Abstract
Background
Iron metabolism plays a significant role in the development of metabolic disorders in women with polycystic ovary syndrome (PCOS). Despite the importance of hepcidin, a key iron regulator, current research on serum hepcidin levels in PCOS patients shows conflicting results.
Methods
PubMed, Embase, Web of Science, Cochrane Library and the China National Knowledge Infrastructure (CNKI) database were systematically searched from their inception to 9 September 2023. The search aimed to identify studies in English and Chinese that examined hepcidin levels in women with PCOS compared to healthy control subjects. Standardized mean differences (SMDs) with corresponding 95% confidence intervals (95% CIs) were calculated to evaluate the difference in serum hepcidin levels between women with and without PCOS.
Results
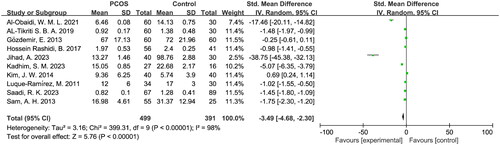
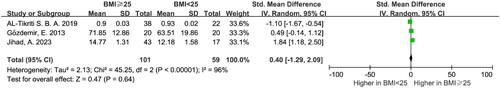
The meta-analysis included a total of 10 eligible studies, which encompassed 499 PCOS patients and 391 control subjects. The pooled analysis revealed a significant reduction in serum hepcidin levels among the PCOS patients compared to the healthy controls (SMD = −3.49, 95% CI: −4.68 to −2.30, p < .05). There was no statistically significant difference in serum hepcidin levels between PCOS patients with a body mass index (BMI) < 25 and those with a BMI ≥ 25 (p > .05).
Conclusion
The serum hepcidin levels of women with PCOS were significantly lower than those of healthy controls, which suggests that serum hepcidin could be a potential biomarker for PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is the most prevalent endocrine metabolic disorder affecting women of reproductive age, with a prevalence ranging from 5% to 18% [Citation1]. PCOS is marked by hyperandrogenaemia (HA) and insulin resistance (IR). It is closely associated with chronic inflammation, obesity, oxidative stress and metabolic syndrome (MetS), which encompasses IR and impaired glucose tolerance [Citation2–5]. However, the role of iron metabolism in PCOS is somewhat uncertain and the subject of theoretical disagreements. Although recent studies have highlighted the role of iron metabolism in IR, obesity and MetS, the specific mechanisms and associations with PCOS are inconclusive [Citation6]. Iron metabolism regulates IR-related metabolic pathways, as well as the generation of reactive oxygen species (ROS) [Citation5,Citation7]. Therefore, studying the regulation of iron metabolism is crucial for understanding PCOS.
Hepcidin, a peptide hormone predominantly synthesized in the liver, acts as the main negative regulator of iron metabolism and serves as a mediator in the innate immune response against inflammation and infection [Citation8,Citation9]. Insufficient levels of hepcidin, often observed in PCOS patients, are associated with both iron overload and impaired glucose metabolism, resulting in iron accumulation within pancreatic islets [Citation10–12]. This iron accumulation subsequently impairs insulin secretion and triggers apoptosis, ultimately contributing to the development of IR [Citation10,Citation13]. However, exactly how hepcidin levels affect PCOS remains controversial. Consequently, understanding alterations in hepcidin levels is important to comprehend PCOS.
Previous studies have suggested a potential involvement of serum hepcidin levels in the pathogenesis of PCOS, given their crucial role in iron metabolism [Citation14–23]. However, these studies have reported inconsistent findings, and their limited sample sizes may have compromised the statistical power of their analyses. Therefore, to gain a more complete understanding of hepcidin levels in PCOS patients, we conducted a meta-analysis of the available evidence.
Methods
The present study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) guidelines [Citation24,Citation25].
Literature search
We conducted a thorough search of English language databases (PubMed, Embase, Web of Science and Cochrane Library) as well as the Chinese National Knowledge Infrastructure (CNKI) database. Our search covered articles published from the inception of these databases until 8 September 2023. We performed the search in both English and Chinese to ensure the inclusion of relevant studies. Furthermore, we manually reviewed the references in the identified articles. Eligible studies were identified using keywords such as “polycystic ovary syndrome” and “hepcidin,’’ along with their synonyms (Supplemental Tables S1–S5).
Table 1. Characteristics of the included studies.
Study selection
After the electronic search, we conducted a systematic selection process based on the titles and abstracts of the identified articles. Then, full-text articles were rigorously assessed for eligibility according to our predefined inclusion and exclusion criteria. To ensure a comprehensive and structured approach to choosing relevant studies, we applied the PICOS (population, intervention, comparison, outcome and study design) framework. Our PICOS components were the following: women diagnosed with PCOS (P), serum levels of hepcidin in PCOS patients (I), serum levels of hepcidin in healthy controls (C), differences in hepcidin between the PCOS patients and the healthy controls (O), and an observational study design (S).
Based on the PICOS framework, we established the following inclusion criteria for the studies: 1) reporting hepcidin means and standard deviations (SDs), or providing sufficient information to calculate these parameters, for both PCOS patients and healthy controls and 2) having an observational design (e.g. cohort, case-control or cross-sectional). Studies were excluded if they met any of the following exclusion criteria: 1) duplicate publication, 2) non-original publications (e.g. meta-analyses, reviews and conference abstracts) and case studies, 3) studying non-human bodies and 4) lack of non-PCOS control group. Endnote X9 (Clarivate Analytics) was used for study organization. Moreover, to identify any other eligible articles that were not found in our search, we manually reviewed the reference lists of the identified papers. Unpublished studies were excluded. If multiple studies utilized the same population, we prioritized the article that provided the most comprehensive information or had the largest sample size. Two reviewers (Jieou Nong and Yifan Sun) independently performed the database search and study selection. Any discrepancies were resolved through consultation with a third reviewer (Hua Li).
Data extraction
The data collected from each study regarded country, study design, sample size, hepcidin measurement method, follow-up time, patient characteristics (e.g. age and BMI) and hepcidin levels (mean and SD or median and interquartile range [IQR]). A maximum of two attempts were made to contact the corresponding author via email to ask for missing or unclear data or to clarify the methods. Two reviewers (Jieou Nong and Yifan Sun) independently extracted the data, and any discrepancies were resolved through discussion.
Quality assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the risk of bias in retrospective and prospective observational studies [Citation26]. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the certainty of evidence [Citation27]. Four reviewers (Jieou Nong, Qi Yin, Hongying He and Yifan Sun) independently conducted the quality assessment, with any differences resolved through discussion.
Statistical analysis
All statistical analyses were performed using RevMan 5.4 and Stata 16. Hepcidin levels were treated as continuous variables, and effect estimates were presented as means and SDs. Pooled standardized mean differences (SMDs) with 95% confidence intervals (CIs) were calculated for the continuous variables. In cases where median and IQR values were reported, they were converted to mean and SD values for analysis [Citation28,Citation29].
Heterogeneity was assessed using Cochran’s Q test and the I2 statistic. Heterogeneity was considered substantial if I2 > 50%; in such cases, a random-effects model was used to calculate pooled estimates of the SMD in hepcidin levels. Conversely, heterogeneity was considered low if I2 < 50%, in which case a fixed-effects model was employed. A p-value < .10 in Cochran’s Q test indicated significant heterogeneity. Sensitivity analyses were performed by sequentially removing each study to assess its impact on the final results. Publication bias was visually evaluated using a funnel plot, while Egger’s test was conducted to quantitatively assess publication bias (significant at p < .05). Two reviewers (Jieou Nong and Yifan Sun) independently carried out the statistical analyses, with any discrepancies resolved through discussion.
Results
Study selection
A comprehensive review of the literature resulted in the identification of 55 studies. After eliminating 26 duplicate articles, a total of 29 papers remained for further evaluation. Upon screening the titles and abstracts of these, 17 studies were selected for a thorough assessment. Subsequently, two articles were excluded due to unavailability of the full text. Following retrieval of the full texts of 15 studies, five were excluded based on irrelevant outcomes (n = 3), lack of a comparison group (n = 1) and duplication of the examined population (n = 1). Consequently, a total of 10 papers were ultimately included in the analysis ().
Study characteristics and quality assessment
The samples of the ten studies amounted to 499 cases of PCOS and 391 control subjects. A summary of the studies’ characteristics can be found in and Supplemental Tables S6 and S7. The studies were conducted in Asia (eight) and Europe (two), and they were published between 2011 and 2023. Serum hepcidin levels were assessed using enzyme-linked immunosorbent assay (ELISA) in nine studies and radioimmunoassay (RIA) in one study. Concerning quality assessment with the NOS, nine studies received ratings between seven and nine stars, while one study received five stars (Supplemental Table S8). The GRADE assessment showed very low certainty of evidence for the overall outcomes (Supplemental Table S9). Nine articles found that women with PCOS had lower hepcidin levels than healthy controls, while one article reported higher hepcidin levels in PCOS patients.
Results of the meta-analysis
The random-effects model indicated a significant decrease in hepcidin levels among PCOS patients (SMD = − 3.49, 95% CI = −4.68 to −2.30, p < .05). Substantial heterogeneity was observed with I2 = 98% and p < .05 (). The exclusion of individual studies for the sensitivity analysis showed little difference in the results, which indicates that the findings of this study have strong credibility. Furthermore, the sensitivity analysis revealed that two studies (Al-Obaidi, W. M. L. and Jihad, A.) strongly influenced the meta-analysis results in the same direction (Supplemental Figure S1). However, even after excluding these two studies, there remained a significant decrease in hepcidin levels among PCOS patients (SMD = −1.34, 95% CI = −2.32 to −0.36, p < .05) (Supplemental Figure S2).
The random-effects model showed no significant difference in hepcidin levels between PCOS patients with BMI < 25 and those with BMI ≥ 25 ().
Risk of publication bias
The funnel plot had an asymmetric shape (Supplemental Figure S3), and Egger’s test demonstrated statistical significance for continuous variables (t = −7.59, p < .05), which signals the presence of significant publication bias.
Subgroup analysis
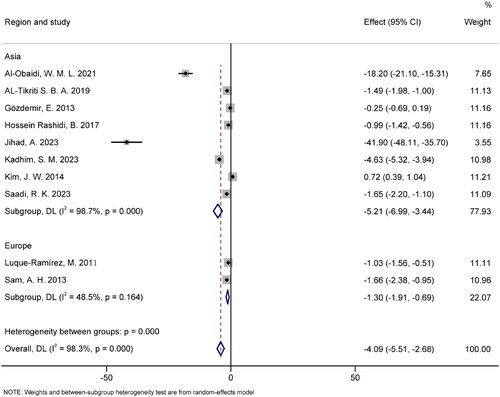
Subgroup analysis was conducted based on region (). The pooled results for the Asia and Europe groups were SMD = −5.21 (95% CI [−6.99, −3.44], p < .05) and SMD = −1.30 (95% CI [−1.91, −0.69], p > .05), respectively. The Asia group exhibited significant heterogeneity (I2 = 98.7%, p < .05), whereas the Europe group demonstrated no significant heterogeneity (I2 = 48.5%, p > .05).
Discussion
Recent studies have found significant changes in hepcidin levels in PCOS patients, which has sparked interest in exploring the relationship between PCOS and hepcidin [Citation14,Citation30]. To the best of our knowledge, this is the first meta-analysis to investigate hepcidin levels in women with PCOS. Our results show that hepcidin levels are significantly reduced in PCOS patients compared to healthy controls. Furthermore, our study demonstrates that hepcidin levels in PCOS patients are not associated with BMI.
Dysregulation of hepcidin, which is linked to phenomena such as inflammation and genetic mutations, underlies iron imbalances in various diseases. Hepcidin is a 25-amino-acid peptide hormone primarily produced by the liver, which serves as the main regulatory hormone for systemic iron homeostasis [Citation9]. By negatively regulating the concentration of transferrin, physiological stimulation or inhibition of hepcidin helps maintain an appropriate plasma iron level. When there is an excess of iron, hepatocytes increase hepcidin production, which results in reduced iron absorption by the duodenum and decreased iron release by macrophages and hepatocytes. Conversely, hepcidin production diminishes during iron deficiency or heightened iron demand, allowing more iron to enter the plasma [Citation9,Citation31].
In addition, IR, along with hyperinsulinemia and hyperandrogenism, is considered the main cause and primary endocrine feature of PCOS; these conditions interact when it comes to in the occurrence and progression of the disorder [Citation2,Citation32]. IR not only promotes obesity but also contributes to the development of functional androgen excess and PCOS [Citation10]. Hepcidin deficiency can lead to iron overload. This excess iron can accumulate in pancreatic islets, resulting in decreased insulin secretion and apoptosis, which ultimately leads to IR [Citation12,Citation33,Citation34]. Recent studies have shown that hepcidin is expressed in pancreatic β cells and is directly influenced by iron levels. Moreover, previous meta-analyses have found that iron and ferritin are significantly elevated in PCOS patients compared to healthy controls [Citation35,Citation36].
Therefore, given the negative regulatory effect of hepcidin on iron, we found that hepcidin was significantly low in women with PCOS, which indicates that decreased hepcidin may be the cause rather than the result of iron overload. This might be because the abnormal reduction of hepcidin leads to iron overload and IR and, then, to the development of PCOS rather than the mechanism of iron overload leading to the up-regulation of hepcidin. The decreased hepcidin levels in PCOS patients suggest that overall reduced hepcidin may be a mechanism linked to IR and iron overload in PCOS.
Reducing inflammation can effectively alleviate clinical symptoms in PCOS patients [Citation37,Citation38]. Inflammation is recognized as a central aspect of this syndrome, with most patients exhibiting low-grade chronic inflammation [Citation39]. C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are typical markers of low-grade inflammation in women with PCOS, and they are strongly associated with IR, obesity and the progression of the condition [Citation39]. Cetuximab has been shown to improve hemoglobin levels and anemia via the IL-6/hepcidin pathway [Citation40]. Therefore, targeting hepcidin levels may improve anemia in PCOS patients [Citation41].
Hepcidin is an acute-phase protein, and obesity is directly associated with inflammation. Under conditions such as obesity and inflammation, hepcidin concentrations are known to increase [Citation42–44]. Hence, we categorized PCOS patients based on BMI using available data from the included literature. Our pooled results indicate that BMI did not have a statistically significant effect on PCOS patients with different BMI values. However, studies have shown that women with PCOS are often obese or overweight, and obesity promotes inflammation and elevated hepcidin [Citation45]. On the one hand, obesity helps the occurrence and development of PCOS and is positively correlated with higher hepcidin [Citation42]. On the other hand, hepcidin is lower than normal in PCOS patients. Therefore, it is unsurprising that there were no significant differences based on BMI.
The diagnosis of PCOS currently poses a challenge due to the absence of specific laboratory biochemical markers. Clinicians heavily rely on clinical symptoms, physical signs, and ultrasound findings for diagnosing this condition. However, such diagnostic criteria have limitations in terms of accuracy and efficiency. Consequently, there is a crucial need to identify specific biochemical markers that can aid in the diagnosis of PCOS. Exploring and discovering these markers has become a key focus in contemporary medical research. Hepcidin, a peptide hormone, plays a pivotal role as the primary negative regulator of iron metabolism. Given the close association between iron metabolism and the risk of PCOS, this meta-analysis revealed that the levels of hepcidin were significantly lower in PCOS patients compared to the control group. This finding suggests that hepcidin could be an inherent characteristic of PCOS. Consequently, hepcidin holds potential as a biomarker for PCOS treatment and could assist in identifying individuals at a higher risk of developing the condition.
Our study has some limitations, which should be acknowledged. First, there is significant heterogeneity in the included studies, possibly due to differences in hepcidin-detection approaches. Although only one of the studies did not use the ELISA method, it is important to note that not all of them used the same ELISA method. Different ELISA methods target different segments of hepcidin, which may account for the significant diversity of results observed among in the included studies. Additionally, most of the studies did not indicate the specific form of hepcidin measured, which is crucial considering that the activity of different fragments can vary. We acknowledge that there are various measurement techniques for hepcidin, which may differ in terms of sensitivity and specificity. Although the measurement methods employed in the included studies adequately addressed the research objectives to some extent, the variability across different methods could still have an impact on the results. To mitigate this issue, we recommend the adoption of standardized measurement methods in future studies to enhance result consistency and comparability.
Second, another potential source of heterogeneity is the variation in patients’ baseline characteristics, such as age and BMI. For example, some studies excluded patients with abnormal weight, while others did not exclude them or did not provide relevant information on BMI. Also, regional differences in diet could also have an impact on hepcidin levels. When we further analyzed the data by region, the European group showed no significant heterogeneity. However, it is important to note that only two studies were from Europe, which limits the generalizability of this finding. Due to a lack of information, our subgroup analysis could only assess a limited number of factors.
Third, as the majority of the included studies were from Asia, the application of our findings to Western populations should be judged carefully due to possible variations in genetics, environment and lifestyles. Furthermore, we recognize that our study is heterogeneous for a variety of reasons, such as regimens, doses, duration, settings and enrolled populations. Because these factors may have significantly affected the consistency of our results, the latter need to be interpreted with care. Hence, it is imperative for future research endeavors to undertake a thorough examination of the impact of regional disparities on hepcidin levels. This necessitates the inclusion of samples from various geographical areas to gain a more nuanced understanding of how environmental, genetic, and lifestyle factors in different regions may influence hepcidin levels. Such a comprehensive approach will serve to improve the generalizability and relevance of the study findings to a broader population.
Fourth, in the meta-analysis, we found that there was significant bias, which had a significant impact on the accuracy and comprehensiveness of the study results. Furthermore, due to the limited number of articles included and the limited amount of data collected, age, weight, and geographic region could also be confounding variables. Therefore, we must be careful when interpreting the results of meta-analysis. To improve the dependability of future research, it is advisable to increase the sample size. Future scholars should strive to conduct studies with larger sample sizes, well-designed methodologies and adjustments for potential confounding factors.
Conclusion
This meta-analysis represents the first attempt to assess hepcidin levels in PCOS patients. Our results show considerably lowered hepcidin levels among women with PCOS compared to healthy controls. However, further high-quality studies are necessary to determine the relationship between serum hepcidin levels and PCOS.
Author contributions
J. N., H. L, H. H., and Q. Y. conceived and designed the study. J. N. and Y. S. performed the data collection, analysis and interpretation of results, and drafted the manuscript. All authors critically reviewed the manuscript and approved the final manuscript.
Supplementary Material.docx
Download MS Word (279.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
Additional information
Funding
References
- Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):1–8. doi: 10.1016/S2213-8587(22)00163-2.
- Wang J, Wu D, Guo H, et al. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940. doi: 10.1016/j.lfs.2019.116940.
- Sadeghi HM, Adeli I, Calina D, et al. Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci. 2022;23(2):583. doi: 10.3390/ijms23020583.
- Fu L, Xie N, Qu F, et al. The association between polycystic ovary syndrome and metabolic syndrome in adolescents: a systematic review and meta-analysis. Reprod Sci. 2023;30(1):28–40. doi: 10.1007/s43032-022-00864-8.
- González-Domínguez Á, Visiedo-García FM, Domínguez-Riscart J, et al. Iron metabolism in obesity and metabolic syndrome. Int J Mol Sci. 2020;21(15):5529. doi: 10.3390/ijms21155529.
- Zhao L, Zhang X, Shen Y, et al. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. 2015;16(12):1081–1093. doi: 10.1111/obr.12323.
- Ni S, Yuan Y, Kuang Y, et al. Iron metabolism and immune regulation. Front Immunol. 2022;13:816282. doi: 10.3389/fimmu.2022.816282.
- Park CH, Valore EV, Waring AJ, et al. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200.
- Nemeth E, Ganz T. Hepcidin and iron in health and disease. Annu Rev Med. 2023;74(1):261–277. doi: 10.1146/annurev-med-043021-032816.
- Escobar-Morreale HF. Iron metabolism and the polycystic ovary syndrome. Trends Endocrinol Metab. 2012;23(10):509–515. doi: 10.1016/j.tem.2012.04.003.
- Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98(15):8780–8785. doi: 10.1073/pnas.151179498.
- Daher R, Lefebvre T, Puy H, et al. Extrahepatic hepcidin production: the intriguing outcomes of recent years. World J Clin Cases. 2019;7(15):1926–1936. doi: 10.12998/wjcc.v7.i15.1926.
- Fernández-Real JM, Equitani F, Moreno JM, et al. Study of circulating prohepcidin in association with insulin sensitivity and changing iron stores. J Clin Endocrinol Metab. 2009;94(3):982–988. doi: 10.1210/jc.2008-1211.
- Luque-Ramírez M, Álvarez-Blasco F, Alpañés M, et al. Role of decreased circulating hepcidin concentrations in the iron excess of women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96(3):846–852. doi: 10.1210/jc.2010-2211.
- Gözdemir E, Kaygusuz I, Kafalı H. Is hepcidin a new cardiovascular risk marker in polycystic ovary syndrome? Gynecol Obstet Invest. 2013;75(3):196–202. doi: 10.1159/000348497.
- Sam AH, Busbridge M, Amin A, et al. Hepcidin levels in diabetes mellitus and polycystic ovary syndrome. Diabet Med. 2013;30(12):1495–1499. doi: 10.1111/dme.12262.
- Kim JW, Kang KM, Yoon TK, et al. Study of circulating hepcidin in association with iron excess, metabolic syndrome, and BMP-6 expression in granulosa cells in women with polycystic ovary syndrome. Fertil Steril. 2014;102(2):548–554.e2. doi: 10.1016/j.fertnstert.2014.04.031.
- Hossein Rashidi B, Shams S, Shariat M, et al. Evaluation of serum hepcidin and iron levels in patients with PCOS: a case-control study. J Endocrinol Invest. 2017;40(7):779–784. doi: 10.1007/s40618-017-0632-z.
- Al-Tikriti SBA, Naji NA. Estimation of the hepcidin level and some Biochemical parameters in patients of polycystic ovary syndrome in Kirkuk city. Tikrit J Pure Sci. 2019;24(4):34–39.
- Al-Obaidi WML, Mhm A-I. Study of hepcidin and many physiological and hematological parameters in women with polycystic qvary in Kirkuk city. Annal Roman Soc Cell Biol. 2021;25:7494–7504.
- Jihad A, Sarhat E. Altered levels of anti-mullerian hormone and hepcidin as potential biomarkers for polycystic ovary syndrome. Georgian Med News. 2023;6(339):47–51.
- Kadhim SM, Al-Fartusie FS, Klichkhanov NK. Evaluation of adiponectin and hepcidin with some biochemical parameters in sera of women with polycystic ovary syndrome. Al-Mustansiriyah J Sci. 2023;34(1):52–57. doi: 10.23851/mjs.v34i1.1299.
- Saadi RK, Alobaidy EJ, Ibrahim HK. Evaluation of some serological indicators in women with polycystic ovary syndrome in Baquba, Iraq. Bangladesh J Med Sci. 2023;22(Special Issue):4–9. doi: 10.3329/bjms.v22i20.66300.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160.
- The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: https://wwwohrica/programs/clinical_epidemiology/oxfordasp.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135.
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Meth Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183.
- Mathew M, Sivaprakasam S, Phy JL, et al. Polycystic ovary syndrome and iron overload: biochemical link and underlying mechanisms with potential novel therapeutic avenues. Biosci Rep. 2023;43(1):BSR20212234. doi: 10.1042/BSR20212234.
- Sangkhae V, Nemeth E. Regulation of the iron homeostatic hormone hepcidin. Adv Nutr. 2017;8(1):126–136. doi: 10.3945/an.116.013961.
- Herman R, Sikonja J, Jensterle M, et al. Insulin metabolism in polycystic ovary syndrome: secretion, signaling, and clearance. Int J Mol Sci. 2023;24(4):3140. doi: 10.3390/ijms24043140.
- Lunova M, Schwarz P, Nuraldeen R, et al. Hepcidin knockout mice spontaneously develop chronic pancreatitis owing to cytoplasmic iron overload in acinar cells. J Pathol. 2017;241(1):104–114. doi: 10.1002/path.4822.
- Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51(8):2348–2354. doi: 10.2337/diabetes.51.8.2348.
- Yin J, Hong X, Ma J, et al. Serum trace elements in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11:572384. doi: 10.3389/fendo.2020.572384.
- Sharma P, Gupta V, Kumar K, et al. Assessment of serum elements concentration and polycystic ovary syndrome (PCOS): systematic review and meta-analysis. Biol Trace Elem Res. 2022;200(11):4582–4593. doi: 10.1007/s12011-021-03058-6.
- Xia Q, Wang W, Liu Z, et al. New insights into mechanisms of berberine in alleviating reproductive disorders of polycystic ovary syndrome: anti-inflammatory properties. Eur J Pharmacol. 2023;939:175433. doi: 10.1016/j.ejphar.2022.175433.
- Jamilian M, Mansury S, Bahmani F, et al. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res. 2018;11(1):80. doi: 10.1186/s13048-018-0457-1.
- Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol. 2011;335(1):30–41. doi: 10.1016/j.mce.2010.08.002.
- Casper C, Chaturvedi S, Munshi N, et al. Analysis of inflammatory and anemia-related biomarkers in a randomized, double-blind, placebo-controlled study of siltuximab (anti-IL6 monoclonal antibody) in patients with multicentric Castleman disease. Clin Cancer Res. 2015;21(19):4294–4304. doi: 10.1158/1078-0432.CCR-15-0134.
- Xu Y, Alfaro-Magallanes VM, Babitt JL. Physiological and pathophysiological mechanisms of hepcidin regulation: clinical implications for iron disorders. Br J Haematol. 2020;193(5):882–893. doi: 10.1111/bjh.17252.
- Tussing-Humphreys LM, Nemeth E, Fantuzzi G, et al. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring). 2010;18(7):1449–1456. doi: 10.1038/oby.2009.319.
- Aguree S, Reddy MB. Inflammatory markers and hepcidin are elevated but serum iron is lower in obese women of reproductive age. Nutrients. 2021;13(1):217. doi: 10.3390/nu13010217.
- Cepeda-Lopez AC, Zimmermann MB, Wussler S, et al. Greater blood volume and Hb mass in obese women quantified by the carbon monoxide-rebreathing method affects interpretation of iron biomarkers and iron requirements. Int J Obes (Lond). 2019;43(5):999–1008. doi: 10.1038/s41366-018-0127-9.
- Toosy S, Sodi R, Pappachan JM. Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord. 2018;17(2):277–285. doi: 10.1007/s40200-018-0371-5.