Abstract
Premature ovarian insufficiency (POI) is a common gynecological endocrine disease, which seriously affects women’s physical and mental health and fertility, and its incidence is increasing year by year. With the development of social economy and technology, psychological stressors such as anxiety and depression caused by social, life and environmental factors may be one of the risk factors for POI. We used PubMed to search peer-reviewed original English manuscripts published over the last 10 years to identify established and experimental studies on the relationship between various types of stress and decreased ovarian function. Oxidative stress, follicular atresia, and excessive activation of oocytes, caused by Stress-associated factors may be the main causes of ovarian function damage. This article reviews the relationship between psychological stressors and hypoovarian function and the possible early intervention measures in order to provide new ideas for future clinical treatment and intervention.
Introduction
Premature ovarian insufficiency (POI) is the loss of normal ovarian function before the age of 40 years. POI is clinically characterized by oligomenorrhea or amenorrhea with increased gonadotrophins (FSH > 25 IU/L) and decreased estradiol (E2) [Citation1] with an incidence of 1–2%, and is still increasing year by year [Citation2]. The pathogenesis of POI is highly intricate, with approximately 90% of cases having an unknown etiology. This may be attributed to various factors including genetic and environmental influences, autoimmune disorders, iatrogenic injuries, as well as idiopathic causes [Citation3]. With the development of social economy and science and technology, anxiety, depression and other negative emotions caused by social, life and environmental factors are considered to be important risk factors for POI [Citation4]. According to previous research, most patients with POI have experienced two or more adverse life events prior to diagnosis, suggesting that the cumulative negative effects of psychological stressors may play a role in the development of POI [Citation4]. Long shifts and intense workloads are associated with menstrual disorders [Citation5]. Lim Ym et al. reported that employed women were more likely to experience POI and early menopause than unemployed people [Citation6]. About 43% of patients with POI had a history of depression, of which 26% were diagnosed with depression within the first 5 years of POI diagnosis [Citation7]. Chronic screaming and unpredictable stress can cause reproductive endocrine disorders in mice and causing ovarian reserve decline by accelerating primordial follicle activation and destroying growing follicles [Citation8–10]. These studies have proved that psychological stressors can indeed inhibit and damage female reproductive and endocrine functions, and induce the occurrence and development of POI. However, the mechanism of action of psychological stressors on POI is not fully understood. And there is currently no effective treatment for POI [Citation1] Therefore, early detection and early prevention may be the direction of future treatment. This article reviews the research progress of the mechanism of psychological stressors on POI and its intervention methods.
The relationship between psychological stressors and ovarian function
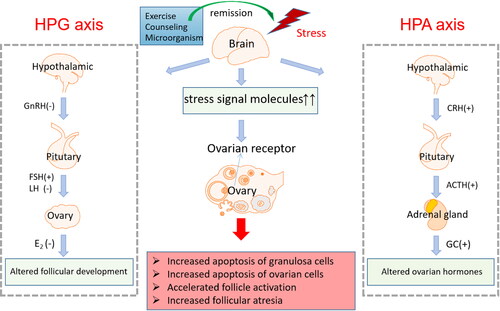
The main endocrine components involved in the human stress system response include hypothalamic-pituitary-adrenal (HPA) axis and locus coeruleus norepinephrine (LC/NE), autonomic nervous system [Citation11]. When the brain detects a homeostatic challenge, it activates the sympathetic nervous system (SNS) and releases catecholamine epinephrine (E) and norepinephrine (NE), leading to a physiological stress response [Citation12]. Within minutes of stress-induced HPA axis activation, the medial small cell area of the paraventricular nucleus stimulates the terminal buckling point of the median protuberans axon to release corticotropin-releasing hormone (CRH). In turn, it stimulates corticotroph cells in the anterior pituitary to release adrenocorticotropic hormone (ACTH) into the systemic circulation [Citation13,Citation14]. Each stressor in the stress system has the potential to regulate reproductive suppression. Studies have proved that, the abnormal abundance of neurotrophins, steroid hormones, metabolic and/or inflammatory cytokines can lead to changes in neurotransmitters, intracellular signaling, gene transcription and translation, and then directly or indirectly affect ovarian development and regulate apoptosis [Citation15–17]).
Psychological stressors can cause ovarian damage by interfering with ovarian hormones
Psychological stressors have gradually become a common and important cause of reproductive endocrine disorders [Citation18]. The activation of the HPA axis suppresses the normal function of the HPO axis through endocrine, paracrine, and neurological mechanisms [Citation19]. The GnRH produced by the hypothalamus enters the pituitary portal venous system, stimulating the secretion of FSH and LH. FSH stimulates follicular maturation and estrogen release during the follicular phase, while LH triggers ovulation and promotes the release of progesterone during the luteal phase [Citation20]. The delayed release of gonadotropin-releasing hormone (GnRH) and the LH surge caused by psychological stressors interfere with the release of reproductive hormones during the follicular phase [Citation21]. Studies have shown that psychological stressors are associated with decreased estradiol (E2) and progesterone (P), as well as increased follicle-stimulating hormone (FSH) [Citation22]. Stressors also affects reproductive hormone levels through some other mechanisms. For example, chronic stress will prolong the catabolic state of the whole body, and continuous HPA overactivity will gradually lead to increased visceral fat and insulin resistance, thereby interfering with HPO axis [Citation23]. Catecholamines and β-adrenergic receptors (ADR β) also affect LH and E2 levels in the body [Citation19]. However, the specific mechanisms of stress-induced endocrine changes and reproductive dysfunction still deserve further study and discussion.
Distribution and regulation of psychological stressors-associated factors in ovary
GnRH and GnRH receptors (GnRHRs) are highly expressed in the granulosa cells (GCs) and granulosa luteal cells (GLCs) of preovulatory follicles [Citation24]. In human GCs, GnRH and GnRH-II induce apoptosis by interfering with the IGF-I/AKT signaling pathway and simultaneously activating proteolytic caspase cascades involved in initiating Caspase-8 and effecting caspase-3 and caspase-7 [Citation25]. GnRH-induced granulosa cell apoptosis is not only related to follicular atresia, but also to low oocyte quality. In luteal cells (LCs), the GnRH/GnRHR system has been proposed to be involved in the processes of luteinization/luteolysis. Specifically, GnRH induces structural luteolysis in corpora lutea, through the up-regulation of molecules that are involved in the remodeling of the extracellular matrix, such as thee matrix metalloproteinase MMP-2 and the membrane type MMP-1 [Citation26,Citation27]. Thus the GnRH/GnRHR system is expressed in a specific manner in the human ovary and is involved in follicular development and luteal function.
CRH concentrations in premenopausal ovaries are higher than those in postmenopausal ovaries, suggesting that ovarian CRH may be associated with normal ovarian function during reproductive life. CRH and its receptor (CRHR-1 and CRHR-2) are found in ovarian tissues, mainly in the membrane, stroma, and cytoplasm of follicle and GCs [Citation28] CRH has a strong inflammatory effect. Reproductive-related CRH regulates reproductive functions, such as ovulation, luteolysis, implantation and delivery, through inflammatory components [Citation29,Citation30]. CRH can also act directly on the ovary to regulate steroidogenesis and/or oocyte production [Citation31,Citation32].
Glucocorticoids, In addition to their effects on ovarian periodicity mediated through the hypothalamus and pituitary, glucocorticoids can also affect ovarian physiology by regulating the function of GCs, oocytes, cumulus cells (CCs), and LCs [Citation33]. In mouse studies, the increased glucocorticoid levels caused by restraint stress impair the developmental potential of oocytes [Citation34]. A study on cats found that glucocorticoid treatment led to morphological changes in oocytes after ovarian stimulation [Citation35]. The use of dexamethasone (DEX) can increase pre-ovulatory anti-Müllerian hormone levels, suggesting that dexamethasone can promote the development of preantral follicles, leading to a reduction in the primordial follicle pool [Citation36]. Glucocorticoids can also affect the production of ovarian steroid hormones, DEX decreased E2 and P production by cultivated ovarian cells during the experiment [Citation37]. Glucocorticoid exposure was also associated with decreased mRNA expression of glucocorticoid receptor (GR) gene (NR3C1) and the ovarian growth factors insulin-like growth factor-1 (IGF-1) and brain-derived neurotrophic factor (BDNF) in parietal granule cells [Citation38].
BDNF is a regulator of human oocyte maturation and early embryonic development. It is expressed in the ovary [Citation39] and involved in oocyte maturation, early embryo cleavage and blastocyst formation [Citation40]. BDNF regulates the expression of genes related to cell proliferation (CCND1, p21, and Bcl2) and apoptosis (Bax), playing a crucial role in cell proliferation, differentiation, and apoptosis [Citation41,Citation42]. After binding to its specific receptor, BDNF activates its downstream phosphoinositide-3 kinase-protein kinase B-mammalian target of rapamycin (PI3K-AKT-mTOR) signaling pathway, participating in the activation of primordial follicles and the meiotic division of oocytes [Citation43,Citation44], Furthermore, studies have shown that the BDNF-activated PI3K-AKT-mTOR signaling pathway play a role in female reproductive diseases such as polycystic and POI caused by chronic unpredictable mild stressors [Citation45].
Nerve growth factor (NGF) and its receptors are expressed in ovarian cells of different species (oocytes, GCs, theca and stromal cells), including humans [Citation46]. NGF promotes follicular development and increases the cellular response to FSH by up-regulating FSH receptors in GCs [Citation47]. NGF is also involved in promoting thecal cells (TC) proliferation, In vitro culture of sinusoidal follicular TC, Plasmid transfection of tyrosine kinase receptor (TrkA) expression in the presence of NGF led to an increase in the proliferation rate of bovine TC cells [Citation48]. NGF is involved in the regulation of steroid hormone production. For example, NGF directly promotes the production of E2 by activating TrkA (high-affinity tyrosine kinase NGF receptor), and inhibits the differentiation of GCs into LCs by suppressing the production of P in isolated human GCs [Citation47, Citation49]. When psychological stressors occurs, the levels of these molecules change accordingly, causing persistent damage to the ovary ().
Table 1. Location and function of stressors on ovary.
The mechanism of psychological stressors induces POI
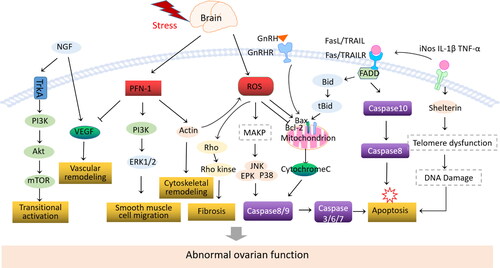
It is well known that the decline of ovarian function is mainly related to follicular atresia and excessive activation of primordial follicles. Due to the unique physiological environment of the ovary and the process of follicular development, the apoptosis of ovarian granulosa cells (GCs) and oocytes is the main cause of follicular atresia. Furthermore, in the process of POI, cell apoptosis and oxidative stress form a ‘vicious cycle’ involving changes in various molecules. POI caused by psychological stress is closely related to these mechanisms ().
ROS
Reactive oxygen species (ROS) are one of the most effective biological effectors of stress-related endocrine, immune/inflammatory, and metabolic pathways on cell health [Citation57,Citation58], and excessive ROS levels can induce the occurrence of ovarian diseases. Increased ROS beyond the antioxidant capacity can induce oxidative stress (OS), leading to oxidative damage to lipids, proteins, and DNA, as well as chromosomal abnormalities and telomere shortening, ultimately resulting in a decrease in the number and quality of oocytes [Citation59,Citation60]. Lipid peroxidation leads to an increase in the level of maturation-promoting factor (MPF), disrupting follicular development [Citation61]. The activation of ROS mediates the induction of ovarian cell hypertrophy, apoptosis, and fibrosis through the protein kinase pathway activated by advanced glycation end products [Citation62]. Excessive ROS production can directly affect the targets of signaling pathways, and can also interact as a second messenger with intermediate reaction steps, inducing abnormal outcomes [Citation63]. Furthermore, HIF-1α (hypoxia-inducible factor-1α) can cause oxidative damage and apoptosis of GCs through the hypoxia-inducible factor-vascular endothelial growth factor (VEGF) signaling pathway [Citation64]. ROS-mediated granulosa cell apoptosis reduces the communication between granulosa cells and oocytes, affecting the supply of nutrients and the quality of pre-ovulatory oocyte maturation, and disrupting the developing follicle-oocyte complex [Citation65,Citation66]. The formation of these oxidative damage signaling pathways promotes ovarian injury.
Psychological stressors-induced oxidative stress can cause ovarian injury by increasing ROS (reactive oxygen species) levels. The elevated levels of stress-related factors activate various physiological functions that require oxygen consumption, leading to the production of superoxide free radicals [Citation67,Citation68]. The increase in cortisol levels reduces the superoxide anion in leukocytes, weakening the body’s antioxidant capacity [Citation69]. Furthermore, stress can suppress the levels of antioxidant enzymes, and the activities of superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT) in the preantral follicles (PF) under stress conditions are significantly reduced [Citation70].
Inflammatory factors
Elevated glucocorticoids can increase the expression of pro-inflammatory genes (iNOS, IL-1β, TNF-α) and reduce the expression of anti-inflammatory genes including IL-1RA, IL-10, and MKP-1 [Citation71]. Dexamethasone up-regulated gene expression of Shelterin complex proteins telomere repeat-binding factor 1 and 2 (TRF1 and TRF2) and protection of telomeres (POT1) in cell lines cultured in vitro. TNF-α up-regulates the expression of telomerase reverse transcriptase (TERT), TRF1, TRF2, and POT1 genes, suggesting that transcriptional regulation of Shelterin complex may be a potential mechanism for stress-related telomere length dynamic changes [Citation72]. Due to the end replication problem, DNA replication machines cannot complete the synthesis of linear chromosome ends, telomere shortens as cells divide. Telomere shortening, collapse of telomere structure or displacement of the Shelterin complex on telomeres cause disruption of telomere structure, which in turn cause DNA damage response and loss of cell proliferation, and finally leads to senescence or apoptosis [Citation73].
Apoptosis
Death receptor and mitochondrial pathways are involved in the process of oocyte apoptosis. In previous studies, total transcriptome analysis of ovaries in depressed mice found significant reductions in genes associated with the mitotic cell cycle. The number of genes related to the regulation of exogenous apoptosis signaling pathway through death domain receptors and the number of genes related to negative regulation of cells increased significantly [Citation74]. Stress signals can activate the tumor necrosis factor-α (TNF-α) system, increase Fas expression, and trigger apoptosis of ovarian cells and oocytes within the ovary by activating the FasL/Fas system [Citation75,Citation76]. Bcl2 protein family members mainly act on mitochondrial membrane, and play an important role in follicular growth/atresia by regulating the apoptosis of germ cells and somatic cells. In the ovary of depression-like mice, more GCs apoptosis occurs and the expression of Bcl2 in GCs mitochondria significantly reduces [Citation77]. BAX expression was abundant in GCs of early atresia follicles, but not in atresia follicles, suggesting that BAX protein expression in early atresia promotes GCs apoptosis [Citation78]. Bcl2-deficient mice exhibit a reduced number of oocytes and primordial follicles [Citation79], whereas Bax-deficient mice exhibit an excess of abnormal follicles [Citation80,Citation81].
Profilin-1(PFN-1)
Profilin-1(PFN-1) is widely present in eukaryotic cells and participates in the regulation of actin skeleton remodeling, cell morphology maintenance, cell adhesion, motility, growth, division and ligand-dependent signal transduction. Long term influence of stressors stimulation significantly increases the expression of PFN-1 [Citation82]. Overexpression of PFN-1 promotes apoptosis of ovarian endothelial cells. Intercellular adhesion molecules up-regulate and vasodilators stimulate phosphorylation of phosphoproteins, mediates endothelial cell dysfunction [Citation83,Citation84]. Extracellular PFN-1 can promote extracellular regulation of protein kinase 1/2(ERK1/2), ribosomal protein S6 kinase, phosphatidylinositol 3 kinase to stimulate the proliferation and migration of ovarian smooth muscle cells, thereby promoting the progression of ovarian dysfunction [Citation85,Citation86]. PFN-1 regulates cytoskeleton-mediated ovarian vascular hypertrophy, increased expression of PFN-1 can directly lead to elongation of sarcoplasmic reticulum, ovarian fiber rearrangement and increased contractile stress, thus activating mechanical stress-related signaling pathways and affecting ovarian physiological function. In addition, PFN-1 may affect the synthesis of nitric oxide in ovarian endothelial cells by binding to actin, causing cytoskeleton remodeling, increasing stress fibers and activating inflammation-related signaling pathways [Citation87,Citation88]. Therefore, PFN-1 induced fibrosis and ovarian cell apoptosis are important pathological processes of ovarian dysfunction.
Adrenergic receptor signaling
Long term influence of stressors can cause an abnormal increase of NE levels, resulting in cell damage or apoptosis, and thus impair ovarian function [Citation89,Citation90]. The activation of adrenergic receptor signaling has an apoptotic effect on cells. Stimulation with NE can significantly enhance the expression of rno-miR-128-3p in GCS and induce apoptosis of rat GCS by inhibiting the expression of Wilms tumor 1 (WT1) [Citation91]. The miR-21/Smad7 pathway mediated by α1A adrenergic receptor activation plays an important role in the occurrence of POI. It has been reported that overexpression of Smad7 in GCs significantly increases apoptosis [Citation92]. α1A adrenergic receptor (α1A-AR), as a negative mediator of Smad7, can regulate the expression of miR-21. Overexpression of Smad7 reduces miR-21 expression, thereby enhancing TGF-β-induced granulosa cell apoptosis and impairs ovarian function [Citation93]. In addition, ROS is produced during the uptake and metabolism of adrenomedullin by GC, which will continue to act even if the receptor is blocked, in turn forming toxic effects through ROS [Citation90, Citation94].
The early detection of POI and psychological stressors
Ovarian histology is the gold standard for assessing follicular status and ovarian reserve. However, the invasive nature of histological examination precludes its use in the long-term monitoring of premature ovarian insufficiency (POI) progression. Consequently, identifying sensitive biochemical markers to evaluate the residual follicular pool in POI patients is of paramount importance. By utilizing commonly employed ovarian function testing parameters in conjunction with stress-related assessments, it may be possible to identify POI patients whose condition is highly correlated with psychological stressors. Implementing early interventions targeting this specific population could prove to be the most effective strategy for preserving ovarian function.
When ovarian dysfunction commences, markers of ovarian reserve exhibit a marked decline, particularly those with heightened sensitivity, such as Anti-Müllerian hormone (AMH), antral follicle count (AFC), inhibin B, and the follicle-stimulating hormone (FSH) to luteinizing hormone (LH) ratio.
AMH
A glycoprotein secreted by the granulosa cells of large, small preantral, and small antral follicles in the female ovary, exhibits a strong correlation with the primordial follicle pool [Citation95]. Among the various ovarian reserve assessment tools, AMH is widely regarded as the most sensitive and earliest marker of diminishing ovarian reserve. The commonly accepted cutoff value for predicting the onset of premature ovarian insufficiency (POI) is 1.211 ng/mL (AUC 0.932, 95%, CI 0.918–0.945) [Citation96].
Antral follicle count (AFC)
AFC represents the total number of antral follicles in both ovaries, as visualized by ultrasonography during the early follicular phase (day 2–4) of the menstrual cycle. AFC is a convenient and practical assessment tool, yielding immediate results and demonstrating good intercycle reliability and interobserver reliability. As ovarian function declines from the stage of normal ovarian reserve (NOR) to the pre-premature ovarian insufficiency (pre-POI) stage, AFC values exhibit an approximately two-fold decrease, from 8 to 4. When employed for the prediction of pre-POI, the recommended cutoff value for AFC is 5 (AUC 0.868, 95% CI 0.848–0.885) [Citation96].
Inhibin B
a glycoprotein hormone secreted by the granulosa cells of developing preantral and early antral follicles, reflects the circulating concentration of the follicular pool responsive to gonadotropins. Its levels peak during the early to mid-follicular phase of the menstrual cycle [Citation97]. Serum Inhibin B concentrations decline with advancing reproductive age, particularly in the early follicular phase [Citation98]. Although low Inhibin B levels (≤31.74 pg/mL) demonstrate high specificity for predicting premature ovarian insufficiency (pre-POI), the sensitivity is suboptimal (AUC 0.704, 95% CI 0.679–0.727) [Citation96]. Consequently, Inhibin B is considered a marker of current ovarian function rather than a reliable predictor of ovarian reserve [Citation98].
FSH/LH ratio
FSH remains the sole ovarian reserve marker currently employed in research for defining premature ovarian insufficiency (POI) and its subgroups. As ovarian function declines from normal ovarian reserve (NOR) to the pre-POI stage, the FSH/LH ratio exhibits a significant increase (1.64 ± 0.66 vs. 2.49 ± 1.22, p < 0.001). The generally recommended cutoff value for the FSH/LH ratio is 2.11 (AUC 0.749, 95% CI 0.726–0.772) [Citation96].
Certain clinical manifestations
Certain clinical manifestations warrant attention, as research has demonstrated that stress-induced Premature Ovarian Failure (POF) in patients may be preceded by alterations in the menstrual cycle, such as oligomenorrhea or menorrhag, prior to the onset oforrhea [Citation99].
In line with our previous discussions, prolonged exposure to high levels of stress may result in compromised reproductive function [Citation100]. The application of psychological assessments (CES-D, BSI-GSI, WHOQOL-Bref) to evaluate the overall psychological state of patients can serve as a basis for the prevention of induced by psychological stressors.
Psychological stressors-associated factors
In a study, the Support Vector Regression (SVR) model was effectively utilized to forecast the nonlinear relationships within the neuroendocrine-immune network of rats with stress-induced POI, thereby presenting a methodological paradigm for evaluating the incidence of POI [Citation18]. Research conducted by Biocycle and Eager discovered that salivary cortisol, apart from its convenience in sampling, can sensitively capture and assess fluctuations in daily stress, making it suitable for measuring both acute and chronic stress [Citation101]. The decrease in biomolecular values within the hypothalamic-pituitary-adrenal (HPA) axis during stress-induced responses leads to a significant decrease in hypothalamic biomolecules (β-EP, IL-1, NOS, and GnRH).
Center for Epidemiologic Studies Depression Scale (CES-D)
The CES-D quantifies the severity of depressive symptomatology and consists of 20 items, which are measured using a scale from 0 to 3. Scores range from 0 to 60, with scores of 16 or higher reflecting clinical relevant depressive symptoms. A value of 22 or more signals a major depression [Citation102].
Brief Symptom Inventory (BSI)
The BSI measures a person’s perceived disturbance by physical and psychological symptoms over a period of seven days. It comprises a total of 53 items, which are grouped into nine scales. These scales describe the areas: somatization, compulsiveness, insecurity in social contact, depression, anxiety, aggressiveness/hostility, phobic anxiety, paranoid thinking and psychoticism [Citation103,Citation104].
The global value Global Severity Index (GSI)
GSI represents the most sensitive indicator of the psychological burden, because it measures the intensity of perceived stress in all 53 items. The response format ranges from 0 (not at all) to 4 (very strong). Higher values mean that the burden is judged to be higher. The standard Global Value BSI GSI value in women is M = 0.35. For the depression scale M = 0.33, for the anxiety scale M = 0.39 and the aggression scale M = 0.37.
WHO Quality of Life Bref (WHOQOL-Bref)
The higher the values of the items in the WHOQOL-Bref, the higher the subjective quality of life is rated to be. The WHOQOL-Bref is a subjective quality of life assessment tool comprising 26 items, including global quality of life, physical well-being, mental well-being, social relationships, and environment. According to the scores of the items in WHOQOL-Bref, the higher the scores, the higher the subjective quality of life is rated to be. The standard value of the WHOQOL Global scale is a total mean (M) of 67.8 with a standard deviation (SD) of 16.7. For women aged 26–35, the mean is 72.49, while for women aged 36–45, the mean is 68.72 [Citation105].
Early intervention of POI caused by psychological stressors
Decreased ovarian reserve may be persistent. Spontaneous premature termination of ovarian function requires a higher level of clinical treatment. Reproductive loss requires multidisciplinary management, including the provision of appropriate counseling and emotional support, nutritional supplementation, hormone replacement therapy, reproductive health care, lifestyle changes, etc [Citation106,Citation107]. Conventional treatment methods for Premature Ovarian Insufficiency (POI) have been widely discussed in other reviews. Here, we will focus exclusively on interventions for stress-induced POI. For POI caused by stress, alleviating stress can restore ovulation, fertility, and improve other neuroendocrine-related symptoms [Citation108]. The hypothalamic-pituitary-adrenal (HPA) axis and the corresponding hypothalamic-pituitary-ovarian (HPO) inhibition are fundamental to the impact of psychological stressors on ovarian function [Citation51]. The response of the HPA axis to stress is dynamic, removing the stressor can quickly halt the action of stress-associated factors, thus preventing damage to the peripheral and central nervous system due to prolonged exposure [Citation109]. Several methods may have a positive impact on improving psychological stressors
Psychological intervention
Substantial evidence suggests that psychotherapy is effective for treating anxiety and depression, including Cognitive Behavioral Therapy (CBT) [Citation110,Citation111], Behavioral Activation Therapy [Citation112], and Interpersonal Psychotherapy (IPT) [Citation113], etc. Psychotherapy is more acceptable than drug therapy [Citation114]. Bahari et al. demonstrated that muscle relaxation and laughter therapy are related to improvements in psychological conditions (trait anxiety and depression) and contribute positively to the treatment outcomes for patients undergoing in vitro fertilization (IVF) (estradiol levels, oocyte retrieval, transfer status, and pregnancy test results). The mechanism may be related to laughter altering the activity of dopamine and serotonin [Citation115], with endorphins released during laughter helping to foster positive emotions and reduce negative feelings such as anxiety and depression [Citation116].
Exercise
Research indicates that exercise has a positive impact on mental health, improving mood, self-esteem, and reducing stress and anxiety levels [Citation117–119]. Exercise combats the pathological effects of stress through physiological effects such as increased endorphin levels [Citation120], improved mitochondrial function [Citation121,Citation122], enhanced production of neurotransmitters [Citation123,Citation124], and a weakened response of the hypothalamic-pituitary-adrenal (HPA) axis [Citation123, Citation125]to stress. Moderate exercise can improve reproductive health. Boudoures et al. found that voluntary exercise can moderately improve mitochondrial structure in oocytes of mice on a high fat diet (HFD), reducing the number of rose petals and oval mitochondria [Citation126]. Another study demonstrated that exercise ameliorated premature decline in ovarian function at the oocyte level in POLG mice, possibly through mitochondrial localization of p53. Enhanced p53 fidelity mechanisms have been demonstrated in somatic tissues after exercise [Citation127,Citation128], and exercise induces translocation of nuclear p53 to mitochondria. This leads to the up-regulation of peroxisome proliferator-activated receptor γ and coactivator 1α, an important regulator of metabolism and mitochondrial biogenesis [Citation119, Citation126]. Furthermore, exercise can help alleviate inflammation, thereby combating the aging or apoptosis of ovarian cells caused by inflammatory factors through the destruction of telomere structure [Citation129,Citation130].
Gut microorganism
There is evidence in animal and human studies that probiotics are associated with anxiety, stress, and depression-like behaviors. Studies over the years have shown that gut microorganism can affect stress-related HPA axis, autonomic nervous system and neurobiological functions, which constitute the basic mechanism of microbial influence on the central nervous system. People taking probiotics had decreased scores on the Hopkins Symptom Global Severity Index (HSSCL-90) checklist and improved anxiety and depressive symptoms [Citation127]. A daily dose of probiotics can prevent mood disorder symptoms in people with mild stress caused by low cortisol [Citation128]. Indeed, these studies highlight new approaches for the prevention and treatment of depression and anxiety. However, there is still a lack of direct studies on whether the intestinal action of microorganisms can rescue ovarian function.
Conclusions and future perspectives
We focus on the direct and indirect molecular basis of POI caused by psychological stressors. Stress-associated factors produced by psychological stressors can directly act on ovarian surface receptors and participate in the decline of ovarian function. It can also change endocrine hormone levels and ROS production through the HPA and HPO axes to participate in ovarian or GCs apoptosis. Understanding the molecular mechanism and signaling pathway of psychological stressors changes follicle physiology will help to further understand the specific mechanism of stress-mediated ovarian dysfunction.Psychological interventions for women with POI have the potential to reduce anxiety and depression and may well improve the outcomes of POI. Exercise and gut microbiome regulation may also be remedies for stress-related POI. However, more quantitative studies are needed to verify the exact relationship between the occurrence and development of POI and stress. For example, the regulatory effect of stress-induced adrenaline or glucocorticoid on the nervous system and reproductive system is still unclear, and the signal interaction between the nervous system and reproductive system, as well as how to find more effective intervention means, are questions worth exploring and answering in the future.
Authors’ contributions
YX and JW conceived the idea. YX and JF were involved in drafting the early manuscript, ZH, DZ prepared tables and helped with revision of the article.CG has contributed to the literature search. PL and JW provided a significant expert contribution in the scientific content revision process. YX and JW completed the literature collection. Subsequent drafts were expanded upon by all authors. All authors read and approved of the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nash Z, Davies M. Premature ovarian insufficiency. BMJ. 2024;384:1. doi:10.1136/bmj-2023-077469.
- Chon SJ, Umair Z, Yoon MS. Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biol. 2021;9:672890. doi:10.3389/fcell.2021.672890.
- Fu X, Zheng Q, Zhang N, et al. CUMS promotes the development of premature ovarian insufficiency mediated by nerve growth factor and its receptor in rats. Biomed Res Int. 2020;2020:1946853–14. doi:10.1155/2020/1946853.
- Sun J, Fan Y, Guo Y, et al. Chronic and cumulative adverse life events in women with primary ovarian insufficiency: an exploratory qualitative study. Front Endocrinol. 2022;13:856044. doi:10.3389/fendo.2022.856044.
- Lawson CC, Johnson CY, Chavarro JE, et al. Work schedule and physically demanding work in relation to menstrual function: the nurses’ health study 3. Scand J Work Environ Health. 2015;41(2):194–203. doi:10.5271/sjweh.3482.
- Lim Y-M, Jeong K, Lee SR, et al. Association between premature ovarian insufficiency, early menopause, socioeconomic status in a nationally representative sample from korea. Maturitas. 2019;121:22–27. doi:10.1016/j.maturitas.2018.12.004.
- Allshouse AA, Semple AL, Santoro NF. Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause. 2015;22(2):166–174. doi:10.1097/GME.0000000000000286.
- Xi W, Mao H, Cui Z, et al. Scream sound-induced chronic psychological stress results in diminished ovarian reserve in adult female rat. Endocrinology. 2022;163(6):42. doi:10.1210/endocr/bqac042.
- Zhou P, Lian H-Y, Cui W, et al. Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice. Biol Reprod. 2012;86(3):83.
- Fu XY, et al. Effects of chronic unpredictable mild stress on ovarian reserve in female rats: feasibility analysis of a rat model of premature ovarian failure. Mol Med Rep. 2018;18(1):532–540.
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992;267(9):1244–1252. doi:10.1001/jama.1992.03480090092034.
- Allen AP, et al. Biological and psychological markers of stress in humans: focus on the trier social stress test. Neurosci Biobehav Rev. 2014;38:94–124.
- Herman JP, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–621.
- Leistner C, Menke A. Hypothalamic-pituitary-adrenal axis and stress. Handb Clin Neurol. 2020;175:55–64.
- Chang H-M, Wu H-C, Sun Z-G, et al. Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: physiological and pathophysiological implications. Hum Reprod Update. 2019;25(2):224–242. doi:10.1093/humupd/dmy047.
- James KA, Stromin JI, Steenkamp N, et al. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front Endocrinol. 2023;14:1085950. doi:10.3389/fendo.2023.1085950.
- Sammad A, Luo H, Hu L, et al. Transcriptome reveals granulosa cells coping through redox, inflammatory and metabolic mechanisms under acute heat stress. Cells. 2022;11(9):1443. doi:10.3390/cells11091443.
- Wang XF, et al. Biological mechanisms of premature ovarian failure caused by psychological stress based on support vector regression. Int J Clin Exp Med. 2015;8(11):21393–21399.
- Morley P, Calaresu FR, Armstrong DT. Catecholamines inhibit steroidogenesis by cultured porcine thecal cells. FEBS Lett. 1990;275(1-2):70–72.
- Sominsky L, Hodgson DM, McLaughlin EA, et al. Linking stress and infertility: a novel role for Ghrelin. Endocr Rev. 2017;38(5):432–467. doi:10.1210/er.2016-1133.
- Lania A, Gianotti L, Gagliardi I, et al. Functional hypothalamic and drug-induced amenorrhea: an overview. J Endocrinol Invest. 2019;42(9):1001–1010. doi:10.1007/s40618-019-01013-w.
- Zhao L-H, Cui X-Z, Yuan H-J, et al. Restraint stress inhibits mouse implantation: temporal window and the involvement of HB-EGF, estrogen and progesterone. PLoS One. 2013;8(11):e80472. doi:10.1371/journal.pone.0080472.
- Wu JX, Lin S, Kong SB. Psychological stress and functional endometrial disorders: update of mechanism insights. Front Endocrinol. 2021;12:690255. doi:10.3389/fendo.2021.690255.
- Choi J-H, Gilks CB, Auersperg N, et al. Immunolocalization of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and type I GnRH receptor during follicular development in the human ovary. J Clin Endocrinol Metab. 2006;91(11):4562–4570. doi:10.1210/jc.2006-1147.
- Hong I-S, Klausen C, Cheung AP, et al. Gonadotropin-releasing hormone-I or -II interacts with IGF-I/Akt but not connexin 43 in human granulosa cell apoptosis. J Clin Endocrinol Metab. 2012;97(2):525–534. doi:10.1210/jc.2011-1229.
- Cheung LW, Leung PC, Wong AS. Gonadotropin-releasing hormone promotes ovarian cancer cell invasiveness through c-Jun NH2-terminal kinase-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res. 2006;66(22):10902–10910. doi:10.1158/0008-5472.CAN-06-2217.
- Walters K, Wegorzewska IN, Chin Y-P, et al. Luteinizing hormone-releasing hormone I (LHRH-I) and its metabolite in peripheral tissues. Exp Biol Med (Maywood). 2008;233(2):123–130. doi:10.3181/0707-MR-201.
- Wypior G, Jeschke U, Kurpisz M, et al. Expression of CRH, CRH-related peptide and CRH receptor in the ovary and potential CRH signalling pathways. J Reprod Immunol. 2011;90(1):67–73.
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351–1362. doi:10.1056/NEJM199505183322008.
- Kalantaridou SN, et al. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. 2010;85(1):33–39.
- Zhou H, Chen A, Lu W. Corticotropin-releasing hormone reduces basal estradiol production in zebrafish follicular cells. Mol Cell Endocrinol. 2021;527:111222.
- Yu J, Li X-F, Tsaneva-Atanasova K, et al. Chemogenetic activation of PVN CRH neurons disrupts the estrous cycle and LH dynamics in female mice. Front Endocrinol (Lausanne). 2023;14:1322662. doi:10.3389/fendo.2023.1322662.
- Luo E, Stephens SBZ, Chaing S, et al. Corticosterone blocks Ovarian cyclicity and the LH surge via decreased kisspeptin neuron activation in female mice. Endocrinology. 2016;157(3):1187–1199. doi:10.1210/en.2015-1711.
- Yuan H-J, Han X, He N, et al. Glucocorticoids impair oocyte developmental potential by triggering apoptosis of ovarian cells via activating the fas system. Sci Rep. 2016;6(1):24036. doi:10.1038/srep24036.
- Andrews CJ, Yapura J, Potter MA, et al. Prolonged glucocorticoid administration affects oocyte morphology in cats (Felis catus) undergoing an ovarian stimulation protocol. Theriogenology. 2023;208:77–87. doi:10.1016/j.theriogenology.2023.05.024.
- Yuan X-H, Yang B-Q, Hu Y, et al. Dexamethasone altered steroidogenesis and changed redox status of granulosa cells. Endocrine. 2014;47(2):639–647. doi:10.1007/s12020-014-0250-x.
- Balasem Z, Salamat N, Mojiri-Forushani H. Using cell culture systems from the Persian Gulf arabian yellowfin sea bream, Acanthopagrus Arabicus, to assess the effects of dexamethasone on gonad and brain aromatase activity and steroid production. Toxicol in Vitro. 2024;97:105803. doi:10.1016/j.tiv.2024.105803.
- Whirledge S, Cidlowski JA. Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab. 2017;28(6):399–415.
- Bódis J, Papp S, Vermes I, et al. “Platelet-associated regulatory system (PARS)” with particular reference to female reproduction. J Ovarian Res. 2014;7(1):55. doi:10.1186/1757-2215-7-55.
- Liu B, Liu Y, Li S, et al. BDNF promotes mouse follicular development and reverses ovarian aging by promoting cell proliferation. J Ovarian Res. 2023;16(1):83. doi:10.1186/s13048-023-01163-9.
- Chen CH, et al. The role of the PI3K/Akt/mTOR pathway in glial scar formation following spinal cord injury. Exp Neurol. 2016;278:27–41.
- Zheng X, Chen L, Chen T, et al. The mechanisms of BDNF promoting the proliferation of porcine follicular granulosa cells: role of miR-127 and involvement of the MAPK-ERK1/2 pathway. Animals. 2023;13(6):115. doi:10.3390/ani13061115.
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33(1):73–83. doi:10.1038/sj.npp.1301571.
- Zheng W, et al. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012;356(1-2):24–30.
- Yan Z, Dai Y, Fu H, et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J Mol Endocrinol. 2018;60(3):261–271. doi:10.1530/JME-17-0214.
- Chaves RN, Alves AMCV, Lima LF, et al. Role of nerve growth factor (NGF) and its receptors in folliculogenesis. Zygote. 2013;21(2):187–197. doi:10.1017/S0967199412000111.
- Romero C, Paredes A, Dissen GA, et al. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology. 2002;143(4):1485–1494. doi:10.1210/en.143.4.1485.
- Dissen GA, Parrott JA, Skinner MK, et al. Direct effects of nerve growth factor on thecal cells from antral ovarian follicles. Endocrinology. 2000;141(12):4736–4750. doi:10.1210/en.141.12.4736.
- Salas C, Julio-Pieper M, Valladares M, et al. Nerve growth factor-dependent activation of trkA receptors in the human ovary results in synthesis of follicle-stimulating hormone receptors and estrogen secretion. J Clin Endocrinol Metab. 2006;91(6):2396–2403. doi:10.1210/jc.2005-1925.
- Kalantaridou SN, Makrigiannakis A, Zoumakis E, et al. Stress and the female reproductive system. J Reprod Immunol. 2004;62(1-2):61–68.
- Zhai Q-Y, Wang J-J, Tian Y, et al. Review of psychological stress on oocyte and early embryonic development in female mice. Reprod Biol Endocrinol. 2020;18(1):101. doi:10.1186/s12958-020-00657-1.
- Wu L-M, Hu M-H, Tong X-H, et al. Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries: relationship to oocytes developmental potential. PLoS One. 2012;7(12):e52331. doi:10.1371/journal.pone.0052331.
- Seifer DB. Brain-derived neurotrophic factor: a novel human ovarian follicular protein. J Clin Endocrinol Metab. 2002;87(2):655–659. doi:10.1210/jc.87.2.655.
- Manni L, et al. Ovarian expression of alpha (1)- and beta (2)-adrenoceptors and p75 neurotrophin receptors in rats with steroid-induced polycystic ovaries. Auton Neurosci. 2005;118(1-2):79–87.
- Saller S, Merz-Lange J, Raffael S, et al. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology. 2012;153(3):1472–1483. doi:10.1210/en.2011-1769.
- Maggi R, et al. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum Reprod Update. 2016;22(3):358–381.
- Gidron Y, Russ K, Tissarchondou H, et al. The relation between psychological factors and DNA-damage: a critical review. Biol Psychol. 2006;72(3):291–304.
- Black CN, Bot M, Révész D, et al. The association between three major physiological stress systems and oxidative DNA and lipid damage. Psychoneuroendocrinology. 2017;80:56–66. doi:10.1016/j.psyneuen.2017.03.003.
- Sasaki H, Hamatani T, Kamijo S, et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front Endocrinol. 2019;10:811. doi:10.3389/fendo.2019.00811.
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62.
- Tiwari M, Prasad S, Tripathi A, et al. Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. ROS. 2016;1(2):99–106. doi:10.20455/ros.2016.817.
- Coyle CH, et al. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radic Biol Med. 2006;40(12):2206–2213.
- Agarwal A, et al. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(8):1375–1403.
- Yang Z, Wenli Hong, K Zheng, et al. Chitosan oligosaccharides alleviate H(2)O(2)-stimulated granulosa cell damage via HIF-1α signaling pathway. Oxid Med Cell Longev. 2022:4247042.
- Agarwal A, Sajal Gupta, Rakesh K Sharma, et al. Role of Oxidative Stress in Female Reproduction. 2005;3(1):1–21.
- Chaube SK, Shrivastav TG, Prasad S, et al. Clomiphene Citrate Induces ROS-Mediated Apoptosis in Mammalian Oocytes. 2014.
- Ebbesen SM, et al. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: a prospective study. Hum Reprod. 2009;24(9):2173–2182.
- Goncharova ND, Alla VS, Tatiana NB, et al. Correlation between activity of antioxidant enzymes and circadian rhythms of corticosteroids in Macaca mulatta monkeys of different age. 2006;41(8):778–783.
- Nelson DH, Ruhmann-Wennhold A. Inhibition of leukocyte superoxide anion production by cortisol administration to normal subjects. J Clin Endocrinol Metab. 1978;46(4):702–705.
- Ghatebi M, et al. Implications from early life stress on the development of mouse ovarian follicles: focus on oxidative stress. J Obstet Gynaecol Res. 2019;45(8):1506–1514.
- ]Escoter-Torres L, Caratti G, Mechtidou A, et al. Fighting the fire: mechanisms of inflammatory gene regulation by the glucocorticoid receptor. Front Immunol. 2019;10:1859. doi:10.3389/fimmu.2019.01859.
- ] Butler KS, et al. Coordinate regulation between expression levels of telomere-binding proteins and telomere length in breast carcinomas. Cancer Med. 2012;1(2):165–175.
- Lin J, Epel E. Stress and telomere shortening: insights from cellular mechanisms. Ageing Res Rev. 2022;73:101507.
- ] Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012;82(3):249–258.
- Liang B, Wei D-L, Cheng Y-N, et al. Restraint stress impairs oocyte developmental potential in mice: role of CRH-Induced apoptosis of ovarian Cells1. Restraint Stress Impairs Oocyte Developmental Potential in Mice. 2013;89(3):64. doi:10.1095/biolreprod.113.110619.
- Li C-Y, Li Z-B, Kong Q-Q, et al. Restraint-induced corticotrophin-releasing hormone elevation triggers apoptosis of ovarian cells and impairs oocyte competence via activation of the fas/FasL system. Biol Reprod. 2018;99(4):828–837. doi:10.1093/biolre/ioy091.
- Xiang Y, Jiang L, Gou J, et al. Chronic unpredictable mild stress-induced mouse ovarian insufficiency by interrupting lipid homeostasis in the ovary. Front Cell Dev Biol. 2022;10:933674. doi:10.3389/fcell.2022.933674.
- Chen Y, et al. Copper exposure induces ovarian granulosa cell apoptosis by activating the caspase-dependent apoptosis signaling pathway and corresponding changes in microRNA patterns. Ecotoxicol Environ Saf. 2023;264:115414.
- Ratts VS, Flaws JA, Kolp R, et al. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136(8):3665–3668. doi:10.1210/en.136.8.3665.
- Knudson CM, Tung KS, Tourtellotte WG, et al. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270(5233):96–99. doi:10.1126/science.270.5233.96.
- Perez GI, Robles R, Knudson CM, et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21(2):200–203. doi:10.1038/5985.
- Keremu A, et al. Research on the establishment of chronic stress-induced premature ovarian failure the rat model and effects of Chinese medicine Muniziqi treatment. Mol Reprod Dev. 2019;86(2):175–186.
- Gau DM, Lesnock J, Krivak T, et al. Abstract LB-70: BRCA1 impacts ovarian cancer cell migration through modulating Pfn1 expression. Cancer Research. 2014;74(19_Supplement):LB-70–LB-70. doi:10.1158/1538-7445.AM2014-LB-70.
- Proteomic analysis of follicular fluid from women with and without endometriosis. New Therapeutic Targets and Biomarkers. 2013;80(6):441–450.
- Ding Z, Gau D, Deasy B, et al. Both actin and polyproline interactions of profilin-1 are required for migration, invasion and capillary morphogenesis of vascular endothelial cells. Exp Cell Res. 2009;315(17):2963–2973. doi:10.1016/j.yexcr.2009.07.004.
- Tomasello L, et al. PFN1 and integrin-β1/mTOR axis involvement in cornea differentiation of fibroblast limbal stem cells. J Cell Mol Med. 2019;23(11):7210–7221.
- Skare P, Karlsson R. Evidence for two interaction regions for phosphatidylinositol(4,5)-bisphosphate on mammalian profilin I. FEBS Lett. 2002;522(1-3):119–124. doi:10.1016/s0014-5793(02)02913-7.
- Sathish K, et al. Phosphorylation of profilin regulates its interaction with actin and poly (L-proline). Cell Signal. 2004;16(5):589–596.
- Pan X, et al. Bushen Jieyu tiaochong formula reduces apoptosis of granulosa cells via the PERK-ATF4-CHOP signaling pathway in a rat model of polycystic ovary syndrome with chronic stress. J Ethnopharmacol. 2022;292:114923.
- Neri M, et al. Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J Cell Mol Med. 2007;11(1):156–170.
- Li M, et al. Rno-miR-128-3p promotes apoptosis in rat granulosa cells (GCs) induced by norepinephrine through Wilms tumor 1 (WT1). In Vitro Cell Dev Biol Anim. 2021;57(8):775–785.
- Quezada M, et al. Smad7 is a transforming growth factor-beta-inducible mediator of apoptosis in granulosa cells. Fertil Steril. 2012;97(6):1452-9.e1-6.
- Zhang L, Gao J, Cui S. miR-21 is involved in norepinephrine-mediated rat granulosa cell apoptosis by targeting SMAD7. J Mol Endocrinol. 2017;58(4):199–210. doi:10.1530/JME-16-0248.
- Patel NJ, Chen MJ, Russo-Neustadt AA. Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. Eur J Pharmacol. 2010;633(1-3):1–9.
- Hansen KR, Hodnett GM, Knowlton N, et al. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175.
- Jiao X, Meng T, Zhai Y, et al. Ovarian reserve markers in premature ovarian insufficiency: within different clinical stages and different etiologies. Front Endocrinol. 2021;12:601752. doi:10.3389/fendo.2021.601752.
- Groome NP, Illingworth PJ, O’Brien M, et al. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81(4):1401–1405. doi:10.1210/jc.81.4.1401.
- Klein NA, et al. Age-related analysis of inhibin A, inhibin B, and activin a relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J Clin Endocrinol Metab. 2004;89(6):2977–2981.
- Webber L, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937.
- Pandey AK, et al. Impact of stress on female reproductive health disorders: possible beneficial effects of shatavari (Asparagus racemosus). Biomed Pharmacother. 2018;103:46–49.
- Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014;94(12):1816–1825. doi:10.2522/ptj.20130597.
- Schein RL, Koenig H. The Center for Epidemiological Studies-Depression (CES-D) scale: assessment of depression in the medically ill elderly. Int J Geriatric Psychiatry. 1997;12(4):436–446.
- Piersma HL, Reaume WM, Boes JL. The brief symptom inventory (BSI) as an outcome measure for adult psychiatric inpatients. J Clin Psychol. 1994;50(4):555–563.
- Haemmerli Keller K, Alder G, Loewer L, et al. Treatment-related psychological stress in different in vitro fertilization therapies with and without gonadotropin stimulation. Acta Obstet Gynecol Scand. 2018;97(3):269–276. doi:10.1111/aogs.13281.
- The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998; 28(3):551–558.
- Reilly J. Premature menopause: a multidisciplinary approach whurr. J Health Psychol. 2001;6(5):606–608. doi:10.1177/135910530100600512.
- Dragojevic-Dikic S, et al. An immunological insight into premature ovarian failure (POF). Autoimmun Rev. 2010;9(11):771–774.
- Warne E, Oxlad M, Best T. Evaluating group psychological interventions for mental health in women with infertility undertaking fertility treatment: a systematic review and meta-Analysis. Health Psychol Rev. 2023;17(3):377–401.
- Lupien SJ, Fiocco A, Wan N, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi:10.1016/j.psyneuen.2004.08.003.
- Santoft F, et al. Cognitive behaviour therapy for depression in primary care: systematic review and meta-analysis. Psychol Med. 2019;49(8):1266–1274.
- Linde K, et al. Effectiveness of psychological treatments for depressive disorders in primary care: systematic review and meta-analysis. Ann Fam Med. 2015;13(1):56–68.
- Cuijpers P, et al. The effects of fifteen evidence-supported therapies for adult depression: a meta-analytic review. Psychother Res. 2020;30(3):279–293.
- Richards DA, et al. Cost and outcome of behavioural activation versus cognitive behavioural therapy for depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet. 2016;388(10047):871–880.
- Cuijpers P, Quero S, Dowrick C, et al. Psychological treatment of depression in primary care: recent developments. Curr Psychiatry Rep. 2019;21(12):129. doi:10.1007/s11920-019-1117-x.
- Bahari K, Lorica JD. The effects of laughter therapy on mental health: an integrative literature review. MJN. 2019;10(03):55–61. doi:10.31674/mjn.2019.v10i03.008.
- Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45(1):1–6.
- Boudoures AL, Chi M, Thompson A, et al. The effects of voluntary exercise on oocyte quality in a diet-induced obese murine model. Reproduction. 2016;151(3):261–270. doi:10.1530/REP-15-0419.
- Safdar A, et al. Amelioration of premature aging in mtDNA mutator mouse by exercise: the interplay of oxidative stress, PGC-1α, p53, and DNA damage. A hypothesis. Curr Opin Genet Dev. 2016;38:127–132.
- Safdar A, et al. Exercise-induced mitochondrial p53 repairs mtDNA mutations in mutator mice. Skelet Muscle. 2016;6:7.
- Han B, Du G, Yang Y, et al. Relationships between physical activity, body image, BMI, depression and anxiety in Chinese college students during the COVID-19 pandemic. BMC Public Health. 2023;23(1):24. doi:10.1186/s12889-022-14917-9.
- Aguiar AS, Stragier E, da Luz Scheffer DJr., et al. Effects of exercise on mitochondrial function, neuroplasticity and anxio-depressive behavior of mice. Neuroscience. 2014;271:56–63., doi:10.1016/j.neuroscience.2014.04.027.
- Bansal Y, Kuhad A. Mitochondrial dysfunction in depression. Curr Neuropharmacol. 2016;14(6):610–618.
- Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016;13:43.
- Sun L, Sun Q, Qi J. Adult hippocampal neurogenesis: an important target associated with antidepressant effects of exercise. Rev Neurosci. 2017;28(7):693–703. doi:10.1515/revneuro-2016-0076.
- Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. J Affect Disord. 2013;148(1):12–27.
- LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003. doi:10.1038/ncb3039.
- Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105(5):755–764. doi:10.1017/S0007114510004319.
- Messaoudi M, Violle N, Bisson J-F, et al. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2(4):256–261. doi:10.4161/gmic.2.4.16108.
- Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi:10.1038/nrn2297.
- Gleeson M, Bishop NC, Stensel DJ, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi:10.1038/nri3041.