Abstract
Objective
Polycystic ovarian syndrome (PCOS) is a common but complex endocrine disorder widely linked to infertility and miscarriage. This study assessed the correlation between PCOS and infertility.
Methods
Using the latest data from the Global Burden of Disease 2019 database, we conducted an in-depth assessment of the disease burden attributed to PCOS in China. This analysis was performed using the joinpoint regression, age-period-cohort, and autoregressive integrated moving average (ARIMA) models.
Results
Between 1990–2019, an upward trend was observed in the age-standardized prevalence of PCOS-related female infertility in China. Joinpoint regression analysis revealed an increasing trend in the age-standardized prevalence of PCOS-related female infertility burden indicators as well as the average annual percentage change and annual percentage change across all age groups in China. In terms of the cohort effect, the period rate ratios associated with the age-standardized prevalence of PCOS-related infertility increased steadily over time. The ARIMA model predicted a relatively swift upward trend in the age-standardized prevalence of PCOS-related infertility in China from 2020–2030.
Conclusion
The age-standardized prevalence of PCOS-related female infertility in China has increased between 1990–2019. The ARIMA model predicted that the age-standardized prevalence of this disease may continue to increase over the next decade. This study can increase the public’s attention, improve women’s health awareness, and have a certain significance for reducing female infertility related to PCOS.
Introduction
Over the past three decades, the age at first marriage of Chinese women has increased, coinciding with a sharp decline in the average fertility rate [Citation1,Citation2]. According to the National Bureau of Statistics, the natural population growth rate in 2021 was 0.34%, a decrease from both 2020 (1.45%) and 2019 (3.34%) [Citation1]. Notably, female infertility has been identified as a contributing factor affecting various aspects of relationships, including marital quality, sexual relationships, mental health, and overall quality of life [Citation3,Citation4]. In this context, infertility has become a significant public health and social concern in China [Citation5–7].
Polycystic ovarian syndrome (PCOS) is a prevalent and complex endocrine disorder that affects 6%–10% of women of reproductive age worldwide [Citation8], it is main clinical symptoms are irregular menstruation, excessive hair growth, weight gain and other health problems [Citation9]. Studies have shown that PCOS is associated with numerous comorbidities, including endometrial cancer, obesity, type 2 diabetes, depression, anxiety disorders, nonalcoholic fatty liver disease, pregnancy complications, sleep apnea, and eating disorders [Citation10].
PCOS is widely acknowledged as being associated with female infertility and miscarriages [Citation11]. Female infertility comprises two main categories: primary infertility, where a woman has not become pregnant despite not using contraception with the same partner for ≥ 12 months; and secondary infertility, where a woman has not become pregnant for ≥ 12 months since her last pregnancy despite not using contraception with the same sexual partner [Citation12,Citation13]. In addition to its association with reproductive health, female infertility has significant psychological, economic, and medical implications, including trauma and stress, particularly in societies and cultures that emphasize fertility [Citation14]. Therefore, extensive research that extends beyond biological aspects and considers the broader consequences for individual well-being and societal dynamics is imperative.
China, the most populous country worldwide, is experiencing a growing burden of polycystic-related infertility [Citation15,Citation16]. In this study, we used GBD 2019 data from 1990–2019 to examine the age-standardized prevalence rates (ASPR) of the burden of polycystic-related infertility in China from the perspectives of primary and secondary infertility. Furthermore, the joinpoint regression analysis, age-period-cohort, and autoregressive integrated moving average (ARIMA) models were used to evaluate disease burden. This study aimed to improve the assessment of the current burden of PCOS-associated infertility, prompting increased awareness of women’s health and advocating for early diagnosis and timely treatment of PCOS. And our study is the first to describe the infertility burden in Chinese women with polycystic disease from these perspectives, which is innovative.
Methods
Data sources
The Global Burden of Disease (GBD) system is a comprehensive and analytical global database arising from long-term collaboration between numerous governments. GBD 2019, a product of this collaboration, meticulously evaluated and updated disease burdens and contributing factors in 204 countries and territories. GBD 2019 has been used to evaluate the burden of PCOS-related infertility, assessing the age-specific prevalence, age-standardized prevalence, and years of living with disability from various perspectives, including a sociodemographic index, on a global, regional, and national scale, and their corresponding trends [Citation14].
The GBD 2019 database (VizHub–GBD Results, healthdata.org) consolidates worldwide population health and demographic data. This comprehensive dataset incorporates information from various sources such as surveys, census data, registries, indicators and estimates, health-related financial data, and administrative health data. The methods for data selection and entry have previously been described [Citation17]. GBD employs the Bayesian meta-regression tool disMOd-MR 2.1 to model the burden of non-fatal diseases [Citation18]. We focused on PCOS in women aged 20–49 years and examined infertility as an impairment, yielding data on primary and secondary infertility.
This study used publicly available data and adhered to the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) for cross-sectional studies [Citation19]. As the data were sourced from a publicly available database, ethics approval was waived.
Descriptive analysis
A descriptive analysis of time and age trends in the burden of PCOS-associated infertility in China was conducted and compared with global levels. This analysis was performed using R version 4.3.1 (R Foundation, Vienna, Austria).
Joinpoint regression analysis
Connection-point regression models are sets of linear statistical models and were used to assess trends in the disease burden of PCOS-associated infertility over time. Calculations involved estimating the change law of the disease rate using the least-squares method, thereby mitigating the subjectivity inherent in typical linear trend analysis. The inflection point of the moving trend was determined by summing the residual squares between the actual and estimated values using the grid search method. The significance of the observed changes was assessed using the Monte Carlo permutation method. The average annual percentage change (AAPC) was calculated to estimate the overall trend in the burden of PCOS-related infertility from 1990–2019. AAPC > 0 indicated an upward trend, whereas AAPC < 0 indicated a downward trend. Joinpoint (version 4.9.1.0; National Cancer Institute, Rockville, MD, USA) was used to establish this model, and p < 0.05 was considered statistically significant.
Age-period-cohort model
A web-based age-period-cohort analysis tool that operates in the R program was used to analyze the influence of age, period, and cohort on the prevalence of polycystic infertility. The probabilistic predictions acquired by the Bayesian age-period-cohort model were well-calibrated and not too wide, implying that the model provided relatively precise predictions [Citation20]. Age was assessed by examining the influence of age-related factors; period denoted alterations in the load of anthropogenic factors during a specific period; and cohort linked differences to distinct exposure conditions of the population during the various birth periods. To address the issue of multicollinearity between age, period, and cohort, an intrinsic estimator based on Poisson distribution was employed to derive the disease parameters in the model. To prevent information overlap in adjacent queues, the duration, time range, and time interval of the queues must be consistent. Thus, the intervals for age, period, and birth cohorts were all set to five years. The net drift signified the overall time trend, and a p < 0.05 was considered statistically significant. A longitudinal age curve was used to evaluate alterations in disease burden attributable to age effects. Period rate ratios (RR) and cohort RR revealed period and birth cohort effects, respectively, with RR > 1 signifying a heightened relative risk of disease compared with the reference cohort and RR < 1 signifying a diminished risk.
ARIMA model
The Forecast function in R was used to construct an ARIMA model to predict trends in the prevalence of the disease burden of PCOS-associated infertility in China from 2020–2030. The optimal prediction model was selected based on the Akaike information criteria (AIC) and Bayesian information criteria (BIC). The Ljung-Box test was performed on the residual sequence of the model to determine goodness-of-fit; p > 0.05 indicated that the ARIMA model was a good fit.
Results
The burden of PCOS-associated infertility in China between 1990–2019
The number of patients with PCOS-related infertility in China increased between 1990–2019. In patients with PCOS-associated primary infertility, ASPR increased from 1591.19% in 1990 (95% UI: 578.4–3422.04%) to 3715.51% in 2019 (95%UI: 1433.47–7704.82%) (Supplementary Table 1). In 2019, 20–24-year-olds had the highest prevalence of primary infertility at 13,858.65% (95%UI: 2750.13–37324.92%), whereas 30–34-year-olds experienced the greatest change, with an increase of 197.23% between 1990–2019 (95%UI: 159.83–260.68%) (Supplementary Table 2; Supplementary Figure 1).
In patients with PCOS-associated secondary infertility, ASPR increased from 13,835.29% (95%UI: 8034.5–21,295.11%) in 1990 to 25,954.7% in 2019 (95%UI: 15326.49–40084.46%) (Supplementary Table 3). In 2019, 30–34-year-olds exhibited the highest prevalence of secondary infertility at 61709.52% (95%UI: 35948.73–95737.97%), whereas the greatest change within an age group was 95.67% in the 20–24-year-olds (95%UI: 74.36–115.7%) (Supplementary Table 4; Supplementary Figure 2).
Joinpoint regression analysis
Joinpoint regression analysis determined that the age-standardized rate of PCOS-related infertility burden indicators, AAPC, and annual percentage change (APC) of all age groups in China showed an increasing trend (Supplementary Tables 1 and 5; , Supplementary Figure 3A). For primary infertility, 20–24-year-olds consistently showed a higher APC over the past 30 years, with a relatively stable APC of 0.06 (95%CI: −0.04–0.17) between 2004–2011 and 2014–2017 and a temporary decline of −1.70 (95%CI: −2.30–-1.09). In contrast, 45–49-year-olds consistently showed a lower APC over the past 30 years (Supplementary Table 5; Supplementary Figure 3A). Overall, the APC between 1990–2019 showed an increasing trend for PCOS-related primary infertility in China. Between 1990–1993, ASPR experienced its most rapid increase, with an APC of 8.40 (95%CI: 8.06–8.74). The APC was 5.46 (95%CI: 4.81–6.11) between 2017–2019 when the prevalence was highest in 20–24-year-olds and 9.37 (95%CI: 8.78–9.97) between 1990–1993 when the prevalence was highest in 25–29-year-olds. The APC was 15.17 (95%CI: 13.18–17.19) between 1990–1992 when the prevalence was highest in 30–34-year-olds and 12.32 (95%CI: 11.27–13.37) between 1990–1992 when the prevalence was highest in 35–39-year-olds. Furthermore, the APC was 6.39 (95%CI: 6.30–6.49) between 1990–2000 when the highest prevalence occurred in 40–44-year-olds and 7.25 (95%CI: 6.87–7.64) between 1990–1999 when the prevalence was highest in 45–49-year-olds (Supplementary Table 5; Supplementary Figure 3A).
Figure 1. Joinpoint regression analysis for age-standardized prevalence rate (ASPR) of PCOS-related infertility in China between 1990–2019. (A) Primary infertility. (B) Secondary infertility.
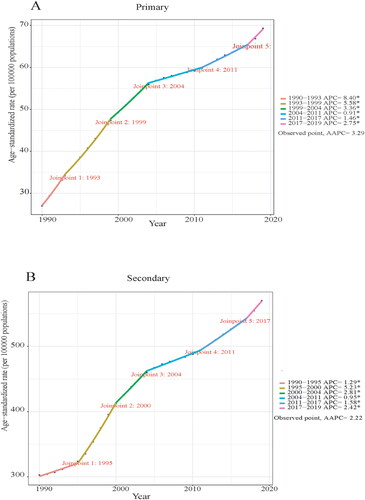
For secondary infertility, the APC in all age groups showed an upward trend between 1990–2019. Notably, the age groups 25–29, 30–34, 35–39, and 40–44 years exhibited a higher burden of secondary infertility and a similar upward trend in APC, whereas the 20–24- and 45–49-year-olds exhibited relatively lighter burdens (Supplementary Table 5; Supplementary Figure 3B). The APC of PCOS-related secondary infertility between 2017–2019 showed an upward trend within the overall timeframe of 1990–2019. The APC with the highest ASPR was 5.23 (95%CI: 4.94–5.51) between 1995–2000. Between 1995–2000, the APC was 6.45 (95%CI: 6.02–6.89) and 5.47 (95%CI: 5.25–5.68) in 20–24- and 25–29-year-olds, respectively. Furthermore, the APC was 5.48 (95%CI: 5.20–5.76) between 1996–2000 when the prevalence rate was the highest in 30–34-year-olds and 5.09 (95%CI: 4.50–5.69) between 1996–1999 when the prevalence was highest in 35–39-year-olds. The APC was 5.37 (95%CI: 5.12–5.61) between 1995–2000 when the prevalence was highest in 40–44-year-olds and 4.67 (95%CI: 3.46–5.89) between 2017–2019 when the prevalence rate was the highest in 45–49-year-olds (Supplementary Table 5; Supplementary Figure 3B).
Age-period-cohort analysis
suggests that the prevalence of PCOS-related primary infertility in China is increasing. After accounting for both cycle and cohort effects, the prevalence of primary infertility generally decreased with age. The results indicated a slight increase in the prevalence in 30–39-year-olds in the age group of 20–49 years. Furthermore, a decline in prevalence was observed among those aged < 30 and ≥ 40 years. From the perspective of cycle effects, the prevalence of primary infertility increased between 1990–2019. Regarding cohort effects, the ASPR consistently increased each year ().
Figure 2. Age-period-cohort analysis for the prevalence of PCOS-related infertility in China. (A) Primary infertility. (B) Secondary infertility.
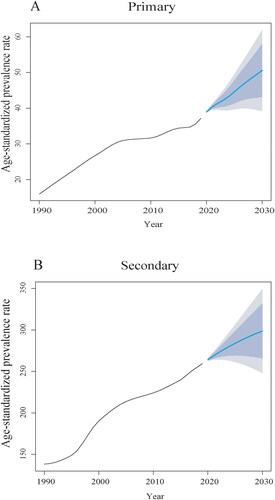
shows an increasing trend in PCOS-related secondary infertility in China. When controlling for both cycle and cohort effects, the prevalence was higher in women aged < 40 years and increased with age, eventually decreasing after age 40. From the perspective of cycle effects, the prevalence of secondary infertility increased between 1990–2019. In terms of cohort effects, the ASPR increased each year ().
Predicting the prevalence of PCOS-associated infertility in China between 2020–2030
By analyzing the time series data of the prevalence of PCOS-associated primary infertility in China, the auto.arima() function automatically selected the model with the optimal AIC and BIC as ARIMA [Citation1,Citation2] (AIC = −33.49, BIC = −30.16), showing that the ARIMA [Citation1,Citation2] model was reliable for analyzing this time series. The ARIMA [Citation1,Citation2] model passed the white noise test, with the Ljung-Box test showing Q = 0.16 and p = 0.69. The predicted outcomes suggest an increase in prevalence of primary infertility in China between 2020–2030. The rates were predicted to rise from 38.94 (per 100,000) in 2020 (95%CI: 38.73–39.15) to 50.60 (per 100,000) in 2030 (95%CI: 39.20–61.99). The corresponding ASPR was predicted to show a rapid upward trend ().
Figure 3. Burden of PCOS-related infertility in China between 1990–2030. (A) Primary infertility. (B) Secondary infertility.
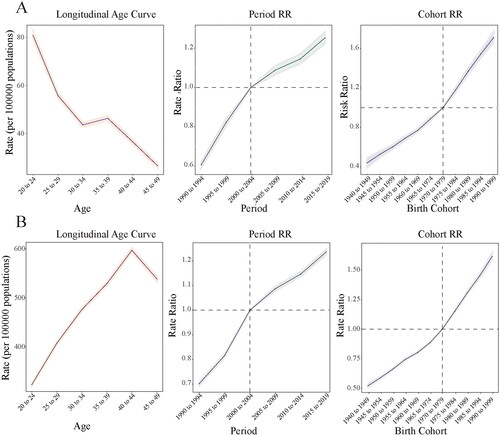
For PCOS-associated secondary infertility, ARIMA [Citation1] was automatically selected as the model with the best AIC and BIC (AIC = 77.98, BIC = 82.08), showing that the ARIMA [Citation1,Citation2] model was reliable for analyzing this time series. The ARIMA [Citation1,Citation2] model passed the white noise test, and the Ljung-Box test showed Q = 0.68 and p = 0.41. The model predicted an increase in the prevalence of secondary infertility between 2020–2030. The rates were predicted to increase from 264.21 (per 100,000) in 2020 (95%CI: 262.64–265.77) to 298.70 (per 100,000) in 2030 (95%CI: 248.01–349.39), with a relatively rapid upward trend ().
Discussion
This study summarized the latest age-standardized prevalence of PCOS-related female infertility in China; elucidated the impact of age, period, and cohort; and predicted the age-standardized prevalence of the disease over the next ten years. The research findings show the prevalence of PCOS-related infertility in China is increasing. Joinpoint regression analysis determined that the age-standardized rate of PCOS-related infertility burden indicators, AAPC, APC of all age groups in China showed an increasing trend. In addition, the ARIMA model predicts that the prevalence will continue to rise over the next 10 years.
PCOS is a leading contributor to infertility in women, affecting up to 20% of women of childbearing age [Citation21]. The pathogenesis of PCOS is complex and multifactorial, encompassing genetic, environmental, and intergenerational factors. These factors contribute to hypothalamic-pituitary-ovarian axis disruption, promoting ovarian and adrenal hyperandrogenemia. Ovulation dysfunction/anovulation and endometrial receptivity are crucial factors in PCOS-related infertility [Citation22]. Interestingly, animal and human studies have revealed that the syndrome has a cross-generational origin, where daughters born to mothers with PCOS have a five-fold greater risk of inheriting the syndrome [Citation23,Citation24]. Using Mendelian randomization analyses, Samvida et al. concluded that women with obesity are more likely to have infertility and that obesity is a comorbidity of polycystic disease [Citation25]. Insulin resistance and hyperinsulinemia have been observed in 50–70% of patients with PCOS [Citation26], and IR impairs endometrial function and affects embryo implantation, resulting in decreased pregnancy rates [Citation27]. Studies have also found reduced transplant success and live birth rates in women with PCOS than in those without the condition [Citation28].
Obesity and overnutrition may be one of the reasons for the increase in the prevalence of PCOS in China. While industrialization and economic development have improved the quality of life, they have also contributed to the obesity epidemic [Citation29,Citation30]. Obesity is a bad metabolic state, and the increase of body weight may lead to insulin resistance. Insulin resistance is an important pathological link in the development of PCOS [Citation31]. The increased stress in modern women’s lives may also lead to more frequent states of anxiety and depression, and these adverse mental states are associated with sleep disorders, which are thought to be one of the first symptoms that lead to a weakening of the body’s protective properties and an enhancement of pathways associated with insulin resistance during PCOS [Citation32]. Therefore, it is also very important to appropriately reduce the pressure of women’s life and improve the quality of sleep in the process of treating polycystic disease.
The prevalence of PCOS-related primary infertility in China was shown to decrease with increasing age, whereas the prevalence of PCOS-related secondary infertility was higher in women aged <40, with a downward trend in women ≥ 40 years. A nationwide epidemiological survey in China revealed that, between 2010–2020, the prevalence of PCOS in 20–44-year-olds increased from 5.6% to 8.6%. This trend was observed across all age groups, suggesting that the number of women with PCOS in China is increasing. Furthermore, the overall phenotype was more severe and the prevalence of obesity, hyperandrogenemia, and infertility was higher in 2020 than in 2010 [Citation33]. The overall population of China is currently aging significantly, and the relaxation of the two/three-child policy has allowed women more flexibility in family planning, leading to an extension of their reproductive years. This may contribute to an increase in the average age of women seeking to start or expand their families, which could increase the prevalence of conditions such as secondary infertility associated with polycystic diseases [Citation34]. Both PCOS-related primary and secondary infertility have increased over the past 30 years. Moreover, we predicted that the prevalence of PCOS-related infertility will continue to increase rapidly over the next 10 years. This is likely related to China’s economic development, which has witnessed significant social progress, driving individuals to pursue education, careers, and higher incomes. Work-related stress and occupational exposure can also affect endocrine function in women, thereby threatening fertility [Citation35].
There is a considerable discrepancy between the current infertility rates in China and the lack of compensatory technologies [Citation36]. Assisted reproduction is known to be effective [Citation37]; however, in China, these technologies are relatively underdeveloped, and a late start is associated with a lower success rate. Furthermore, an imbalance in economic development between Eastern and Western China has led to an uneven distribution of health resources and services [Citation38,Citation39]. China’s heavy reliance on the import of assisted reproductive devices and drugs, coupled with limited insurance coverage, presents significant challenges in addressing infertility [Citation36]. To protect the reproductive rights of citizens, the medical insurance department of China has announced the inclusion of certain eligible fertility support drugs, with the aim of enhancing medical insurance and medication services for patients with infertility. Measures will be developed to provide additional support without affecting the stability of current medical insurance funds [Citation36]. In addition, under the guidance of the State Medical Insurance Bureau, Beijing, Guangxi, Inner Mongolia and Gansu provinces have included assisted reproduction in medical insurance reimbursement. The National Medical Insurance Bureau previously issued the Guide for the Establishment of Assisted Reproductive Medical Service Price Projects (Trial), which classified and integrated assisted reproductive projects into 12 items. We will further standardize the prices of medical services for assisted reproduction [Citation40]. With increasing public attention and awareness of women’s health, along with improved medical technology, protection of the reproductive rights of patients with infertility is anticipated to develop rapidly in China [Citation36].
However, our study was limited in certain aspects. First, determining the changing trends of PCOS-related infertility in various provinces in China was not possible due to a lack of relevant data. Second, information on PCOS phenotypes was lacking, and determining the relationship between different phenotypes and infertility was not possible. In future studies, we can focus on these limitations and refine them. We can further explore its relationship with the prevalence of female infertility from the perspective of different polycystic phenotypes, and also compare the prevalence of female infertility related to polycystic in China with that in the world. Despite these limitations, this study is of national significance and is the first to use the Joinpoint regression, age-period cohort model, and ARIMA model to explore trends in the prevalence of PCOS-related female infertility rates in China.
Conclusion
In conclusion, the age-standardized prevalence of PCOS-related female infertility in China increased between 1990–2019, and the ARIMA model predicted that the age-standardized prevalence of the disease would continue to increase over the next decade.
Authors’ contributions
D.Y. Shen wrote the original manuscript and conceived the study; Y. Wang performed the data analysis; D.Y. Shen and P.W. Hu revised the final manuscript; C. Qi and H. Yang supervised the study process.
Declarations
The data were sourced from a publicly available database and did not require ethical review.
Disclosure of interest
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
| Abbreviations | ||
| UI | = | uncertainty interval |
| CI | = | confidence interval |
| GBD | = | global burden of disease |
| APC | = | annual percentage change |
| AAPC | = | average annual percent change |
| ASPR | = | age-standardized prevalence rates |
Supplemental Material
Download Zip (795.2 KB)Acknowledgments
We appreciate the work of the 2019 Global Burden of Disease Study collaborators. We thank Bullet Edits Limited for linguistic editing and proofreading of the manuscript.
Data availability statement
The data underlying this article are available in the Global Health Data Exchange at http://ghdx.healthdata.org/gbd-results-tool.
Additional information
Funding
References
- Luo D, Yan X, Xu R, et al. Chinese trends in adolescent marriage and fertility between 1990 and 2015: a systematic synthesis of national and subnational population data. Lancet Glob Health. 2020;8(7):e954–e64. doi:10.1016/S2214-109X(20)30130-3.
- Zhu C, Yan L, He C, et al. Incidence and risk factors of infertility among couples who desire a first and second child in Shanghai, China: a facility-based prospective cohort study. Reprod Health. 2022;19(1):155. doi:10.1186/s12978-022-01459-x.
- Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76. doi:10.1001/jama.2021.4788.
- Bala R, Singh V, Rajender SA-O, et al. Environment, lifestyle, and female infertility. Reprod Sci. 2021;28(3):617–638. doi:10.1007/s43032-020-00279-3.
- Shi Z, Nie H, Geng L, et al. Evaluating health-related quality of life and subjective wellbeing among infertility patients: a cross-sectional study in mainland China. Qual Life Res. 2023;32(5):1469–1480. doi:10.1007/s11136-022-03330-9.
- Wang L, Tang Y, Wang Y. Predictors and incidence of depression and anxiety in women undergoing infertility treatment: a cross-sectional study. PLoS One. 2023;18(4):e0284414. doi:10.1371/journal.pone.0284414.
- Lau JT, Wang Q, Fau- Cheng Y, et al. Infertility-related perceptions and responses and their associations with quality of life among rural chinese infertile couples. J Sex Marital Ther. 2008;34(3):248–267. doi:10.1080/00926230701866117.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855. doi:10.1093/humrep/dew218.
- Joshi A. PCOS stratification for precision diagnostics and treatment. Front Cell Dev Biol. 2024;12:1358755. doi:10.1530/JOE-23-0342.
- Zhu T, Goodarzi MO. Causes and consequences of polycystic ovary syndrome: insights from mendelian randomization. J Clin Endocrinol Metab. 2022;107(3):e899–e911. doi:10.1210/clinem/dgab757.
- Woolner AM, Bhattacharya S. Intergenerational trends in reproduction: infertility and pregnancy loss. Best Pract Res Clin Obstet Gynaecol. 2023;86:102305. doi:10.1016/j.bpobgyn.2022.102305.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406. doi:10.1016/j.fertnstert.2017.06.005.
- Garolla A, Pizzol D, Carosso AR, et al. Practical clinical and diagnostic pathway for the investigation of the infertile couple. Front Endocrinol (Lausanne). 2020;11:591837. doi:10.3389/fendo.2020.591837.
- De Geyter C, Calhaz-Jorge C, Kupka MS, et al. ART in Europe, 2014: results generated from European registries by ESHRE: the European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod. 2018;33(9):1586–1601. doi:10.1093/humrep/dey242.
- Yu J, Fu Y, Zeng L, et al. Burden of female infertility in China from 1990 to 2019: a temporal trend analysis and forecasting, and comparison with the global level. Sex Health. 2023;20(6):577–584. doi:10.1071/SH23029.
- Safiri S, Noori M, Nejadghaderi SA, et al. Prevalence, incidence and years lived with disability due to polycystic ovary syndrome in 204 countries and territories, 1990-2019. Hum Reprod. 2022;37(8):1919–1931. doi:10.1093/humrep/deac091.
- Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9.
- Vosoughi K, Stovner LJ, Steiner TJ, et al. The burden of headache disorders in the Eastern Mediterranean Region, 1990-2016: findings from the Global Burden of Disease study 2016. J Headache Pain. 2019;20(1):40. doi:10.1186/s10194-019-0990-3.
- Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. PLoS Med. 2016;13(6):e1002056. doi:10.1371/journal.pmed.1002056.
- Lee TC, Dean CB, Semenciw R. Short-term cancer mortality projections: a comparative study of prediction methods. Stat Med. 2011;30(29):3387–3402. doi:10.1002/sim.4373.
- Notaro ALG, Neto FA-OX. The use of metformin in women with polycystic ovary syndrome: an updated review. J Assist Reprod Genet. 2022;39(3):573–579. doi: 10.1007/s10815-022-02429-9.
- Cohen AM, Ye XY, Colgan TJ, et al. Comparing endometrial receptivity array to histologic dating of the endometrium in women with a history of implantation failure. Syst Biol Reprod Med. 2020;66(6):347–354. doi:10.1080/19396368.2020.1824032.
- Stener-Victorin E, Padmanabhan V, Walters KA, et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):bnaa010. doi:10.1210/endrev/bnaa010.
- Risal S, Pei Y, Lu H, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894–1904. doi:10.1038/s41591-019-0666-1.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: A Mendelian randomisation study. PLoS Med. 2022;19(2):e1003679. doi:10.1371/journal.pmed.1003679.
- Shorakae S, Ranasinha S, Abell S, et al. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin Endocrinol (Oxf). 2018;89(5):628–633. doi:10.1111/cen.13808.
- Qi J, Wang W, Zhu Q, et al. Local cortisol elevation contributes to endometrial insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103(7):2457–2467. doi:10.1210/jc.2017-02459.
- Steiner N, Ates S, Shaulov T, et al. A comparison of IVF outcomes transferring a single ideal blastocyst in women with polycystic ovary syndrome and normal ovulatory controls. Arch Gynecol Obstet. 2020;302(6):1479–1486. doi:10.1007/s00404-020-05699-9.
- Wang Y, Xue H, Sun M, et al. Prevention and control of obesity in China. Lancet Glob Health. 2019;7(9):e1166-e7. doi:10.1016/S2214-109X(19)30276-1.
- Mathis BA-O, Tanaka K, Hiramatsu Y. Factors of obesity and metabolically healthy obesity in Asia. Medicina (Kaunas). 2022;58(9):1271. doi:10.3390/medicina58091271.
- Szczuko M, Kikut J, Szczuko U, et al. Nutrition strategy and life style in polycystic ovary syndrome-narrative review. Nutrients. 2021;13(7):2452. doi:10.3390/nu13072452.
- Yang Y, Deng H, Li T, et al. The mental health of Chinese women with polycystic ovary syndrome is related to sleep disorders, not disease status. J Affect Disord. 2021;282:51–57. doi:10.1016/j.jad.2020.12.084.
- Yang R, Li Q, Zhou Z, et al. Changes in the prevalence of polycystic ovary syndrome in China over the past decade. Lancet Reg Health West Pac. 2022;25:100494. doi:10.1016/j.lanwpc.2022.100494.
- Group ECW, ESHRE Capri Workshop Group. A prognosis-based approach to infertility: understanding the role of time. Hum Reprod. 2017;32(8):1556–1559. doi:10.1093/humrep/dex214.
- Smiechowicz J, Skoczynska A, Nieckula-Szwarc A, et al. Occupational mercury vapour poisoning with a respiratory failure, pneumomediastinum and severe quadriparesis. SAGE Open Med Case Rep. 2017;5:2050313X17695472. doi:10.1177/2050313X17695472.
- Wang L, Zhu Y, Wang T, et al. Feasibility analysis of incorporating infertility into medical insurance in China. Front Endocrinol. 2022;13:967739. doi:10.3389/fendo.2022.967739.
- Farquhar CM, Bhattacharya S, Repping S, et al. Female subfertility. Nat Rev Dis Primers. 2019;5(1):7. doi:10.1038/s41572-018-0058-8.
- Liu Q, Guo Y. Regional differences of individual and allocation efficiencies of health resources in China. Front Public Health. 2023;11:1306148. doi:10.3389/fpubh.2023.1306148.
- Bai F, Wang DY, Fan YJ, et al. Assisted reproductive technology service availability, efficacy and safety in mainland China: 2016. Hum Reprod. 2020;35(2):446–452. doi:10.1093/humrep/dez245.
- Jingtong Q, Liang L. National Medical Insurance Bureau: four provinces have included assisted reproduction in medical insurance reimbursement: CCTV network; 2024. Available from: https://news.cctv.com/2024/03/30/ARTIaH3Lj01Em9w1Zgzm60e9240330.shtml.
