Abstract
Background
Telomeres maintain chromosome stability, while telomerase counteracts their progressive shortening. Telomere length varies between cell types, with leukocyte telomere length (LTL) decreasing with age. Reduced telomerase activity has been linked to reproductive issues in females, such as low pregnancy rates and premature ovarian failure, with recent studies indicating correlations between telomere length in granulosa cells and IVF outcomes.
Objectives
The study aims to explore the relationship between telomere length, telomerase activity, and euploid blastocyst rate in infertile women undergoing IVF/ICSI PGT-A cycles.
Methods
This prospective study involves 108 patients undergoing controlled ovarian stimulation and PGT-A. Telomere length and telomerase activity were measured in peripheral mononuclear cells and granulosa cells (GC), respectively.
Results
The telomere repeat copy number to single gene copy number ratio (T/S) results respectively 0.6 ± 0.8 in leukocytes and 0.7 ± 0.9 in GC. An inverse relationship was found between LTL and the patient’s age (p < .01). A higher aneuploid rate was noticed in patients with short LTL, with no differences in ovarian reserve markers (p = .15), number of oocytes retrieved (p = .33), and number of MII (p = 0.42). No significant association was noticed between telomere length in GC and patients’ age (p = 0.95), in ovarian reserve markers (p = 0.32), number of oocytes retrieved (p = .58), number of MII (p = .74) and aneuploidy rate (p = .65).
Conclusion
LTL shows a significant inverse correlation with patient age and higher aneuploidy rates. Telomere length in GCs does not correlate with patient age or reproductive outcomes, indicating differential telomere dynamics between leukocytes and granulosa cells.
Introduction
Telomeres have a fundamental pivotal role in maintaining genome stability hence preventing chromosome ends from being recognized as double-strand breaks and processed by DNA damage repair [Citation1–3]. Because of incomplete DNA replication at the ends of chromosomes, telomeres shorten progressively with cell divisions [Citation4,Citation5]. In somatic cells, this leads to progressive telomere attrition/shortening which triggers replicative senescence or apoptosis. Telomerase is a ribonucleoprotein composed of a catalytic subunit called ‘Telomerase Reverse Transcriptase’ and an RNA matrix subunit called ‘Telomerase RNA component’. Telomerase maintains telomere length by extending the guanine-rich single strand of telomeres, allowing DNA polymerase to achieve synthesis of the opposite strand and thus avoiding the progressive loss of DNA at each replication cycle, adding TTAGGG repeats at the end of chromosomes and allowing cell proliferation [Citation6].
In humans, mean telomere length (TL) ranges from 4 to 12 kb in somatic cells and from 10 to 20 kb in germinal cells. These mean values differ within an individual depending on the cell type, tissues, and organs. Leukocyte telomere length (LTL), the most studied telomere length in clinical and epidemiological studies due to the easy accessibility of leukocytes, decreases with age [Citation7–10]. The LTL attrition rate is higher in utero and during the first years of life and then it decreases during adulthood. In adults, LTL attrition rates are estimated at 25 to 35 bp/year. LTL is highly heritable (60 to 70%) and displays a wide range of mean values (4 kb range) between individuals of the same age in a population [Citation11–13].
Reduced telomerase activity in granulosa cells (GC) seems to play an important role in the maturation of the oocytes and may be associated with specific clinical conditions related to reproductive biology in females. It has been observed that women with low pregnancy rates after IVF treatment, premature ovarian insufficiency (POI), or pregnancies complicated with IUGR have shorter telomeres compared with healthy controls [Citation14].
Some studies found shorter telomere length in granulosa cells and/or leukocytes and/or lower telomerase activity in premature ovarian failure patients [Citation15]. A recent study showed that granulosa cell telomere length (GCTL) or telomerase activity correlated with embryo quality and pregnancy outcomes in women undergoing IVF [Citation16].
The cumulus cells from mature follicles have significantly longer telomeres than leukocytes, suggesting that the follicular environment could possess different mechanisms to cope against telomere shortening compared with other somatic tissues [Citation17].
Increased female age is associated with an increased risk of chromosomal abnormality in the embryos and it is mainly due to chromosomal abnormalities occurring in the egg. In a previous study performed in our center [Citation18], we found that the aneuploidy rate increases by ∼10% per year of female age, with 48.1%, 41.3%, 29.7%, and 10.3% euploid blastocysts in patients with mean female ages ≤32, 33–36, 37–41, and ≥42 years, respectively. The abnormalities include modifications in the mechanism for the assembly of the meiotic spindle, leading to errors in chromosome alignment and the microtubule matrix [Citation19], increased rates of chromosome degeneration into unassociated chromatids [Citation20], and increased rates of chromosome nondisjunction [Citation21].
Aneuploidy affects more than one-half of human embryos and is the main reason for implantation failure and miscarriages in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles [Citation22–24].
The objective of the present study is to investigate the association between telomere length and telomerase activity in leukocytes and granulosa cells and the number and rate of euploid blastocysts in infertile women undergoing IVF/ICSI PGT-A cycles.
Materials and methods
This is a prospective study in which 108 patients were consecutively enrolled between June 2022 and September 2023, including only patients undergoing PGT-A for the first time. PGT-A indications were: advanced maternal age, recurrent miscarriage, repeated implantation failure, or severe male infertility, as well as to all good-prognosis patients who desire information regarding the health status of their embryos. In all women who underwent the ICSI procedure TL and TA were measured in the peripheral mononuclear cell and in granulosa cells after follicular aspiration and intermediate and definitive outcomes have been then recorded. All of the patients provided informed consent for their anonymized clinical records to be used for research purposes. The study was approved by the local Institutional Review Board (protocol number 01/2022) and was carried out in accordance with the Helsinki Declaration.
Ovarian Stimulation. Controlled ovarian stimulation started on the second day of the menstrual cycle, with an initial dose of Menotropin (Meriofert, IBSA) chosen according to age, antral follicle count or serum AMH, and body mass index (BMI). Ganirelix has been administered by subcutaneous daily injections (Orgalutran 0.25 mg/50 ml, MSD, Italy) in the evening, from the seventh day of hormonal stimulation until trigger day. When at least one follicle reached ≥ 18 mm, 0.3 mg of triptorelin acetate (Decapeptyl, Merck, Italy) was administered to trigger ovulation; 36 h later, follicles were aspirated under sedation in all patients.
Oocyte insemination, embryo culture, and biopsy
All biological procedures were performed as described elsewhere [Citation18]. Only ICSI was used as an insemination technique and only oocytes with the first polar body extruded (metaphase II, MII) were subjected to ICSI. On Day 3, for all of the developing embryos, a medium change (Quinn Advantage Blastocyst Medium; Sage-CooperSurgical Denmark) for sequential culture was performed and a noncontact 1.48-μm diode laser was used to create a circular opening with a diameter of 6–9 μm in the zona pellucida in cleavage-stage embryos to allow the trophectoderm cells to herniate. On the day of the blastocyst biopsy, 5– 10 trophectoderm cells were gently aspirated into the biopsy pipette (Cook, Ireland) followed by laser-assisted removal from the rest of the blastocyst. The obtained trophectoderm cells were washed in sterile PBS, then placed in microcentrifuge tubes containing 2 μL PBS, spun down for a few seconds and sent to the GENOMA laboratory for analysis [Citation25].
PGT-A
PGT-A was performed using NGS-based platform VeriSeq (Vitrolife). Cells were lysed, and genomic DNA was fragmented randomly and amplified using the SurePlex DNA Amplification System (Vitrolife) according to the manufacturer’s protocol. The whole-genomic amplified DNA product of each sample was processed to prepare a genomic DNA library using VeriSeq PGS workflow (Vitrolife). Purified DNA libraries were normalized to equalize the quantity of each sample in the final pool using VeriSeq’s library normalization protocol. Equal volumes of normalized samples were pooled, denatured, and sequenced. The MiSeq Reagent Kit v.3 (Illumina) was used on a MiSeq System (Illumina). The sequencing data were analyzed using BlueFuse Multi Software (Vitrolife). NGS profiles <20% were considered euploid, and those >80% were considered aneuploid.
Telomerase activity. Peripheral blood mononuclear cells (PBMC) have been separated from whole blood by Histopaque‐1077 (Sigma‐Aldrich, St.Louis, USA) and stimulated with 1% phytohaemagglutinin (PHA HA15, Remel, Santa Fe, USA) for 48 h; at the end of the incubation period cells have been harvested by centrifugation and cell extracts obtained by incubation with NP‐40 lysis buffer containing 1% protease inhibitor mix (GE Healthcare, UK). Protein concentration has been measured using the Bio‐Rad Protein Assay kit (Bio‐Rad, Munchen, Germany). TA has been determined by the real‐time, quantitative TRAP (Q‐TRAP) protocol using a fluorescence‐based assay. In the first step of the protocol, the telomerase substrate and dNTPs have been used for the addition of telomeric repeats by telomerase, while in the second steps, specific primers for these products have been used for amplification. For the assay, 20 μl of TRAP master mix per sample contained 1X Sybr Green Master Mix (including ROX as passive reference dye, Bioline), 100 ng TS primer(5′‐AATCCGTCGAGCAGAGTT‐3′), 100 ng ACX primer (5′‐GCGCGGCTTACCCTTACCCTTACCCTAACC‐3′), 1 mM EGTA, and water. The real‐time PCR conditions have been as follows: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and at 60 °C for 60 s. Each sample has been prepared in triplicate. Values have been expressed as relative TA (%RTA) in comparison to that of the positive reference sample.
TL Determination. Genomic DNA has been isolated from whole blood samples using Puregene Core Kit (Qiagen, Hilden, Germany) and stored at −80 °C. A nonradioactive chemiluminescent assay has been applied to determine TL using the TeloTAGGG TL Assay Kit (Roche Diagnostics, Indianapolis, USA). Briefly, 1.5 μg of DNA has been digested with 20 units of RsaI and HinfI for 2 h at 37 °C. The sequence specificity of enzymes is such that telomeric DNA and subtelomeric DNA are not cut, because of the sequence characteristics of the repeats, while nontelomeric‐DNA is digested to low molecular weight fragments. After digestion, samples have been loaded on a .5% agarose gel and run for 21 h at 35 V. Gels are treated with HCl, denaturated and neutralized, and then transferred to a nylon membrane by Southern blotting for 12–18 h. DNA fragments have been indirectly visualized by hybridization with a digoxigenin (DIG)‐labeled probe complementary to the telomeric repeat sequence (3 h, 42 °C). Images have been digitalized using a densitometer and the mean telomeric length (MTL) was determined using the formula: MTL = ∑(MWi x ODi)/∑(ODi), where ODi is the densitometer output and MWi is the length of the DNA at position i. Sums have been calculated over the range 1.6–12.2 kb.
Sample size calculation and data analysis. This is a prospective study with the main objective of correlating telomere length and telomerase activity to the rate of euploid embryos. Assuming an alpha of .01 and a Beta of .2 and considering a clinically significant correlation of at least r: .04, 86 patients must be included in the study. Considering that in our center 20% of women entering the PGT-A program will not have blastocysts available for the analysis, the total number of women to include in the study is 108.
Data has been presented as mean ± SD when they had a Gaussian distribution or median (interquartile range) when they were not normally distributed. Parametric and nonparametric tests were used to compare baseline characteristics and outcomes as appropriate. Multiple and logistic regression analyses have been used to assess the predictive ability of independent variables. All statistical tests will use a two-tailed α of .05. Statistical analysis has been done with the use of Stata 12 software.
Results
A total of 108 patients have been included in the study and have completed the PGTA cycle. Baseline patients’ characteristics are reported in .
Table 1. Patient and cycle characteristics.
Preconception leukocyte and granulosa cell telomere length is expressed in telomere repeat copy number to single gene copy number ratio (T/S) and results respectively 0.6 ± 0.8 in leukocytes and 0.7 ± 0.9 in granulosa cells. Firstly, we studied the telomere length in leukocytes and its correlation with patients’ age and reproductive outcomes. Analysis of data obtained so far revealed a statistically significant inverse relationship between telomere length in leukocytes and patient’s age, as showed in (p < .01). About reproductive outcomes, measured by ovarian and embryonic performance, a significant higher aneuploid rate was noticed in patients with short telomerase length in leucocytes(p < .01) (). However, no differences were found in ovarian reserve markers (p = .15), number of oocytes retrieved (p = .33) and number of MII (p = .42).
Figure 1. (A) Correlation between patient age (in years) and the relative telomere length of leukocyte samples. The line indicates that the relative telomere length of leukocytes decreases with age. (B) Correlation between patient age (in years) and the relative telomere length of granulosa cell (GC) samples. The line indicates that the relative telomere length of GCs does not change significantly with increasing age.
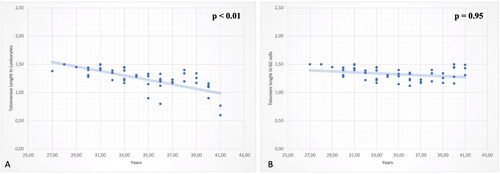
Figure 2. (A) Correlation between aneuploidy rate and relative telomere length of leukocytes. The line indicates that there is a negative correlation: lower telomere length is associated to a higher aneuploidy rate (%). B) Correlation between aneuploidy rate and relative telomere length of granulosa cell (GC) samples. The line shows that there is no significant correlation.
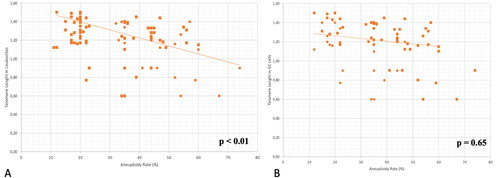
To complete the analysis, the telomerase length in granulosa cells has been considered. Nevertheless, no significant association was noticed between telomere length in granulosa cells and participants’ age, as reported in (p = .95). These data suggest that the progressive shortening of telomeres that occurs within most tissues over time is not present in granulosa cells. About reproductive outcomes, no statistical association was found in ovarian reserve markers (p = .32), number of oocytes retrieved (p = .58), number of MII (p = .74) and aneuploidy rate (p = .65) ().
Discussion
Telomere shortening has been associated with impaired fertility in women. Telomeres protect the genome from chromosomal imbalance and altered gene expression [Citation26]. The telomere-based theory of reproductive aging proposes that ovarian dysfunction related to age is a consequence of the progressive shortening of the telomeres due to the chronic effects of oxidative stress [Citation27].
A study aimed to determine if granulosa cell telomere length (GCTL) or telomerase activity correlates with embryo quality and pregnancy outcomes in women with normal ovarian function undergoing IVF concluded that telomerase activity, rather than length, represents a better predictor of IVF treatment outcomes [Citation16].
Relative cumulus cell telomere length (CCTL) from mature oocytes is longer than CCTL of immature oocytes, and, similarly, CC from oocytes generating good-quality embryos present longer telomeres in contrast to those generating poor-quality embryos [Citation28]. Therefore, measurements of telomere length (TL) and telomere integrity in follicular cells appear to be potential predictors for oocyte and embryo quality, clinical outcomes, and ovarian function. Because TL measurements of blood cells can be performed from a routine blood extraction, leukocyte telomere length (LTL) is an interesting candidate for a surrogate of telomere DNA content in other types of tissues. A recent study demonstrated that LTL and GCTL of patients with biochemical primary ovarian insufficiency were shortened significantly compared with control subjects [Citation29].
This study aimed to assess if TL measured in peripheral blood could be a reliable surrogate for TL in follicular cells because of the extensively reported association between telomere shortening and age-related ovarian dysfunction. Granulosa cells (GCs) are the most important somatic cells for determining the final size of preovulatory follicles. It has been previously reported that LTL is a good indicator of TL in vascular tissue [Citation30], buccal cells and fibroblasts in patients with dyskeratosis congenita [Citation31]. Providing a similar correlation between LTL and TL in granulosa cells would be of great clinical relevance.
A possible relationship between telomeres and reproduction has recently been reported. There is evidence that telomere length (TL) is longer in the oocytes of women who become pregnant than in those who fail after IVF treatment [Citation32]. Telomeres in GCs are significantly longer than in leukocytes, suggesting that the progressive shortening of telomeres that occurs within most tissues over time is not present in granulosa cells. The follicular environment could be supposed to be regulated by different mechanisms regarding telomere maintenance suggesting that different tissue types differ in their mechanisms of telomere maintenance [Citation33].
The GCs have significantly longer telomeres than leukocytes, as recently demonstrated regarding CC [Citation34]. This, together with the lack of correlation between LTL and TL in follicular cells, strongly confirms that the microenvironment in ovarian follicles deals differently with telomere biology than other somatic tissues. Also, studies in other mammals have reported telomere lengthening in ovarian follicles during folliculogenesis [Citation35]. Liu et al. (2002) discovered that infertility in telomerase knockout mice is associated with gradual germinal cell telomere shortening.
Interestingly, telomerase activity seems to decrease during folliculogenesis, posing a contradiction to telomere lengthening during follicular development [Citation36].
Telomerase activity was found to predict IVF outcomes better than telomerase length [Citation16], and it was supposed to be considered a good biomarker of IVF treatment outcomes. Several studies demonstrate that female fertility disorders are likely to be associated with fewer cell divisions, supposing that the cells may have prolonged cell cycle. This also indicated that TL alone was not enough to fully reveal the reproductive potential. TA is a better indicator of pregnancy outcomes than other factors, and TL alone is not a sufficient proxy for ovarian reserves and reproductive potential. Telomerase can compensate for telomere shortening resulting from cell division, and maintain genetic integrity [Citation37].
The study included a specific number of women, adhering to statistical assumptions (with an alpha of .01, a beta of .2, and a clinically significant correlation of at least r: .04). It also considered that in our center, 20% of women entering the PGT-A program may not have blastocysts available for analysis. While our sample size met the mentioned criteria, it’s important to note that the small size of our study population reduces the reliability of our results, representing the main limitation of our study. Therefore, interpretations should be made cautiously, especially in comparison to larger samples, to avoid overgeneralization.
Summary of the evidence
Our findings provided by this study indicate that LTL is a poor surrogate for TL in the ovarian follicle. In addition, it was observed that GCs have longer telomeres than L, which suggests a different mechanism of telomere maintenance in the ovarian follicle microenvironment. Moreover, relative TL in the cell types analyzed was not associated with subject characteristics such as age, ovarian reserve, and response to COS.
This study contributes to our understanding of telomere biology in reproductive medicine.
It seems that telomere shortening in oocytes may induce apoptosis in human preimplantation embryos, which would be consistent with a telomere theory of reproductive senescence in women.
Exploring telomere length in granulosa cells (GCs) and other follicular environment cells could potentially pave the way for a new research avenue in understanding the mechanisms of oocyte aging, directly impacting functional ovarian reserve.
Further research is needed to fully understand the underlying mechanisms behind our findings. However, our data provides a solid foundation for further studies, particularly with regard to the assumption that the LTL may not be the most accurate marker of follicular functioning.
Authors’ contributions
Maria Longo, Ermanno Greco and Pierfrancesco Greco acquired data, designed and drafted the manuscript, critically reviewed the manuscript for important intellectual content, approved the final version to be published, and agreed to be responsible for all aspects of the work. Ilaria Listorti, Maria Teresa Varricchio, Katerina Litwicka, Cristiana Arrivi and Cecilia Mencacci contributed to the design and draft of the manuscript. They also agree to be responsible for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to restrictions, e.g. their containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–573. doi: 10.1038/350569a0.
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42(1):301–334. doi: 10.1128/MCB.01352-08.
- O'Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11(3):171–181. doi: 10.1038/nrm2848.
- Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88(5):657–666. doi: 10.1016/s0092-8674(00)81908.
- Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116(2):273–279. doi: 10.1016/s0092-8674(04)00038-8.
- Greider CW, Blackburn EH. Telomeres, telomerase and cancer. Sci Am. 1996;274(2):92–97. doi: 10.1038/scientificamerican0296-92.
- Factor-Litvak P, Susser E, Kezios K, et al. Leukocyte telomere length in newborns: implications for the role of telomeres in human disease. Pediatrics. 2016;137(4):e20153927. doi: 10.1542/peds.2015-3927.
- Samassekou O, Gadji M, Drouin R, et al. Sizing the ends: normal length of human telomeres. Ann Anat. 2010;192(5):284–291. doi: 10.1016/j.aanat.2010.07.005.
- Baird DM, Kipling D. The extent and significance of telomere loss with age. Ann N Y Acad Sci. 2004;1019(1):265–268. doi: 10.1196/annals.1297.044.
- Aubert G, Baerlocher GM, Vulto I, et al. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLOS Genet. 2012;8(5):e1002696. doi: 10.1371/journal.pgen.1002696.
- Arias-Sosa LA. Understanding the role of telomere dynamics in Normal and dysfunctional human reproduction. Reprod Sci. 2019;26(1):6–17. doi: 10.1177/1933719118804409.
- Benetos A, Verhulst S, Labat C, et al. Telomere length tracking in children and their parents: implications for adult onset diseases. Faseb J. 2019;33(12):14248–14253. doi: 10.1096/fj.201901275R.
- Hjelmborg JB, Dalgård C, Möller S, et al. The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52(5):297–302. doi: 10.1136/jmedgenet-2014-102736.
- Ozturk S, Sozen B, Demir N. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod. 2014;20(1):15–30. doi: 10.1093/molehr/gat055.
- Fattet AJ, Toupance S, Thornton SN, et al. Telomere length in granulosa cells and leukocytes: a potential marker of female fertility? A systematic review of the literature. J Ovarian Res. 2020;13(1):96. doi: 10.1186/s13048-020-00702-y.
- Wang W, Chen H, Li R, et al. Telomerase activity is more significant for predicting the outcome of IVF treatment than telomere length in granulosa cells. Reproduction. 2014;147(5):649–657. doi: 10.1530/REP-13-0223.
- Lara-Molina EE, Franasiak JM, Marin D, et al. Cumulus cells have longer telomeres than leukocytes in reproductive-age women. Fertil Steril. 2020;113(1):217–223. doi: 10.1016/j.fertnstert.2019.08.089.
- Minasi MG, Colasante A, Riccio T, et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31(10):2245–2254. doi: 10.1093/humrep/dew18.
- Battaglia DE, Goodwin P, Klein NA, et al. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 2001;11(10):2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080.
- Lim AS, Tsakok MF. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68(2):265–271. doi: 10.1016/s0015-0282(97)81513-0.
- Pellestor F, Andréo B, Arnal F, et al. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112(2):195–203. doi: 10.1007/s00439-002-0852-x.
- Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002;8(4):333–343. doi: 10.1093/humupd/8.4.333.
- Fragouli E, Wells D. Aneuploidy screening for embryo selection. Semin Reprod Med. 2012;30(4):289–301. doi: 10.1055/s-0032-1313908.
- Fragouli E, Alfarawati S, Spath K, et al. The origin and impact of embryonic aneuploidy. Hum Genet. 2013;132(9):1001–1013. doi: 10.1007/s00439-013-1309-0.
- Greco E, Bono S, Ruberti A, et al. Comparative genomic hybridization selection of blastocysts for repeated implantation failure treatment: a pilot study. Biomed Res Int. 2014;2014:457913–457910. doi: 10.1155/2014/457913.
- Raynaud CM, Sabatier L, Philipot O, et al. Telomere length, telomeric proteins and genomic instability during the multistep carcinogenic process. Crit Rev Oncol Hematol. 2008;66(2):99–117. doi: 10.1016/j.critrevonc.2007.11.006.
- Keefe DL. Telomeres, reproductive aging, and genomic instability during early development. Reprod Sci. 2016;23(12):1612–1615. doi: 10.1097/OGX.0000000000000907.
- Cheng EH, Chen SU, Lee TH, et al. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum Reprod. 2013;28(4):929–936. doi: 10.1093/humrep/det004.
- Xu X, Chen X, Zhang X, et al. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum Reprod. 2017;32(1):201–207. doi: 10.1093/humrep/dew283.
- Wilson WR, Herbert KE, Mistry Y, et al. Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur Heart J. 2008;29(21):2689–2694. doi: 10.1093/eurheartj/ehn386.
- Gadalla SM, Cawthon R, Giri N, et al. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany NY). 2010;2(11):867–874. doi: 10.18632/aging.100235.
- Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci. 2007;64(2):139–143. doi: 10.1007/s00018-006-6466-z.
- Wang JC, Warner JK, Erdmann N, et al. Dissociation of telomerase activity and telomere length maintenance in primitive human hematopoietic cells. Proc Natl Acad Sci U S A. 2005;102(40):14398–14403. doi: 10.1073/pnas.0504161102.
- Morin SJ, Tao X, Marin D, et al. DNA methylation-based age prediction and telomere length in white blood cells and cumulus cells of infertile women with normal or poor response to ovarian stimulation. Aging . 2018;10(12):3761–3773. doi: 10.18632/aging.101670.
- Russo V, Berardinelli P, Martelli A, et al. Expression of telomerase reverse transcriptase subunit (TERT) and telomere sizing in pig ovarian follicles. J Histochem Cytochem. 2006;54(4):443–455. doi: 10.1369/jhc.4A6603.2006.
- Kosebent EG, Uysal F, Ozturk S. Telomere length and telomerase activity during folliculogenesis in mammals. J Reprod Dev. 2018;64(6):477–484. 2018 Sep 28. doi: 10.1262/jrd.2018-076.
- Mondello C, Riboni R, Casati A, et al. Chromosomal instability and telomere length variations during the life span of human fibroblast clones. Exp Cell Res. 1997;236(2):385–396. doi: 10.1006/excr.1997.3756.
