 2011 and 2021 in the following countries: Australia, Brazil, India, Italy, Thailand, and Turkey. The number of study participants varied from 44 to 548, totaling 1,718 teens.
All articles followed the Rotterdam Criteria available at the time of the study for the diagnosis of PCOS. Only the article by Ybarra et al. [Citation29] used the following criteria, in addition to the Rotterdam Consensus: Guideline for PCOS in adolescence, Androgen Excess and PCOS Society, and American National Institute of Health’s criteria [Citation30].
Clinical hyperandrogenism was defined based on the following variables: Ferriman-Gallwey (FG) score > 6 [Citation31,Citation32] to 8 [Citation29,Citation33,Citation34], acne [Citation31,Citation35] serum testosterone > 48 to 82 ng/dl [Citation29,Citation31,Citation32,Citation34], hair loss [Citation31,Citation36], hirsutism [Citation33,Citation37], oily skin [Citation36]. And ovarian morphology was determined by abdominal ultrasonographic imaging of ovaries and uterus. According to the Rotterdam criteria, >12 follicles with diameters of 2–9 mm in ovaries or ovary volume of ≥10 ml in pelvic USG was accepted as consistent with the polycystic ovary morphology [Citation29,Citation31–35,Citation38].
In most studies, the diagnosis of PCOS was made during the research, only three studies included patients previously diagnosed with PCOS [Citation34,Citation37,Citation38], most of them were from urban areas and were diagnosed in a hospital setting. However, in one study [Citation31], the rural population (n = 51) was compared with the urban population (n = 51), and PCOS was found in 30% of the rural girls and in 76% of the urban girls.
Menstrual pattern
The definition of forms of abnormal uterine bleeding varied between studies. Oligomenorrhea was defined as the absence of menstruation for a period between 35 [Citation37], 45 [Citation31,Citation32] or 90 days [Citation34], and/or < = 6 [36] and 8 [Citation31,Citation32] menstruations per year. Amenorrhea is defined by Hickey et al. [Citation35] as absence of menstruation >60 days, for Siklar et al. [Citation34] > 90 days, and for Ybarra et al. [Citation29], chronic anovulation and/or menstrual irregularity was considered when the patient reported that her menstrual cycle had been irregular for the last 6 months.
displays the characteristics of the menstrual pattern in each study quantitatively and with the main perceived repercussions. The frequency of PCOS in the studies ranged from 5.3% [Citation31] to 53% [Citation33]. Only four studies evaluated phenotypes. The prevalence of phenotype A ranged from 26.19% [Citation32] to 73.4% [Citation37]), for phenotype B, from 2.38% [Citation32] to 21.1% [Citation37], for phenotype C, from 2.38% [Citation32] to 27.59% [Citation31], and for phenotype D, from 0.9% [Citation37] to 69.05% [Citation32].
Table 2. Menstrual pattern data extracted from selected studies.
Menstrual irregularity was observed in adolescents with PCOS and ranged from 65.52% [Citation31] to 100% [Citation38]. One study [Citation37] stratified the participants according to age, finding there was no significant difference between adolescents (3–5 years after menarche) and young adults (6–9 years after menarche) in terms of mean BMI, prevalence of obesity, and hormone levels. However, younger patients presented with the B phenotype more often than the A phenotype. In the NPCOS group, menstrual irregularity was much lower, ranging from 1.29 to 29%.
Menstrual irregularity in the PCOS group manifested as oligomenorrhea, which the prevalence ranged from 27.3% [Citation38] to 78.9% [Citation36], and amenorrhea, which ranged from 8% [Citation35] to 72.7% [Citation38]. The findings of the present study suggest that the presence of amenorrhea, would be more indicative of persistent chronic anovulation than of temporary anovulation.
Age at menarche and body mass index
Patients included in the studies were at least 2 years postmenarche. Only in Nidhi et al. [Citation32] and Hickey’s et al. [Citation35] studies did this period last 1.5 years and 6 months, respectively.
presents the articles, with the number of study participants and their mean age, age at menarche, and BMI. Upon quality assessment by NOS, all articles scored between 5 and 9 stars, out of a total of 10 stars. The articles used in the meta-analysis scored between 7 and 9 stars [Citation29,Citation31,Citation33,Citation35].
Table 3. Age, age at menarche, and BMI of the adolescents in the PCOS and NPCOS groups. Article quality rating by the NOS scale.
The mean age at menarche of the participants from the PCOS group varied from 11.8 [Citation38] to 12.55 [Citation31] years and of those from the NPCOS group varied from 12.0 [Citation33] to 12.6 [Citation35] years. The BMI of the NPCOS group in the studies ranged from 20.7 [Citation31] to 34.4 [Citation29] kg/m2, and the BMI of the PCOS group ranged from 20.24 [Citation32] to 35.5 [Citation29] kg/m2.
Meta-analysis of age at menarche and BMI
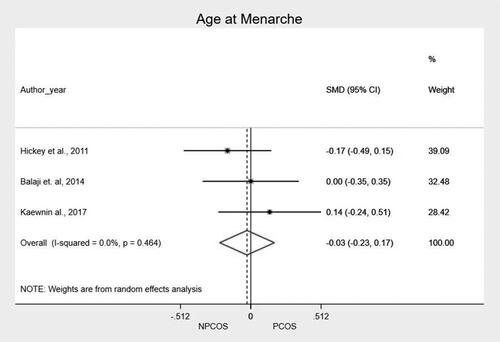
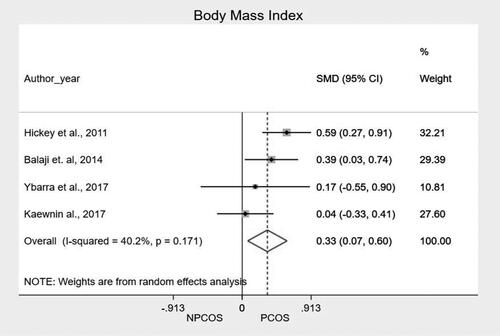
We performed a meta-analysis of the mean and standard deviation of age at menarche and BMI, comparing the two groups. The main findings, along with the mean of the standardized difference and the confidence interval, are shown in and in and . The meta-analysis showed that patients from PCOS group presented higher BMI as compared to NPCOS group (SMD 0.334; CI95% 0.073 − 0.595; p = .012). In the heterogeneity analysis of the meta-analysis, the result of I2 (40.2%) with a value of p = 0.171 shows that there is homogeneity in the included studies, reinforcing the result of the grouped studies that show that there is a difference in BMI between patients with and without PCOS. We found no significant differences related to age at menarche (SMD −0.027; CI95% −0,227 − 0.172; p = 0.790).
Table 4. Meta-analysis of age at menarche and BMI of analyzed adolescent groups (PCOS and NPCOS).
Discussion
Diagnosis of PCOS in adolescence is a challenging topic. Indeed, many clinical features of PCOS are common during adolescence, such as menstrual irregularity, multicystic ovaries, and acne, and they are due to an immature HPO axis.
However, the findings of the present study suggest that the presence of amenorrhea, would be more indicative of persistent chronic anovulation (including PCOS as a possible cause) than of temporary anovulation (such as the HPO immaturity in our data). Additionally, our findings reveal that the BMI of adolescents with PCOS is higher than that found in patients with HPO axis immaturity. These points can help identify adolescents with a potential risk of presenting PCOS.
In our study, the percentage of PCOS varied widely (5.3%−53%), possibly because there is currently no gold standard diagnosis for PCOS in adolescents. This is a major point raised by Teede et al. [Citation13]. For this reason, some investigators suggest that the diagnosis in the first two years after menarche should remain incomplete and the patient should be considered at risk for PCOS. This fact is frustrating for both the health professional and the adolescent, as it causes great anxiety and stress. The delay in reaching a definitive diagnosis not only prolongs the period of uncertainty but also hinders the implementation of appropriate management strategies, exacerbating the psychological impact on both parties involved.
In our review of the literature, the most evident irregular menstrual pattern was amenorrhea, that is, absence of menstrual flow for more than three months. This point had already been suggested by Teede et al. [Citation13] and other researchers, as an important diagnostic marker.
In our results, the age at menarche was not related to the diagnosis of PCOS among the adolescents included in the meta-analysis. The relationship between age at menarche and the diagnosis of PCOS is linked to the complexity of hormonal and reproductive development in adolescents. Earlier menarche, associated with earlier onset of ovarian activity, may increase exposure to hormonal imbalances during development [Citation39], potentially influencing regulation of the HPO axis. On the other hand, a later menarche may not rule out the possibility of having PCOS, as such girls who are later diagnosed with PCOS can present with primary amenorrhea [Citation40].
Our study also showed that adolescents from PCOS group have a 33% higher BMI than adolescents from the NPCOS group (HPO axis immaturity). Another study also found that BMI would have a negative impact from birth to adulthood, mainly on females with PCOS [Citation41]. In fact, adipose tissue produces adipokines and growth factors, such as leptin, that interfere with the reproductive axis. It has been shown in various studies that, in addition to leptin, adipokines such as endotrophin and oncostatin M may also play a role in the pathogenesis of PCOS [Citation42,Citation43], influencing both the appetite (satiety) and the gonadotrophins of the reproductive axis. Insulin-like growth factors I and II, which are also produced by adipose tissue, may influence ovarian activity. The targets of leptin and insulinoid growth factors in the hypothalamus are neuropeptide Y, proopiomelanocortin, and kisspeptin. In addition, leptin directly affects the release of gonadotropin-releasing hormone from the hypothalamus, of the pituitary LH, and of ovarian follicular steroidogenesis [Citation44]. Recently, neuroendocrine mechanisms have been particularly emphasized in the etiology of PCOS, and genetic mechanisms play an important role here. Polymorphisms and mutations in the ERK-1 and ERK-2 genes affect GnRH secretion, causing changes in the HPO axis [Citation43,Citation45,Citation46]. Therefore, increased adipose tissue would influence the HPO axis, a hypothesis clinically verified by Tay et al. [Citation41].
This fact could lead to an earlier onset of menarche in adolescents with PCOS; however, our meta-analysis, comprised only of three studies, did not confirm such a conjecture. Age at menarche is associated with excess body adiposity. Vitalle et al. [Citation47] have established that a minimum of 17% of total body weight in the form of fat was required for menarche to occur by the age of 13, and at least 22% of body fat was required for the maintenance of menstrual cycles at 16 years of age. Nevertheless, the genetic component seems to be the crucial one [Citation48].
In general, epidemiological data show that obesity has a strong relationship with PCOS: between 38% and 88% of women with PCOS are also obese [Citation49–51]. According to a meta-analysis conducted by Lim et al. [Citation52], obese women have a 2.77-fold higher odds ratio of developing PCOS than eutrophic women. This fact shows the close relationship of BMI with the pathophysiology of PCOS, and our study confirms this, showing that adolescents of the PCOS group have, on average, a BMI that is one-third higher than that of the NPCOS group.
There is divergence between the studies in this regard, these disparities may arise from differences in factors like age, ethnicity, and hormonal profiles may contribute to the inconsistencies observed across studies. As in the study by Faria et al. [Citation53], they evaluated 485 adolescents aged 15 to 18 years, who were divided into two groups: G1 (with PCOS) and G2 (without PCOS). In their study, there was no association between height, BMI, use of contraceptives, or the presence of chronic noncommunicable diseases and the diagnosis of PCOS. However, upon simple logistic regression analyses, late menarche was a protective factor for the development of PCOS. In addition, another point that deserves attention is sudden weight gain during adolescence, which in the case of patients with a low BMI could mask PCOS [Citation54,Citation55].
The main limitation of our study was the lack of details in the articles regarding the description of the menstrual patterns. This was due to the fact that the primary goal of most of the studies included in the metanalysis was not to evaluate menses; this may have hindered the standardization of results. Also, few studies in the literature were of good quality, and there were no long-term evaluations to confirm the data. In addition, menstrual data were collected from the patients during medical appointments, and not monitored with a menstrual diary. This introduces the potential for bias related to recall accuracy and completeness, as self-reported data may be subject to memory errors or incomplete recording of symptoms. Despite these limitations, a main strength of our study was finding that amenorrhea was present in patients with PCOS but absent in girls with HPO immaturity.
Another limitation of our study refers to the use of ultrasound in diagnosing PCOS in adolescents. The new 2023 recommendations of the international evidence-based guideline for the assessment and treatment of PCOS, based on practice points, say that there are no definitive criteria to define polycystic ovary morphology upon ultrasound in adolescents [Citation13]; hence, this criteria it is no longer recommended when evaluating adolescents. As the studies were developed before 2020, they used ultrasound to characterize PCOM, and as in the 2004 Rotterdam consensus, there was no such recommendation for adolescents.
Following the new recommendations, perhaps a prospective cohort study with adolescents who 2 years after menarche still develop menstrual irregularities, and were followed prospectively for a period of 2 years, could help in standardizing the diagnosis of PCOS in adolescents.
In conclusion, the main menstrual feature that differentiates PCOS adolescents from those with HPO immaturity is amenorrhea. Also, the BMI was 33% higher in the group of adolescents with PCOS than in the group of adolescents without the syndrome.
Acknowledgements
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the support received. Process No.2020/11553-4.
Disclosure statement
The authors have nothing to disclose.
Data availability statement
The data that support the findings of this study were derived from the following resources available in the public domain: PubMed (https://www.ncbi.nlm.nih.gov/pubmed), EMBASE (https://www.embase.com), Web of Science (https://isiknowledge.com), Virtual Health Library (http://bvsalud.org), and Scopus (https://www.scopus.com)
Additional information
Funding
References
- Elmaoğulları S, Aycan Z. Abnormal uterine bleeding in adolescents. J Clin Res Pediatr Endocrinol. 2018;10(3):191–197. doi: 10.4274/jcrpe.0014.
- Benetti-Pinto CL, De Sá Rosa-E-Silva ACJ, Yela DA, et al. Abnormal Uterine Bleeding. Rev Bras Ginecol e Obstet. 2017;39(7):358–368.
- Lemarchand-Béraud T, Zufferey MM, Reymond M, et al. Maturation of the hypothalamo-pituitary-ovarian axis in adolescent girls. J Clin Endocrinol Metab. 1982;54(2):241–246. doi: 10.1210/jcem-54-2-241.
- Polis RL, Hertweck SP. Treatment options for the adolescent patient experiencing abnormal uterine bleeding. Curr Treat Options Pediatr. 2016;2(3):184–195.
- Arao Y, Hamilton KJ, Wu SP, et al. Dysregulation of hypothalamic-pituitary estrogen receptor α-mediated signaling causes episodic LH secretion and cystic ovary. Faseb J. 2019;33(6):7375–7386. doi: 10.1096/fj.201802653RR.
- Cardoso CBMA, Bordallo MAN. Distúrbios menstruais na adolescência. Adolescência Saude. 2004;1(4):23–25.
- Sun BZ, Kangarloo T, Adams JM, et al. Healthy post-menarchal adolescent girls demonstrate multi-level reproductive axis immaturity. J Clin Endocrinol Metab. 2019;104(2):613–623. doi: 10.1210/jc.2018-00595.
- Spritzer PM, Motta AB. Adolescence and polycystic ovary syndrome: current concepts on diagnosis and treatment. Int J Clin Pract. 2015;69(11):1236–1246. doi: 10.1111/ijcp.12719.
- Adone A, Fulmali DG. Polycystic ovarian syndrome in adolescents. Cureus. 2023;15(1):e34183. doi: 10.7759/cureus.34183.
- Khan L. Polycystic ovarian syndrome in adolescents: keys to diagnosis and management. Pediatr Ann. 2021;50(7):e272–e275. doi: 10.3928/19382359-20210622-01.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551.
- Rosenfield RL. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics. 2015;136(6):1154–1165. doi: 10.1542/peds.2015-1430.
- Teede HJ, Tay CT, Laven J, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2023;120(4):767–793.
- Fauser BCJM, Tarlatzis BC, RWz R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97(1):28–38.e25.
- WHO, World Health Organization. Young people’s health – a challenge for society. Report of a WHO study group on young people and health for all. Technical Report Series 731. Geneva: WHO, 1986.
- Escobar-Morreale HF, Roldán B, Barrio R, et al. High prevalence of the polycystic ovary syndrome and hirsutism in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2000;85(11):4182–4187. doi: 10.1210/jc.85.11.4182.
- Codner E, Soto N, Lopez P, et al. Diagnostic criteria for polycystic ovary syndrome and ovarian morphology in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2006;91(6):2250–2256. doi: 10.1210/jc.2006-0108.
- Samara-Boustani D, Colmenares A, Elie C, et al. High prevalence of hirsutism and menstrual disorders in obese adolescent girls and adolescent girls with type 1 diabetes mellitus despite different hormonal profiles. Eur J Endocrinol. 2012;166(2):307–316. doi: 10.1530/EJE-11-0670.
- Escobar-Morreale HF, Roldan-Martin MB. Type 1 diabetes and polycystic ovary syndrome: systematic review and meta-analysis. Diabetes Care. 2016;39(4):639–648. doi: 10.2337/dc15-2577.
- Baillargeon JP, Nestler JE. Commentary: polycystic ovary syndrome: a syndrome of ovarian hypersensitivity to insulin? J Clin Endocrinol Metab. 2006;91(1):22–24. doi: 10.1210/jc.2005-1804.
- Janssen OE, Mehlmauer N, Hahn S, et al. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;150(3):363–369. doi: 10.1530/eje.0.1500363.
- Petríková J, Lazúrová I, Yehuda S. Polycystic ovary syndrome and autoimmunity. Eur J Intern Med. 2010;21(5):369–371. doi: 10.1016/j.ejim.2010.06.008.
- Nisar S, Shah PA, Kuchay MS, et al. Association of polycystic ovary syndrome and grave’s disease: is autoimmunity the link between the two diseases. Indian J Endocr Metab. 2012;16(2):982–986.
- Baracat EC, Soares JM. Ovários policísticos, resistência insulínica e síndrome metabólica. Rev Bras Ginecol e Obstet. 2007;29(3):117–119.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592.
- Arie WMY, Fonseca AM, Bagmoli VR, et al. Polycystic ovary syndrome and metformin: evidence-based review. Femina. 2009;37(11):585–602.
- Hermont AP, Oliveira PAD, Martins CC, et al. Tooth erosion and eating disorders: a systematic review and meta-analysis. PLoS One. 2014;9(11):e111123. doi: 10.1371/journal.pone.0111123.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. 2014. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Ybarra M, Franco RR, Cominato L, et al. Polycystic ovary syndrome among obese adolescents. Gynecol Endocrinol. 2018;34(1):45–48. doi: 10.1080/09513590.2017.1359250.
- Witchel SF, Oberfield S, Rosenfield RL, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83(6):376–389. doi: 10.1159/000375530.
- Kaewnin J, Vallibhakara O, Arj-Ong Vallibhakara S, et al. Prevalence of polycystic ovary syndrome in Thai university adolescents. Gynecol Endocrinol. 2018;34(6):476–480.
- Nidhi R, Padmalatha V, Nagarathna R, et al. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011; 24(4):223–227. doi: 10.1016/j.jpag.2011.03.002.
- Balaji S, Amadi C, Prasad S, et al. Urban rural comparisons of polycystic ovary syndrome burden among adolescent girls in a hospital setting in India. Biomed Res Int. 2015;2015:158951.
- Sıklar Z, Berberoğlu M, Çamtosun E, et al. Diagnostic characteristics and metabolic risk factors of cases with polycystic ovary syndrome during adolescence. J Pediatr Adolesc Gynecol. 2015;28(2):78–83. doi: 10.1016/j.jpag.2014.05.006.
- Hickey M, Doherty DA, Atkinson H, et al. Clinical, ultrasound and biochemical features of polycystic ovary syndrome in adolescents: implications for diagnosis. Human Rep. 2011;26(6):1469–1477. doi: 10.1093/humrep/der102.
- Kumari S, Pankaj S, Kavita K, et al. Study of adolescent girls with menstrual irregularities for polycystic ovaries and insulin resistance. Jemds. 2015;4(32):5472–5483. doi: 10.14260/jemds/2015/802.
- Fruzzetti F, Baldari F, Palla G, et al. Comparison of PCOS phenotypes in adolescent and young adult mediterranean women with possible PCOS. J Endocrinol Invest. 2021;44(5):995–1000. doi: 10.1007/s40618-020-01394-3.
- Rehme MFB, Pontes AG, Goldberg TBL, et al. Manifestações clínicas, bioquímicas, ultrassonográficas e metabólicas da síndrome dos ovários policísticos em adolescentes [Clinical manifestations, biochemical, ultrasonographic and metabolic of polycystic ovary syndrome in adolescents]. Rev Bras Ginecol Obstet. 2013;35(6):249–254. doi: 10.1590/s0100-72032013000600003.
- Macklon NS, Fauser BCJM. Aspects of ovarian follicle development throughout life. Horm Res. 1999;52:161–170.
- Guzick D. Polycystic ovary syndrome: symptomatology, pathophysiology, and epidemiology. Am J Obstet Gynecol. 1998;179(6 Pt 2):S89–S93. doi: 10.1016/s0002-9378(98)70238-8.
- Tay CT, Hart RJ, Hickey M, et al. Updated adolescent diagnostic criteria for polycystic ovary syndrome: impact on prevalence and longitudinal body mass index trajectories from birth to adulthood. BMC Med. 2020;18(1):389. doi: 10.1186/s12916-020-01861-x.
- Guney G, Taskin MI, Baykan O, et al. Endotrophin as a novel marker in PCOS and its relation with other adipokines and metabolic parameters: a pilot study. Ther Adv Endocrinol Metab. 2021;12:20420188211049607. doi: 10.1177/20420188211049607.
- Camili FE, Akis M, Adali E, et al. Oncostatin M is related to polycystic ovary syndrome-case control study. Biomedicines. 2024;12(2):355. doi: 10.3390/biomedicines12020355.
- Hausman GJ, Barb CR. Adipose tissue and the reproductive axis: biological aspects. Endocr Dev. 2010;19:31–44. doi: 10.1159/000316895.
- Bliss SP, Navratil AM, Xie J, et al. ERK signaling, but not c-Raf, is required for gonadotropin-releasing hormone (GnRH)-induced regulation of Nur77 in pituitary gonadotropes. Endocrinology. 2012;153(2):700–711. doi: 10.1210/en.2011-0247.
- Guney G, Taşkın MI, Sener N, et al. The role of ERK-1 and ERK-2 gene polymorphisms in PCOS pathogenesis. Reprod Biol Endocrinol. 2022;20(1):95. doi: 10.1186/s12958-022-00967-6.
- Vitalle MSS, Tomioka CY, Juliano Y, et al. Índice de massa corporal, desenvolvimento puberal e sua relação com a menarca. Rev Assoc Med Bras. 2003;49(4):429–433. doi: 10.1590/S0104-42302003000400036.
- Roberts SA, Kaiser UB. Genetics in endocrinology: genetic etiologies of Central precocious puberty and the role of imprinted genes. Eur J Endocrinol. 2020;183(4):R107–R117. doi: 10.1530/EJE-20-0103.
- Barber TM, McCarthy MI, Wass JA, et al. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf). 2006;65(2):137–145. doi: 10.1111/j.1365-2265.2006.02587.x.
- Legro RS. The genetics of obesity. Lessons for polycystic ovary syndrome. Ann N Y Acad Sci. 2000;900:193–202. doi: 10.1111/j.1749-6632.2000.tb06230.x.
- Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10(8):2107–2111. doi: 10.1093/oxfordjournals.humrep.a136243.
- Lim SS, Davies MJ, Norman RJ, et al. Overweight, obesity and Central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–637. doi: 10.1093/humupd/dms030.
- Faria FR, Gusmão LS, Faria ER, et al. Síndrome do ovário policístico e fatores relacionados em adolescentes de 15 a 18 anos. Revista da Associação Médica Brasileira. 2013;59(4):341–346. doi: 10.1016/j.ramb.2013.02.003.
- Barber TM, Hanson P, Weickert MO, et al. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health. 2019;13:1179558119874042. doi: 10.1177/1179558119874042.
- Fu L, Qu F, Pan J, et al. Polycystic ovary syndrome in adolescents with obesity. Rev Assoc Med Bras (1992). 2021;67(3):468–473. doi: 10.1590/1806-9282.20200890.