Abstract
Objective
To determine whether ultrasonic manifestations of Hashimoto’s thyroiditis (HT) related to embryo qualities or pregnancy outcomes in women with thyroid autoimmunity (TAI) undergoing in vitro fertilization/intracytoplasmic sperm injection.
Methods
Our study was a retrospective cohort study. A total of 589 euthyroid women enrolled from January 2017 to December 2019. 214 TAI women and 375 control women were allocated in each group according to serum levels of thyroid peroxidase antibodies (TPOAb) and/or anti-thyroglobulin antibodies (TgAb). Basal serum hormone levels and thyroid ultrasound were assessed, embryo qualities, pregnancy outcomes were collected from medical records. Diagnosis of thyroid ultrasound was used for subanalysis. Logistic regression was used to evaluate outcomes of embryo development and pregnancy.
Results
Implantation rate was significantly lower in euthyroid women with TAI compared with control group (TAI group: 65.5% vs. Control group: 73.0%, adjusted OR (95% CI): 0.65 (0.44, 0.97), p = 0.04). We further stratified TAI group into two groups: one group with HT features under ultrasound and another group with normal thyroid ultrasound. After regression analysis, TAI women with HT morphological changes had a lower chance of implantation compared with control group (TAI group with HT: 64.1% vs. Control group: 73.0%, adjusted OR (95% CI): 0.63 (0.41, 0.99), p = 0.04), while there was no significant difference on implantation rate between TAI women with normal thyroid ultrasound and control group. Other outcomes, such as embryo qualities and pregnancy rate, were comparable between TAI and control groups.
Conclusions
A higher risk of implantation failure was seen among euthyroid women with TAI, especially women with HT morphological changes under ultrasound. The underlying mechanisms of implantation failure among euthyroid HT patients need further research.
Introduction
Thyroid autoimmunity (TAI) is an autoimmune endocrine disease commonly seen in women at reproductive age, especially in women with infertility [Citation1]. It is characterized by elevated serum levels of thyroid peroxidase antibodies (TPOAb) and/or anti-thyroglobulin antibodies (TgAb). Previous studies have controversies on the association between TAI and pregnancy outcomes after in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) [Citation1–3]. Venables et al. [Citation1] and Huang et al. [Citation2] considered TAI had no effects on pregnancy outcomes of euthyroid women, such as clinical pregnancy rate, clinical miscarriage rate, biochemical pregnancy loss, and live birth rate. Busnelli et al. [Citation3] found that the presence of TAI was associated with a higher risk of miscarriage, lower chance of embryo implantation, and live birth. However, all the previous studies described TAI characteristics with only serum hormone measurements but failed to provide thyroid ultrasound results [Citation1–3]. So as for women with serological TAI, whether those accompanied with thyroid ultrasonic changes have adverse effects on embryo qualities or pregnancy outcomes remains uncertain.
Hashimoto’s thyroiditis (HT) is the commonest disease of TAI. It is mainly characterized by the presence of serum antibodies against thyroid antigens, thyroid enlargement, or infiltrated lymphocytes under microscope [Citation4,Citation5]. Ultrasonography was proved to be valuable and was currently applied to help diagnose HT as thyroid uptake of radioactive iodine and cytological examination of thyroid aspirate were used more rarely [Citation5]. Acar et al. suggested structural and hemodynamic changes started long before serological changes and clinical symptoms [Citation6]. Pedersen et al. also suggested that biochemical dysfunction was rarely associated normal thyroid echogenicity in TAI patients [Citation7]. However, there is lack of knowledge on the role of thyroid ultrasonography in the process of assisted reproduction. Therefore, the aim of this study was to evaluate whether ultrasonic manifestations of HT related to embryo qualities or pregnancy outcomes in euthyroid TAI women after IVF or ICSI.
Materials and methods
This was a retrospective cohort study. Euthyroid women who intended to conduct their first IVF/ICSI cycle during January 2017 to December 2019 in Hospital for Reproductive Medicine affiliated to Shandong University was recruited. The process was approved by Ethics Committee of Center for Reproductive Medicine of Shandong University (Ethical Review No. 69, 2022).
Patients
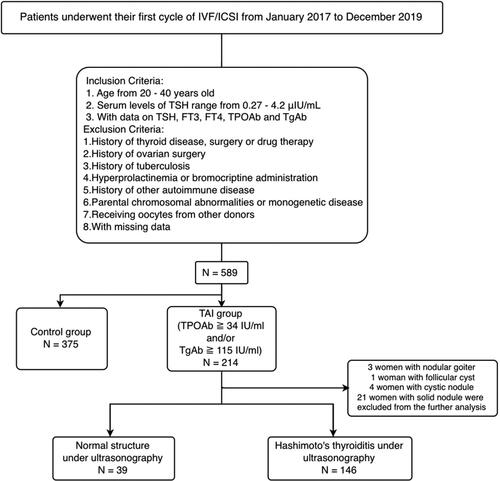
A total of 589 patients, who underwent their first IVF/ICSI cycles between January 2017 and December 2019 at Center for Reproductive Medicine of Shandong University, were included in our study (). Inclusion criteria were as follows: (1) women aged ≧ 20 and ≦40; (2) with normal serum levels of TSH (0.27–4.2 μIU/mL) and available data on thyroid stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), TPOAb, and TgAb. Excluded criteria included: (1) history of thyroid disease (e.g. hyperthyroidism, hypothyroidism, thyroid cancer), surgery, or drug therapy; (2) history of ovarian surgery; (3) history of tuberculosis; (4) hyperprolactinemia or bromocriptine administration; (5) history of other autoimmune disease; (6) parental chromosomal abnormalities or monogenetic disease; (7) receiving donated oocytes from others. TAI was defined as TPOAb levels above 34 IU/mL and/or TgAb levels above 115 IU/mL. Accordingly, 214 euthyroid TAI women and 375 Controls were allocated in each group. We further stratified TAI patients based on thyroid ultrasonography into three groups: normal, HT, and other structural changes (including nodular goiter, follicular cyst, cystic, or solid nodule). TAI patients with other structural changes (including 3 women with nodular goiter, 1 woman with follicular cyst, 4 women with cystic nodule, 21 women with solid nodule) was excluded in the further analysis.
Figure 1. Flow chart of study population selection.
Abbreviations: IVF = in vitro fertilization; ICSI = intracytoplasmic sperm injection; TSH = thyroid stimulating hormone; FT3 = free triiodothyroinine; FT4 = free thyroxine; TPOAb = antithyroperoxidase antibody; TGAb = antithyroglobulin antibody; TAI = thyroid autoimmunity.
Serological measurements and ultrasonography
The measurements of serum hormone levels were conducted as previous study described [Citation8]. In detail, follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), prolactin, and total testosterone were measured by chemiluminescence immunoassay (Roche Diagnostics, Basel, Switzerland) at the early follicular phase, mostly on days 1–3 of the menstrual cycle. Anti-Müllerian hormone (AMH) was measured using an ultra-sensitive enzyme-linked immunosorbent assay (Kangrun Biotech, China). TSH, FT3, FT4, TPOAb, and TgAb were measured by automatic chemiluminescence immunoassays (Roche Diagnostics, Basel, Switzerland). Number of antral follicle counts (AFC) was counted at the early follicular phase under transvaginal ultrasound. Diagnosis of thyroid ultrasonography was performed by experienced examiners who were blinded to all patients enrolled in this study. Briefly, diagnostic criteria of HT included decreased echogenicity, heterogeneity, hypervascularity, and presence of hypochoic micronodules with echogenic rim [Citation9].
Ovarian stimulation and embryo transfer
All women were treated under the guidance of experienced investigators using gonadotropin-releasing hormone (GnRH) agonist, GnRH antagonist or gonadotropin (Gn) according to their individualized treatment. The protocols applied in ovarian stimulation process were described previously [Citation10]. Briefly, the long protocol was started with GnRH from mid-luteal phase of the last menstrual period for pituitary down-regulation and then using Gn for synchronized follicular development. The short protocol was started with GnRH on day 2 or 3 of the menstrual period and then using Gn 2 days later. The antagonist protocol was started with using Gn on day 3 of the menstrual period. When the follicle diameter reached 12 mm or more, GnRH-antagonist was additionally applied. For each protocol, human chorionic gonadotropin (hCG) was administered alone or together with GnRH agonist when at least two follicles reached an average diameter of 18 mm. After 34–36 h, experienced clinicians retrieved oocytes by transvaginal ultrasonography.
Fertilization process was conducted through IVF or ICSI. All selected embryos were good-quality according to Brinsden [Citation11] and Gardner Criteria [Citation12]. Either cleavage-stage embryos on day 3 of embryo culture or blastocysts on day 5 were transferred in the order from top to good.
As previously described [Citation13], the choice of fresh or frozen embryo transfer was determined by both ultrasonography and serum E2 at the second day after oocyte retrieval. If the number of oocytes retrieved ≧15 or the serum E2 ≧ 1500 pg/ml, patients would be recommended to a “freeze-all” approach instead of fresh embryo transfer to prevent ovarian hyperstimulation syndrome (OHSS). In this study, none patient was found to have OHSS symptoms such as nausea, vomiting, abdominal pain, and distension.
Fresh embryo transfer was performed at day 3 or day 5 after oocyte retrieval. As for frozen embryo transfer, endometrium preparation was also under investigation including nature cycle, hormone replacement therapy (HRT) cycle, and human menopausal gonadotrophins (hMG) stimulated cycle regimens. In summary, nature cycle started on days 1–3 of a new menstrual cycle and patients were monitored by ultrasound untill ovulation. HRT cycle started at the similar time with oral estradiol valerate (Progynova, Delpharm Lille, Lys-Lez-Lannoy, France). When endometrial thickness reached over 8 mm, vaginal progesterone gel (Crinone, Merck Serono, Watford, UK) and oral dydrogesterone (Duphaston, Abbott, OLST, Netherlands) were added and that day was regarded as ovulation day. hMG cycle was started on day 3 of a new cycle, patients were continuously injected hMG (Le Baode, Livzon, Zhuhai, China) for 5 days and then adjusted based on follicle development. When the follicle diameter reached over 17 mm or the occurrence of an LH surge, hCG was administered alone or together with the GnRH agonist for ovulation. After ovulation, oral dydrogesterone was administered by practitioners for luteal support for 2 weeks. Biochemical pregnancy, clinical pregnancy, miscarriage was monitored at 4 weeks, 7 weeks, and 28 weeks gestation, respectively.
Outcome measurements
Normal fertilization rate was defined as the number of 2PN zygotes divided by the number of retrieving oocytes (IVF) or the number of MII oocytes (ICSI). Implantation rate was defined as the number of biochemical pregnancies (serum hCG level > 10 IU/L at 2 weeks after embryo transfer) divided by the number of women underwent embryo transfer. Clinical pregnancy rate was defined as the number of women who had gestational sac at their 7 weeks’ gestation divided by the number of women underwent embryo transfer. Miscarriage rate was defined as the number of clinical pregnancy loss divided by the number of clinical pregnancy. Live birth rate was defined as the number of successful live birth delivery divided by the number of women underwent embryo transfer regardless of single or twin pregnancy.
Statistical analyses
All data analysis was performed using SPSS software (SPSS Inc., Version 26.0, Chicago, USA). t test and ANOVA were used to compare continuous variables with normal distribution, such as age and body mass index (BMI). Mann-Whitney U test and Kruskal-Wallis test were used to compare continuous variables with non-normal distribution. χ2 test was used to compare categorical variables. Potential confounders included FT4. Binary logistic regression was used to evaluate the difference of pregnancy outcomes between two groups, TAI group with normal thyroid structure under ultrasonography, and TAI group with HT under ultrasonography. P-value < 0.05 was considered statistically significant.
Results
showed the population selection process of this study. presented the baseline characteristics of TAI group and control group. No significant difference was found in age, BMI, duration of infertility, serum levels of LH, estradiol, prolactin, and total testosterone between two groups. For ovarian reserve markers, such as serum levels of FSH, AMH, and number of antral follicle counts, these two groups were still comparable with each other. However, TAI group had a higher serum level of TPOAb (Median (interquartile range): 61.8 (24.4, 205.5) vs. 9.0 (0.9, 15.5), p < 0.001) and TgAb (Median (interquartile range): 221.9 (57.8, 438.2) vs. 10.1 (0.5, 14.1), p < 0.001) than control group. Also, despite the serum level of FT4 remained within normal ranges in both two groups, TAI group was higher compared with control group (Median (interquartile range): 16.1 (14.2, 17.9) vs. 14.9 (11.3, 17.0), p < 0.001).
Table 1. Baseline characteristics of patients.
Subsequently, we divided TAI patients into two groups based on the morphological changes under ultrasonography (). 39 patients with normal thyroid structure and 146 patients with HT were allocated in each group. No difference was observed in baseline characteristics between these two groups.
Table 2. Baseline characteristics of patients with Hashimoto’s thyroiditis or with normal structure under ultrasonography.
and Citation4 presented embryo qualities and pregnancy outcomes of these study groups. The binary logistic regression model showed that TAI group had lower chance of implantation than control group (adjusted OR (95% CI): 0.65 (0.44, 0.97), adjusted p = 0.04) after adjusting potential confounders (). However, after stratified by ultrasonography, TAI group with HT still had lower chance of implantation compared with control group (adjusted OR (95% CI): 0.63 (0.41, 0.99), adjusted p = 0.04) while TAI group with normal thyroid structure had no difference in implantation rate with control group (adjusted OR (95% CI): 0.85 (0.37, 1.93), adjusted p = 0.70). Other embryo qualities and pregnancy outcomes, such as number of antral follicle counts, normal fertilization rate of IVF or ICSI, number of good-quality embryos, clinical pregnancy rate, miscarriage rate, and live birth rate were comparable among among these three groups.
Table 3. Outcomes of embryo development and pregnancy in TAI and control group.
Table 4. Outcomes of embryo development and pregnancy based on ultrasonography.
Discussion
In this retrospective cohort study, we found that TAI patients had lower implantation rate than non-TAI euthyroid patients. However, stratified by thyroid ultrasonography, TAI patients with HT still showed lower implantation rate compared with control group while TAI patients with normal thyroid structure showed comparable rate in implantation with control group. Besides, embryo qualities and other pregnancy outcomes were similar among these study groups.
Lower implantation rate in TAI patients was observed not only in our study but also in several previous researches [Citation3,Citation14,Citation15]. Busnelli et al. conducted the most recent meta-analysis and concluded that a significantly lower chance of embryo implantation was observed in TAI-positive women [Citation3]. Zhong et al. retrospectively analyzed 90 patients (156 cycles) with positive antithyroid antibodies and 676 infertile women (1062 cycles) with negative antithyroid antibodies who underwent IVF or ICSI then found significantly lower implantation rate in those with antithyroid antibodies [Citation14]. Chen et al. also reported lower implantation rates in both aβ2GPI-IgG-positive group and TPOAb-positive group compared to the negative group [Citation15]. Although some studies held the opinion that implantation rate was similar between TAI patients and controls[Citation1,Citation16], lack of studies reported the association between changes under thyroid ultrasonography and embryo qualities or pregnancy outcomes among TAI women. Hence, whether thyroid structural changes in TAI patients contributed to the conflicting conclusions remains unknown.
Our results showed TAI patients with ultrasonic manifestations of HT had lower implantation rate compared to non-TAI euthyroid patients while TAI patients with normal thyroid structure had comparable implantation rate with non-TAI euthyroid patients. It was the first study demonstrating the role of thyroid ultrasonography in assessing embryo qualities and pregnancy outcomes. We accordingly assumed that positive antithyroid-antibody patients with ultrasonic manifestations of HT suffered a longer term of TAI than patients with normal thyroid structure so that resulted in the lower implantation rate.
This hypothesis was supported by several studies [Citation6,Citation7]. Acar et al. suggested thyroid structural changes may start long before the symptoms and hormonal imbalances [Citation6]. Pedersen et al. [Citation7] examined 3077 patients by thyroid ultrasonography and considered reduced thyroid echogenicity under ultrasonography was a strong predictor for autoimmune thyroid diseases before clinical disorders as biochemical dysfunction of TAI was rarely seen with normal structure. Thus, evaluation of thyroid ultrasonography is essential before IVF or ICSI cycles, as it may indicate adverse pregnancy outcomes.
Besides, no difference was found in parameters of embryo qualities, such as oocyte yield, normal fertilization rate, and number of good-quality embryos, among these study groups regardless of thyroid morphological changes. Although it was consistent with some findings [Citation3,Citation17] but others also found TAI had significantly adverse effects on embryo qualities [Citation18,Citation19]. Herein, lack of association between TAI and embryo qualities perhaps because the relatively younger age and smaller sample size in our study population. In the future, studies with a large study population and more specific parameters of embryo development need to be performed to explore the potential association.
Underlying mechanisms of lower implantation rate in TAI patients are still uncertain, but endometrial dysfunction, abnormal maternal-placenta structure may be detrimental factors for implantation [Citation20,Citation21]. For example, Rahnama et al. analyzed TPO gene and protein levels in proliferative-phase endometrium, pre-implantation embryos and placenta then found TPO expression was localized in endometrium and placenta but not in embryos [Citation20]. Nevertheless, lack of evidence elucidated pathophysiological changes in euthyroid HT patients. Only some animal experiments substantiated that euthyroid HT could reduce weight of placenta, change its normal structure [Citation22] and inhibit expression of receptivity markers (e.g. estrogen receptor α (ERα), integrin β3, leukemia inhibitory factor (LIF), and cell adhesion molecule-1 (ICAM-1)) [Citation23]. Studies with human samples are needed to further demonstrate the mechanisms of embryo implantation failure in euthyroid HT women.
The strength of our study was that it first focused on the relationship between thyroid ultrasonic changes and embryo qualities or pregnancy outcomes in TAI patients. Also, it provides a possible reason for controversial conclusions on embryo implantation among previous studies. However, there are also some limitations in our study. First, this was a retrospective study. Thus, during follow-up period, self-reported and retrospective measures may be somewhat inaccurate and lead to recall bias. Second, as small sample size of other thyroid structure changes, we excluded them in the subanalysis. The relationship between these changes and embryo qualities or pregnancy outcomes need studies with a larger population to further explore. Third, other ultrasound parameters (such as thyroid size, gray-scale grade, etc.) were not collected in our study, so which parameter (including hemodynamics and morphology) may be strongly related to the poor pregnancy outcomes remains to be verified. Fourth, as the relatively younger patients and smaller sample size in our study, we did not find embryo quality difference between study groups. Population-based studies especially including women with advanced age need to be conducted in the future.
Conclusions
Our findings suggest that implantation rate was decreased in TAI patients with an ultrasonic manifestation of HT. Further work is essential to reveal the underlying mechanisms of implantation failure in euthyroid TAI or HT patients.
Authors contribution
Zengxiang Ma and Yingxin Zhang designed the study. Jiahui Wang and Wei Zhou wrote the manuscript, made tables and figures. Jincheng Li, Shuo Zhang, and Tong Wu performed data collection and data analysis. Zhiyi Song and Chenxi Li supervised the manuscript. All authors have read and agreed to the final manuscript.
Acknowledgment
The authors thank all the staffs involved in the process of data measurements and collection.
Disclosure statement
No conflict of interest was reported by the authors.
Data availability statement
The data of this study is available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Venables A, Wong W, Way M, et al. Thyroid autoimmunity and IVF/ICSI outcomes in euthyroid women: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2020;18(1):1. doi: 10.1186/s12958-020-00671-3.
- Huang N, Chen L, Lian Y, et al. Impact of thyroid autoimmunity on in vitro fertilization/intracytoplasmic sperm injection outcomes and fetal weight. Front Endocrinol (Lausanne). 2021;12:698579. doi: 10.3389/fendo.2021.698579.
- Busnelli A, Beltratti C, Cirillo F, et al. Impact of thyroid autoimmunity on assisted reproductive technology outcomes and ovarian reserve markers: an updated systematic review and meta-analysis. Thyroid. 2022;32(9):1010–7. doi: 10.1089/thy.2021.0656.
- Bothra N, Shah N, Goroshi M, et al. Hashimoto’s thyroiditis: relative recurrence risk ratio and implications for screening of first-degree relatives. Clin Endocrinol (Oxf). 2017;87(2):201–206. doi: 10.1111/cen.13323.
- Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13(4–5):391–397. doi: 10.1016/j.autrev.2014.01.007.
- Acar T, Ozbek SS, Erdogan M, et al. US findings in euthyroid patients with positive antithyroid autoantibody tests compared to normal and hypothyroid cases. Diagn Interv Radiol. 2013;19(4):265–270. doi: 10.5152/dir.2013.041.
- Pedersen OM, Aardal NP, Larssen TB, et al. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000;10(3):251–259. doi: 10.1089/thy.2000.10.251.
- Ke H, Hu J, Zhao L, et al. Impact of thyroid autoimmunity on ovarian reserve, pregnancy outcomes, and offspring health in euthyroid women following in vitro fertilization/intracytoplasmic sperm injection. Thyroid. 2020;30(4):588–597. doi: 10.1089/thy.2018.0657.
- Ralli M, Angeletti D, Fiore M, et al. Hashimoto’s thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020;19(10):102649. doi: 10.1016/j.autrev.2020.102649.
- Wei D, Liu JY, Sun Y, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310–1318. doi: 10.1016/S0140-6736(18)32843-5.
- Brinsden PR. A textbook of in vitro fertilization and assisted reproduction: the Bourn Hall guide to clinical and laboratory practice. New York: Parthenon Pub.Group; 1999.
- Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty infertility & genetics beyond. Carnforth: Parthenon Press; 1999. p. 378–388.
- Liu F, Jiang Q, Sun X, et al. Lipid metabolic disorders and ovarian hyperstimulation syndrome: a retrospective analysis. Front Physiol. 2020;11:491892. doi: 10.3389/fphys.2020.491892.
- Zhong YP, Ying Y, Wu HT, et al. Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int J Med Sci. 2012;9(2):121–125. doi: 10.7150/ijms.3467.
- Chen X, Mo ML, Huang CY, et al. Association of serum autoantibodies with pregnancy outcome of patients undergoing first IVF/ICSI treatment: a prospective cohort study. J Reprod Immunol. 2017;122:14–20. doi: 10.1016/j.jri.2017.08.002.
- Łukaszuk K, Kunicki M, Kulwikowska P, et al. The impact of the presence of antithyroid antibodies on pregnancy outcome following intracytoplasmatic sperm injection-ICSI and embryo transfer in women with normal thyreotropine levels. J Endocrinol Invest. 2015;38(12):1335–1343. doi: 10.1007/s40618-015-0377-5.
- Poppe K, Autin C, Veltri F, et al. Thyroid disorders and in vitro outcomes of assisted reproductive technology: an unfortunate combination? Thyroid. 2020;30(8):1177–1185. doi: 10.1089/thy.2019.0567.
- Andrisani A, Sabbadin C, Marin L, et al. The influence of thyroid autoimmunity on embryo quality in women undergoing assisted reproductive technology. Gynecol Endocrinol. 2018;34(9):752–755. doi: 10.1080/09513590.2018.1442427.
- Weghofer A, Himaya E, Kushnir VA, et al. The impact of thyroid function and thyroid autoimmunity on embryo quality in women with low functional ovarian reserve: a case-control study. Reprod Biol Endocrinol. 2015;13(1):43. doi: 10.1186/s12958-015-0041-0.
- Rahnama R, Mahmoudi AR, Kazemnejad S, et al. Thyroid peroxidase in human endometrium and placenta: a potential target for anti-TPO antibodies. Clin Exp Med. 2021;21(1):79–88. doi: 10.1007/s10238-020-00663-y.
- Liu S, Xu F, Wei H, et al. The correlation of thyroid autoimmunity and peripheral and uterine immune status in women with recurrent miscarriage. J Reprod Immunol. 2020;139:103118. doi: 10.1016/j.jri.2020.103118.
- Borodina E, Katz I, Antonelli A, et al. The pathogenic role of circulating Hashimoto’s thyroiditis-derived TPO-positive IgG on fetal loss in naïve mice. Am J Reprod Immunol. 2021;85(1):e13331. doi: 10.1111/aji.13331
- Wu Z, Cai Y, Xia Q, et al. Hashimoto’s thyroiditis impairs embryo implantation by compromising endometrial morphology and receptivity markers in euthyroid mice. Reprod Biol Endocrinol. 2019;17(1):94. doi: 10.1186/s12958-019-0526-3.
