ABSTRACT
At the onset of the winter breeding season, male humpback whales begin a prominent breeding behaviour, singing. Early songs are produced on summer feeding grounds prior to migration, but little is known about the proximate cues for the initiation of this behaviour, nor where or when it begins. We document the phenology of humpback whale singing along the western North Atlantic coast ranging from Newfoundland and Labrador, Canada to Massachusetts, USA through the fall-winter of 2015–16 (seven stations) and 2016–17 (three stations). Acoustic data from static recorders were categorised as containing humpback whale non-song calls, song fragments, or full songs. First heard in September, singing occurred throughout the fall-winter, but was not regular until October. Latitude, temperature, photoperiod, sea surface pressure, and wind speed were considered as potential explanatory variables for four definitions of song onset using forward stepwise regression. Final models included the environmental variables with photoperiod negatively correlated to singing (coefficient = −657; p-value = 0.04). Reliable environmental cues, such as photoperiod, may produce a heritable physiological response, resulting in whales acquiring the capacity and motivation to sing, with the subsequent timing and nature of song production influenced by other factors.
KEYWORDS:
Introduction
Seasonal breeding is common throughout the animal kingdom. The timing of behavioural and physiological shifts to initiate mating are species-specific, and often population-specific, typically functioning to maximise reproductive potential. Environmental cues can influence the onset of breeding behaviours (e.g. Bubenik et al. Citation1982; Sharp Citation1993; Perret and Aujard Citation2001; Oseen and Wassersug Citation2002). Social interactions, temperature, rainfall, and food can impact mating behaviour timing, with photoperiod often identified as the determinant proximate factor (Lofts and Murton Citation1968; Ball Citation1993; Inai et al. Citation2003; Rosa and Bryant Citation2003). Acoustic signals produced by males, presumably to advertise fitness and attract mates, have received attention as they can be energetically expensive or high risk (e.g. predator or competitor attraction). From insects, to frogs, birds, fish and mammals, seasonal mating vocalisations are heard globally. Here, we investigate the timing of male singing for one of the more well-studied seasonal singers in the ocean, the humpback whale (Megaptera novaeangliae).
Humpback whales are medium-sized mysticete whales distributed globally that make large-scale seasonal migrations from the high-latitudes in the summer to feed to low-latitudes in the winter to mate and give birth, with the exception of the non-migratory Arabian Sea population (Kellogg Citation1929; Bettridge et al. Citation2015). As with many animals, numerous physiological and behavioural changes are observed in male humpback whales at the onset of the breeding season in the fall, beyond undertaking migration. Their testes size (Chittleborough Citation1955), testosterone levels (Cates et al. Citation2019), and aggressive male–male interactions (Tyack and Whitehead Citation1983) all increase with the onset of the breeding season.
Humpback whale vocalisations vary with season and have been most generally categorised as song or non-song. Non-song calls seem to be produced by males, females, adults, and calves throughout the year. A range of non-song vocalisations have been described and are believed to be related to social and feeding behaviours (Cerchio and Dahlheim Citation2001; Dunlop et al. Citation2008; Zoidis et al. Citation2008; Videsen et al. Citation2017). Non-song calls are simple, in that they are not organised into complex patterns (Rekdahl et al. Citation2017). In contrast to non-song calls, songs are produced only by males in association with the breeding season (Payne and McVay Citation1971; Winn and Winn Citation1978). Songs have complex patterns and are hierarchical in structure with discrete units forming phrases, that repeat to create themes, which together make up a song (Payne and McVay Citation1971; Payne et al. Citation1983; Cholewiak et al. Citation2013; Schneider and Mercado III Citation2019). Songs are repeated to form song bouts that can continue for hours (Au et al. Citation2006; Parsons et al. Citation2008). While the ultimate reason for singing is successful reproduction, several proximate functions have been proposed, including to attract females (Winn and Winn Citation1978; Tyack Citation1981), stimulate receptivity in females (Smith et al. Citation2008), form male coalitions (Darling et al. Citation2006), establish dominance (Darling and Bérubé Citation2001), and/or mediate competitive interactions between males as part of a lek mating system (Cholewiak et al. Citation2018). Male song is predominant on warm water breeding grounds, but it also occurs during migration and on the feeding grounds pre and post migration (Mattila et al. Citation1987; McSweeney et al. Citation1989; Magnúsdóttir et al. Citation2014; Kowarski et al. Citation2018, Citation2019). There is some evidence that humpback whales occur at high latitudes throughout the year, suggesting that perhaps not all individuals migrate, or perhaps that not all individuals migrate at the same time (Pomilla et al. Citation2014).
In the western Atlantic a population of humpback whales, thought to be distinct from their eastern Atlantic counterparts (Stevick et al. Citation2006), migrate between their summer feeding grounds that include the waters off eastern Canada and the USA and their winter breeding grounds of the Caribbean (Whitehead and Moore Citation1982; Martin et al. Citation1984; Katona and Beard Citation1990; Palsbøll et al. Citation1997; Smith et al. Citation1999; Jann et al. Citation2003). In the fall and winter, humpback whale songs have been recorded on known western North Atlantic feeding grounds including the Bay of Fundy, Canada, and the Stellwagen Bank National Marine Sanctuary, USA, as well as in the offshore Gully submarine canyon and eastern Scotian Slope of Nova Scotia (Vu et al. Citation2012; Stanistreet et al. Citation2013; Kowarski et al. Citation2018, Citation2019). Kowarski et al. (Citation2019) described the period of song onset in the fall in the Bay of Fundy and defined two categories of singing. The first was ‘song fragments’ which occurred during the early months of song onset and ranged from one phrase or subphrase to two or more themes that are not repeated. The second was ‘songs’ or ‘full songs’ which began later in the season and were defined as three or more themes that are repeated at least once, similar to the classic definitions of song (Frumhoff Citation1983; Cholewiak et al. Citation2013).
Though song onset has been described at one location in the western North Atlantic (Kowarski et al. Citation2019), it is unclear if the patterns observed were representative of typical song onset in other areas or how the onset of singing in the fall occurs over larger spatial and temporal scales. Indeed, we do not know when or where seasonal song onset first occurs in the western North Atlantic nor whether it is driven by any proximate cues as has been described for many land animals (e.g. Ball Citation1993; Meitzen et al. Citation2007). Here, we investigate acoustic recordings collected from static long-term recorders ranging from Labrador, Canada to Massachusetts, USA, over two consecutive fall and winter seasons. We aim to provide a broad scale picture of humpback whale acoustic behaviour during this transitional period and describe where and when humpback whale singing begins. We investigate whether cues such as latitude, temperature, or photoperiod are correlated with the seasonal change in acoustic behaviour. By investigating the onset of singing, we gain further insight into humpback whale song function and shed light on connections between the acoustic behaviour of these animals and their environments.
Methods
Data collection
Passive acoustic monitoring (PAM) data was collected from eight locations (stations) on the seafloor that spanned 10.9° of latitude, ranging from Newfoundland and Labrador, Canada to Massachusetts, USA from the years 2015–2017 ( and ). Data were selected to be included in the present analysis where previous work (e.g. Delarue et al. Citation2018; Kowarski et al. Citation2019) found the recordings captured humpback whale vocalisations in the fall. This was the case for seven stations (1–3 and 5–8) in fall 2015 to winter 2016 (2015–16) and three stations (4, 5, and 7) in fall 2016 to winter 2017 (2016–17) ().
Table 1. Region, timeframe analysed, deployment and retrieval dates, location, and water depth for the eight recording stations (Stn)
Figure 1. Location of eight recording stations located off eastern Canada (2–8) and the United States of America (1)
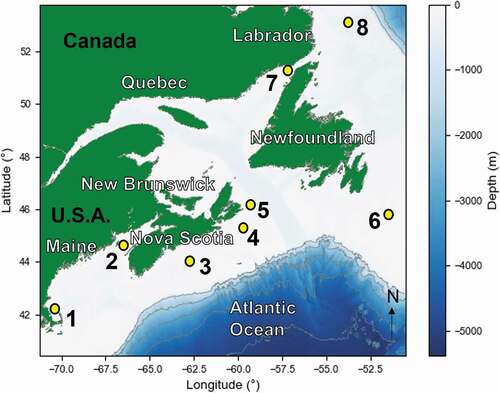
Recordings were collected as part of four different projects coordinated by three different organisations resulting in a range of devices utilised and recording schedules applied that included both low sampling rates (2–16 kHz) and high sampling rates (250–375 kHz) (Detailed recording schedules provided in ). Data from stations 4, 6, 7, and 8 were collected as part of JASCO Applied Sciences (JASCO) Environmental Science Research Fund (ESRF) programme, dedicated to describing the soundscape of the waters off eastern Canada, including the occurrence of vocalising marine mammals (Delarue et al. Citation2018). Stations 3 and 5 were part of the Department of Fisheries and Oceans Canada Maritimes Region ongoing acoustic programme to monitor marine mammal occurrence on the Scotian Shelf and used the same recording scheme as the ESRF data set. We previously analysed the data at station 2 (see 2015 fall in Kowarski et al. Citation2019) that were supplied by JASCO. The Passive Acoustic research Group of the Northeast Fisheries Science Center (NEFSC) of the National Oceanic and Atmospheric Administration (NOAA) provided the data for station 1 which was originally deployed as part of a larger array, primarily aimed at tracking North Atlantic right whales (Eubalaena glacialis). Despite the range of data sources, all recordings were sufficient in duration (10 min cycles to continuous) and sampling frequency (2 to 16 kHz during low frequency cycle) to adequately capture the vocalisations of humpback whales for the purpose of the present study ().
Table 2. Recording equipment specifications and recording schedule for the eight stations
Acoustic analysis
Acoustic data analysis was undertaken on recordings from 1 September 2015 to 31 January 2016 (2015–16; stations 1–3 and 5–8) and 1 September 2016 to 31 January 2017 (2016–17; stations 4, 5, and 7) (). Analysis occurred in two phases, following the protocol described in detail in Kowarski et al. (Citation2019) and summarised here.
In phase 1, humpback whale acoustic occurrence was determined by the systematic review of a portion of data by an experienced analyst using PAMlab software (JASCO). Samples of data were extracted from the central temporal period of the low-frequency recording files (e.g. the middle 60 s of every 8 kHz file was extracted for stations 3–8 resulting in 72 samples 20 min apart each day; ). The manual review of these samples resulted in the analysis of 1.2 h per day to determine humpback whale acoustic occurrence (). By regularly and frequently reviewing short samples, we improved our chances of detecting humpback whale vocalisations, especially their long-duration songs, in comparison to the review of fewer, longer samples (Thomisch et al. Citation2015). Slight variation in sample selection across data sets was necessary due to differences in recording schedules ().
Table 3. Description of samples of acoustic data systematically selected for manual review to determine humpback whale acoustic occurrence
In phase 2, humpback whale acoustic behaviour was categorised as non-song, song fragment, or full song as per the definitions in Kowarski et al. (Citation2019). The file from phase 1 with the highest SNR vocalisation for each six-hour period (00:00–06:00, 06:00–12:00, 12:00–18:00, and 18:00–00:00) was analysed to assign vocal categories to that period. By sampling across different six-hour periods, we strove to find a balance between achieving results at a fine scale while reducing the chance of continually resampling the same individual; however, it is expected that the same vocalising animal was occasionally sampled in consecutive timeframes. Multiple vocal categories could be assigned to the same acoustic file. For example, non-song and full song could be identified within the same timeframe where the analyst either identified signals outside of the pattern of the song or at a different SNR. When multiple songs occurred simultaneously, it became difficult to identify coinciding non-songs; thus, the analysis was biased towards under-identifying non-songs. In the duty cycled data, where the recorders regularly entered sleep mode, it was at times challenging to differentiate song fragments from full songs. Where two or more complete song cycles occurred within one file, the file was categorised as full song. Where the song was so long that two or more full cycles could not be observed within a single file, the analyst would expand analysis to the preceding and following file. If either also had singing, it was assumed the behaviour continued through any periods without recordings and were categorised as full song. Where the adjacent files did not have singing, the vocalisations were categorised as song fragment. Any files with vocalisations too low in SNR to confidently discern a vocal category were categorised as unknown. The vocal categories assigned to each six-hour period of each recording day were plotted for all data sets. A naïve matching test performed on station 2 in Kowarski et al. (Citation2019) revealed that this method of separating humpback whale vocalisations into categories is repeatable with a match rate of 100% between two analysts.
Defining song onset
Defining the onset of humpback whale singing behaviour in the fall required some consideration given the inherent limitations of PAM data. We could not be certain when the first song occurred in that region (e.g. the whale might not have been within range of a recorder), and it was difficult to discriminate song fragments from full songs where data were not recorded continuously. Therefore, for each of the 10 data sets (seven in 2015–16 and three in 2016–17) we had four definitions of song onset:
The first day singing was confirmed
The first day singing became regular
The first day full song was confirmed
The first day full songs became regular.
For this analysis, we considered singing as any type of song behaviour (both song fragments or full song) and ‘regular’ is the first day of five consecutive days with humpback whale full songs or singing present (where days lacking any humpback whale vocalisations were excluded when counting consecutive days). By investigating singing (vs just presence of full songs), any inaccuracies in our interpretation of song fragments vs full song are mitigated. Therefore, singing onset (first occurrence and regular) analysis is the focus of the results as it captures the earliest instance of humpback whales recorded producing organised vocal patterns in the fall and it is less prone to human misclassification. Full song onset (first occurrence or regular) findings are described in the Results and in .
Environmental variable analysis
To investigate correlations between humpback whale song onset in the fall and environmental variables, data were acquired using Environmental Data Management software (EDM; JASCO; Python based) that sourced NOAA’s griddap server. Variables included in this analysis were chosen because they were accessible (wind speed and sea surface pressure), had previously been used in humpback whale habitat suitability models (sea surface temperature and chlorophyll A concentration) (e.g. Bombosch et al. Citation2014), and/or had previously been associated with the onset of singing in other species (day length and latitude) (Lofts and Murton Citation1968; Dawson Citation2013). Wind speed (m/s; 10 m above sea level) and sea surface pressure (kPa; SSP) were supplied by the Fleet Numerical Meteorology and Oceanography Center (FNMOC) at a six-hour resolution of ½ degree. Sea surface temperature (°C; SST) was provided by NASA, providing an average daily SST at 1/100th degree. All data were compiled and averaged daily except for SSP and wind speed that were averaged over two-week periods to account for the large variations in these variables that can occur between days. Chlorophyll A (CHLA) concentration (mg/m3) was fed by the National Aeronautics and Space Administration (NASA) at a daily resolution of 1/24th degree, but with the majority of values recorded as zero, this variable was deemed inappropriate to include in analysis. Day length (photoperiod) for each recording day was sourced from Ark software (JASCO; where Ark uses an algorithm sourced from Blanco-Muriel et al. Citation2001) and was calculated as the number of hours between civil dawn and civil dusk, a definition used in previous studies investigating photoperiod (e.g. Bauchinger and Klaassen Citation2005).
The environmental variables (wind speed, SST, SSP, latitude, day length) at each station were inherently strongly correlated with each other and with day of year. Therefore, values were taken not from the day of song onset, but rather the average day of song onset across recording sites. This was done both as the average of each year separately and as the average across both years (). The variable value was the daily average (on the average day of song onset), except for SSP and wind speed where the averages over a two-week period (centred on the average day of song onset) were investigated. To reduce the impact of multicollinearity between the environmental variables, each SSP, SST, wind speed, and day length value was centred, or standardised, by subtracting the mean of the variable. For each of the four definitions of song onset, a forward stepwise regression was performed (R-studio Version 1.2.5019). Analysis was completed both for variables selected from song onset averaged across years and for each year separately, resulting in a total of eight models considered.
Results
Humpback whales were acoustically present in all analysed data sets (). Vocalisations were present at the onset of the analysis period in September for all data sets except off eastern Labrador (station 8), where vocalisations were not recorded until October 2015. In many areas, humpback whale acoustic occurrence continued until the end of January when analysis ceased. However, vocalisations were absent from eastern Labrador (station 8) and the Bay of Fundy (station 2) by the end of December 2015. The Strait of Belle Isle (station 7) had a marked difference in occurrence between years with vocalisations absent by mid-November 2015 in 2015–16 but continuing until early January 2017 in 2016–17 ().
Figure 2. The occurrence of humpback whale vocalisations in 6 hour periods (00:00–06:00, 06:00–12:00, 12:00–18:00, and 18:00–00:00) for every day with recordings from 1 September 2015 to 31 January 2016 (2015–16) for stations 1–3 and 5–8 and 1 September 2016 to 31 January 2017 (2016–17; red) for stations 4, 5, and 7. Hashed grey periods indicate no recordings. Where multiple acoustic behaviours were present in the same timeframe a single behaviour is presented with the priority of full song, song fragment, non-song, unknown
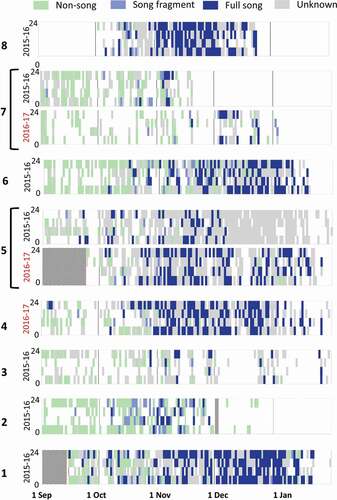
The predominant humpback whale vocal category varied throughout the timeframe analysed with non-songs, song fragments, and full songs observed in all data sets. Non-song calls were the most common vocalisation category from September to November but are likely under-represented in the results once singing began. Song fragments were most common in Oct and Nov. With few exceptions, full songs were the predominant vocal category from November to January ().
Singing could be heard as early as 4 September (song fragment; station 7, 2015–16) but on average, singing began on 29 September and did not become regular until 27 October (; ). For all data sets, a lag was observed between the first day of singing and the first day of regular singing (9–51 days), with an average of 30 days before singing became regular (). Singing never became regular at Emerald Basin where humpback whale vocalisations became sporadic after mid-November (station 3 2015–16) (; ).
Table 4. First day of singing, first day when singing became regular, the number of days between the two for each recording station and year, and the average for both years combined as well as 2015–16 and 2016–17 separately
Figure 3. The average first day of singing and first day when singing became regular in 2015 and 2016 with 95% confidence intervals
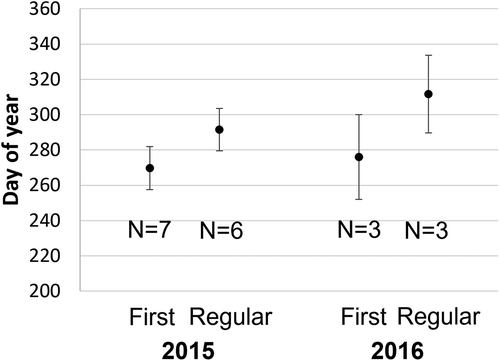
Results varied when comparing singing onset between years. At St. Ann’s Bank (station 5), the timing of singing onset was similar in 2015–16 and 2016–17 (; ). In contrast, singing began notably later in the Strait of Belle Isle (station 7) in 2016–17 as compared to 2015–16 (53 days later for first day of singing and 30 days later for first day of regular singing). Cape Breton South (station 4) 2016–17 did not have recordings from 2015-16, but song onset occurred in a similar manner to nearby stations in 2015–16 (; ).
Forward stepwise regression analysis resulted in three of the final models being statistically significant (). Variables with significant coefficients in these models were day length, SST, and SSP. Day length had a significant negative correlation to first regular singing, both when values were taken for onset averaged across both years (coefficient = −657 ± 241; p-value = 0.04) and averaged separately for each year (coefficient = −99 ± 42; p-value = 0.0496). While never a significant coefficient, latitude is inherently linked to day length and was included in the final model for first regular singing (coefficient = 13 ± 11; p-value = 0.26) with variable values taken from the onset averaged across both years (model p-value = 0.03; ). With the value taken from the onset day averaged across both years, SST was significantly correlated to first regular full song (coefficient = −4 ± 0.9; p-value = 0.02) and first regular singing (coefficient = 14 ± 5; p-value = 0.04). SSP was significantly correlated to first regular full song when the value was taken from onset averaged across both years (coefficient = 39 ± 14; p-value = 0.0493). Though not a significant coefficient, wind speed (coefficient = 4 ± 2; p-value = 0.18) was included in the final model for first regular full song when the values were taken from the onset day averaged across both years (model p-value = 0.04; ).
Table 5. Final models, including coefficient and p-values, of forward stepwise regression for each definition of song onset when environmental variable values (latitude, SST, SSP, wind speed, and day length) were taken from the song onset day averaged across both years and for each year separately
Discussion
Acoustic recordings ranging from Labrador, Canada to Massachusetts, USA captured the onset of humpback whale seasonal singing in fall 2015 at seven stations and fall 2016 at three stations. There are inherent limitations and caveats in our research design that are important to discuss and clarify. The use of omnidirectional static PAM restricted the present study to describing the acoustic behaviour of a group or population of whales, rather than how individual whales began singing. While the analysis protocol was designed to reduce the resampling of the same animal, individuals may well have been resampled over time at the same station or even across stations given the onset of migration south in the fall. With the exception of the Bay of Fundy, where 80 whales were once estimated to feed through the summer (Paquet et al. Citation1997), it is unclear how many whales frequent the other stations in the present study and to what extent whales remain in each area for weeks (increasing the chance of resampling) versus simply moving through during migration (reducing the chance of resampling). Resampling was not necessarily problematic to the present research questions and considering the spatial span of the stations and the restriction to analysing high SNR vocalisations, it is likely that on any day we were sampling different individuals at different stations.
A lack of singing could indicate an absence of males, that males were present but not singing, that males were singing but during times when either recorders were in sleep mode or the data were not analysed, or that singing males were out of detection range of the acoustic recorders, all inherent limitations to PAM. Both the duty cycled recordings and sampling regime during manual analysis were selected, in part, to increase the chance of identifying humpback whale vocalisations based on findings by Thomisch et al. (Citation2015). The chances of missing singing male humpback whales may have decreased throughout the season as songs became more prolific in the data, but even early in the season this species would be rarely missed given their propensity towards regular vocal activity (Thomisch et al. Citation2015). Given the range of soundscape characteristics of the stations (e.g. vessel noise, currents, and water depth that all impact sound propagation) (see ambient analysis in Delarue et al. Citation2018), it is likely that the detectability of humpback whales varied somewhat depending on station and time of year and may account for some of the variability in occurrence across stations. In previous studies using similar recorders and mooring setups offshore Nova Scotia, humpback whale vocalisations were modelled to be detectable at a range of 1 to 75 km in winter and 3 to 100 km in summer, depending on the ambient sound levels (Kowarski et al. Citation2018). The reduced detection range in winter is common in the North Atlantic, and is caused, in part, by an increase in adverse weather conditions, such as high wind speeds. The increase in songs into the winter despite the decrease in detection range, would suggest that the overall trends observed here were not influenced significantly by seasonal variation in detectability. Our analysis protocol and the nature of humpback whale vocalisations biased our results to highlight the occurrence of song over non-song acoustic signals where the former has higher source levels and is more prolific than the latter (Au et al. Citation2006; Dunlop et al. Citation2013). It is unclear whether song fragments have an intermediate source level between full songs and non-songs. The bias towards identifying full song is not problematic for the present study where identifying song onset was the primary objective. Although it is important to recognise the aforementioned caveats, we were able to effectively observe humpback whale vocal behaviour through time despite these inherent limitations.
Occurrence and implications for migratory behaviour
Previously, humpback whales were thought to leave inshore Canadian waters by November and December (Kowarski et al. Citation2019) and make their way south either through a more coastal route such as Massachusetts Bay (Vu et al. Citation2012; Stanistreet et al. Citation2013) or an offshore route (Kowarski et al. Citation2018). They then migrate to the Caribbean where they seem to prefer the waters off the Dominican Republic over the eastern Caribbean (Stevick et al. Citation2018), occurring from December through June (Heenehan et al. Citation2019). In looking at humpback whale northern occurrence on a broader scale, we have concluded that they are common in Canada through January, even in coastal regions. Such was unsurprising in the more southern Massachusetts Bay given previous observations by Vu et al. (Citation2012) and Stanistreet et al. (Citation2013). These findings indicate that individuals undertake migration at different times with some already in the West Indies in December while others remain in Canada and the USA at least through January, undergoing a later migration, short migration, or not migrating at all. Indeed, evidence suggests that in some areas, such as the Grand Banks of Newfoundland (station 6), humpback whales are present year-round (Station 7 in Delarue et al. Citation2018).
In south Labrador, the Strait of Belle Isle, and the Bay of Fundy, humpback whales were acoustically absent by early December, presumably having moved southward. The decrease in humpback whale occurrence during mid-November to early January at the northern stations (South Labrador and the Strait of Belle Isle) coincides with an increase during December and January previously seen in submarine canyons and the eastern continental slope area off Nova Scotia (Kowarski et al. Citation2018), a possible migration route for these northern individuals. It is unclear where the whales from the Bay of Fundy move after seemingly departing in December; they could travel further south through Massachusetts Bay or take an offshore route. Future PAM work in more offshore areas, especially seamounts, such as those found off Bermuda combined with satellite tags, would be informative.
Singing onset and evidence for proximate cues
Humpback whale vocalisations transitioned through the fall from being predominantly non-song to song fragments to full songs as was previously described by Kowarski et al. (Citation2019) irrespective of where the acoustic recorders were located. This was true in both 2015–16 and 2016–17. In some cases, there was inter-annual variability in the timing of song onset, the extent of which could not be assessed given the low sample size (three stations in 2016–17). Considering the potential errors in separating song fragments from full songs in duty cycled data that include sleep mode (all stations but 1 and 2) and the challenge in identifying fragments when full songs are present, the present discussion is focused on singing results, which encapsulates both fragments and full songs. More continuous, acoustic tag or directional data sets in the future may allow these vocalisation categories to be considered separately with more confidence.
There was a lag averaging one month between when singing first occurred (predominantly fragments) and when it became regular (predominantly full songs). These results strongly reinforce the previous findings of a transition period described by Kowarski et al. (Citation2019); this ontogeny of singing behaviour appears to be a consistent feature of at least the humpback whale population in the western North Atlantic. The onset of infrequent singing could reflect one or a combination of the following: variation among individuals with some singing earlier than others, mature males are practicing, immature males are learning, or the behaviour is hormone-induced and testosterone levels have not reached minimal threshold for more regular singing (see discussion of Kowarski et al. (Citation2019)). If the hormonal influence is important, one could expect some environmental cue to trigger the regulatory process as has been described in other species (e.g. Bubenik et al. Citation1982; Asher et al. Citation1989; Smith et al. Citation1997).
The first day of singing occurred over the same time frame at all stations (September–October; average 29 September) despite the wide north-south spread of recorders (spanning 10.9° of latitude). At some stations (Massachusetts Bay and St. Ann’s Bank) singing may have occurred earlier than reported, but there were no data for early September. With the present data, it is impossible to know whether these initial songs are produced rarely by all males or are only produced by few individuals. These behavioural outliers may not be representative of the behavioural shift of the population. Therefore, it is arguably more meaningful to consider the timeframe when singing became regular than when it first occurred. Here, we have defined regular as five days with song, an arbitrary human imposed category. Future work may well identify a better unit for song onset, but we have provided a first attempt at describing these trends.
The onset of humpback whale regular singing ranged from late September to early December (averaging 27 October) and was correlated with day length, SST, and latitude. Day length had a particularly strong correlation, with song onset occurring earlier in the year with longer photoperiods (and therefore lower latitudes). Models defining song onset as first day of full song additionally identified SSP and wind speed as contributing environmental variables. Given the small sample size and the migratory nature of the whales that can move across stations, the present evidence for environmental correlation with song onset, even for only a portion of the song onset definitions, is biologically meaningful. The addition of further data sets in the future will be valuable to fully confirm and understand the observed trends. It is difficult to infer causation from correlation in situ, especially when dealing with non-independent variables during the fall equinox, but we can draw from observations in other taxa.
It has been understood for decades that the number of hours with sunlight in a day affects reproduction in many, if not most, animal species outside the tropics as it provides reliable information regarding upcoming seasonal changes (Lofts and Murton Citation1968; Rojansky et al. Citation1992). In some of the more well-studied species, it has further been found that other variables can accelerate or inhibit the impacts of photoperiod. For example, for birds in temperate regions, photoperiod has been shown to influence the development of song control nuclei in the brain that affect testosterone levels (Lofts and Murton Citation1968; Nottebohm et al. Citation1986; Meitzen et al. Citation2007; Dawson Citation2013). Avian gonadal response to day length can be adjusted by any number of other factors including social interactions, weather, food supply, and temperature (Lofts and Murton Citation1968; Rojansky et al. Citation1992). Similarly, photoperiod has been identified as the determinant factor of seasonal breeding in sheep with temperature, social interactions, and nutrition modulating the timing of breeding (Rosa and Bryant Citation2003).
Humpback whale physiological and behavioural shifts may also be driven by photoperiod and modified by additional variables. All whales in the present study would experience a reliable cue (in the form of shorter photoperiods) to begin behaviours associated with the breeding season during the fall equinox (e.g. southward migration and male singing). As has been well documented in birds, the photoperiod cue may trigger the onset of a heritable physiological response in humpback whales resulting in increased testis size, increased testosterone production, and development of song control processes in the brain (Lofts and Murton Citation1968; Nottebohm et al. Citation1986; Meitzen et al. Citation2007; Dawson Citation2013). Congruent with this suggestion, historical whaling data indicates an increase in humpback whale testes size during the breeding season (Chittleborough Citation1955) and Cates et al. (Citation2019) described a seasonal increase in male humpback whale testosterone levels through the fall in the North Pacific.
Once the physiological processes are triggered, the exact timing of breeding season-related behaviours may then be modified by other variables, such as physical condition of the animal, an important consideration for a migratory species that largely fasts on the breeding grounds. Whales on higher latitude feeding grounds (e.g. Labrador) will undertake longer migrations northward and southward than those on lower latitude feeding grounds (e.g. Massachusetts Bay). With shorter migrations, whales in Massachusetts Bay may arrive back to the feeding grounds earlier in the spring, begin foraging earlier, and as a result, be physically prepared earlier in the fall to begin breeding behaviours when compared to their longer migrating counterparts. Additionally, whales in high latitudes may forage for longer to ensure sufficient reserves for their long migration. Indeed, Szesciorka et al. (Citation2020) concluded that the variable timing of blue whale migrations is linked to prey availability. Warmer temperatures may also allow physiological changes such as testes growth to occur more quickly. Additional variables not explored here could further explain the variability in humpback whale song onset observed across stations such as social interactions in a species where cultural behaviours are well described (Rendell and Whitehead Citation2001).
One population that may shed light on the question of what drives the onset of singing is the non-migratory Arabian Sea humpback whales. Little is known about this genetically distinct population, but they have been found to sing on a Northern Hemisphere seasonal cycle and female reproduction is similarly seasonal based upon examination of foetal lengths in Soviet whaling data, similar to their migratory counterparts (Mikhalev Citation1997; Cerchio et al. Citation2016). Moreover, the population is derived from a founder event originating in the Southern Hemisphere (Pomilla et al. Citation2014), inferring that during the adaptation to residency in the Arabian Sea, they switched breeding cycle to a Northern Hemisphere timing. Therefore, whatever drives timing in breeding must be a strong selective force. Living near the equator, these whales do not experience the variation in light hours, or variation in those variables impacted by light hours, that are experienced by humpback whales foraging in high latitudes. It may therefore be that humpback whales of a given hemisphere begin singing at around the same time due to an internal clock or even social tradition, rather than environmental cues. However, the Arabian Sea humpback whales are exposed to a biannual monsoon season that brings a range of environmental cues, including temperature changes, that could trigger song onset. This juxtaposition of varying proximate cues is seen in bird species where temperate birds respond to photoperiod while equatorial birds respond to other cues such as rainfall (though even tropical birds maintain capacity to be influenced by light in a lab setting) (Lofts and Murton Citation1968).
Conclusions
This study revealed that humpback whale songs are common in coastal regions of the western North Atlantic in September to January. On average, singing began in late September and transitioned over the course of four weeks from song fragments and sporadic full songs to regular singing. Understanding this transition period can inform future PAM analysis techniques and automated detector development and application for a species with a dynamic repertoire that can be challenging to acoustically differentiate from other baleen whales. Management bodies should consider that the prolific occurrence of humpback whale songs from Massachusetts Bay, USA to Labrador, Canada could indicate that these regions are not only important feeding grounds, but also important habitats for onset of mating behaviours prior to migration to breeding habitat. We found evidence that the onset of humpback whale song is correlated with environmental variables, with the most apparent trend being that singing occurs earlier at stations with longer photoperiods. We propose that photoperiod may be a trigger for humpback whale breeding behaviour in the fall, but many other variables, such as temperature, body condition, food availability, and social interactions likely influence the timing of song onset. Future work on other baleen whale species that sing seasonally would be an enlightening addition to this line of research. By understanding where, when, and why whales begin to sing we can piece together the significance of these behaviours, what drives them in terms of proximate and ultimate factors, and how they relate to their environment.
Ethical statement
The present study followed the ethical guidelines for scientific research of Dalhousie University.
Acknowledgements
From JASCO, we would like to acknowledge Briand Gaudet for his continual PAMlab support and innovation, Julien Delarue and Emily Maxner for their input and contributions during manual analysis, Bruce Martin for his ongoing support, and Karen Scanlon for her editorial guidance. Thank you, Andrew Horn and David Barclay of Dalhousie University, for your feedback on the manuscript. Sofie Van Parijs, Genevieve Davis, and Danielle Cholewiak of the NEFSC, and Christopher Tessaglia-Hymes of Cornell University, thank you for sharing the data and information for the Massachusetts Bay data set. Data collected by DFO was supported by DFO Species at Risk Implementation Funds and National Conservation Plan funds.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
For access to the acoustic data used in this study please contact the corresponding author at [email protected].
Additional information
Funding
References
- Asher G, Peterson A, Bass J. 1989. Seasonal pattern of LH and testosterone secretion in adult male fallow deer, Dama dama. Reproduction. 85(2):657–665.
- Au WWL, Pack AA, Lammers MO, Herman LM, Deakos MH, Andrews K. 2006. Acoustic properties of humpback whale songs. J Acoust Soc Am. 120(2):1103–1110.
- Ball GF. 1993. The neural integration of environmental information by seasonally breeding birds. Am Zool. 33(2):185–199.
- Bauchinger U, Klaassen M. 2005. Longer days in spring than in autumn accelerate migration speed of passerine birds. J Avian Biol. 36(1):3–5.
- Bettridge SOM, Baker CS, Barlow J, Clapham P, Ford MJ, Gouveia D, Mattila DK, Pace RM, Rosel PE, Silber GK 2015. Status review of the humpback whale (Megaptera novaeangliae) under the Endangered Species Act.
- Blanco-Muriel M, Alarcón-Padilla DC, López-Moratalla T, Lara-Coira M. 2001. Computing the solar vector. Sol Energy. 70(5):431–441.
- Bombosch A, Zitterbart DP, Van Opzeeland I, Frickenhaus S, Burkhardt E, Wisz MS, Boebel O 2014. Predictive habitat modelling of humpback (Megaptera novaeangliae) and Antarctic minke (Balaenoptera bonaerensis) whales in the Southern Ocean as a planning tool for seismic surveys. Deep Sea Research Part I: Oceanographic Research Papers. 91:101–114.
- Bubenik GA, Morris JM, Schams D, Claus A. 1982. Photoperiodicity and circannual levels of LH, FSH, and testosterone in normal and castrated male, white-tailed deer. Can J Physiol Pharmacol. 60(6):788–793.
- Cates KA, Atkinson S, Gabriele CM, Pack AA, Straley JM, Yin S. 2019. Testosterone trends within and across seasons in Male Humpback Whales (Megaptera novaeangliae) from Hawaii and Alaska. Gen Comp Endocrinol. 279:164–173.
- Cerchio S, Dahlheim ME. 2001. Variation in feeding vocalizations of humpback whales (Megaptera novaeangliae) from Southeast Alaska. Bioacoustics. 11(4):277–295.
- Cerchio S, Willson A, Muirhead C, Minton G, Collins T, Baldwin R, Al Harthi S 2016. Preliminary report on long-term detection of Arabian Sea Humpback Whale vocalizations off Oman. International Whaling Commission. SC/66b/SH/32.
- Chittleborough RG. 1955. Puberty, physical maturity, and relative growth of the female humpback whale, Megaptera nodosa (Bonnaterre), on the Western Australian coast. Mar Freshwater Res. 6(3):315–327.
- Cholewiak DM, Cerchio S, Jacobsen JK, Urbán-R J, Clark CW. 2018. Songbird dynamics under the sea: acoustic interactions between humpback whales suggest song mediates male interactions. J R Soc Open Sci. 5:2.
- Cholewiak DM, Sousa‐Lima RS, Cerchio S. 2013. Humpback whale song hierarchical structure: historical context and discussion of current classification issues. Mar Mamm Sci. 29(3):E312–E332.
- Darling JD, Bérubé M. 2001. Interactions of singing humpback whales with other males. Mar Mamm Sci. 17(3):570–584.
- Darling JD, Jones ME, Nicklin CP. 2006. Humpback whale songs: do they organize males during the breeding season? Behaviour. 143(9):1051–1101.
- Dawson A. 2013. The effect of latitude on photoperiodic control of gonadal maturation, regression and molt in birds. Gen Comp Endocrinol. 190:129–133.
- Delarue JJ-Y, Kowarski KA, Maxner EE, MacDonnell JT, Martin SB 2018.Acoustic monitoring along Canada’s East Coast: August 2015 to July 2017. Dartmouth (NS, Canada). 120 pp + appendices: Technical report by JASCO Applied Sciences for Environmental Studies Research Fund.
- Dunlop RA, Cato DH, Noad MJ. 2008. Non-song acoustic communication in migrating humpback whales (Megaptera novaeangliae). Mar Mamm Sci. 24(3):613–629.
- Dunlop RA, Cato DH, Noad MJ, Stokes DM. 2013. Source levels of social sounds in migrating humpback whales (Megaptera novaeangliae). J Acoust Soc Am. 134(1):706–714.
- Frumhoff PC. 1983. Aberrant songs of humpback whales (Megaptera novaeangliae): clues to the structure of humpback songs. In: Payne R, editor. Communication and behavior of Whales. 1st ed. Boulder (CO): Westview Press for the American Association for the Advancement of Science; p. 81–127.
- Heenehan H, Stanistreet JE, Corkeron PJ, Bouveret L, Chalifour J, Davis GE, Henriquez A, Kiszka J, Kline L, Reed C. 2019. Caribbean Sea soundscapes: monitoring humpback whales, biological sounds, geological events, and anthropogenic impacts of vessel noise. Front Mar Sci. 6:347.
- Inai Y, Nagai K, Ukena K, Oishi T, Tsutsui K. 2003. Seasonal changes in neurosteroid concentrations in the amphibian brain and environmental factors regulating their changes. Brain Res. 959(2):214–225.
- Jann B, Allen J, Carrillo M, Hanquet S, Katona SK, Martin AR, Reeves RR, Seton R, Stevick PT, Wenzel FW. 2003. Migration of a humpback whale (Megaptera novaeangliae) between the Cape Verde Islands and Iceland. J Cetacean Res Manage. 5(2):125–130.
- Katona SK, Beard JA. 1990. Population size, migrations and feeding aggregations of the humpback whale (Megaptera novaeangliae) in the western North Atlantic Ocean. Rep Int Whaling Commission. 12( Special Issue):295–306.
- Kellogg R. 1929. What is known of the migrations of some of the whalebone whales. Washington: US Government Printing Office.
- Kowarski KA, Evers C, Moors‐Murphy H, Martin B, Denes SL. 2018. Singing through winter nights: seasonal and diel occurrence of humpback whale (Megaptera novaeangliae) calls in and around the Gully MPA, offshore eastern Canada. Mar Mamm Sci. 34(1):169–189.
- Kowarski KA, Moors-Murphy H, Maxner EE, Cerchio S. 2019. Western North Atlantic humpback whale fall and spring acoustic repertoire: insight into onset and cessation of singing behavior. J Acoust Soc Am. 145(4):2305–2316.
- Lofts B, Murton RK. 1968. Photoperiodic and physiological adaptations regulating avian breeding cycles and their ecological significance. J Zool. 155(3):327–394.
- Magnúsdóttir EE, Rasmussen MH, Lammers MO, Svavarsson J. 2014. Humpback whale songs during winter in subarctic waters. Polar Biol. 37(3):427–433.
- Martin AR, Katona SK, Matilla D, Hembree D, Waters TD. 1984. Migration of humpback whales between the Caribbean and Iceland. J Mammal. 65(2):330–333.
- Mattila DK, Guinee LN, Mayo CA. 1987. Humpback whale songs on a North Atlantic feeding ground. J Mammal. 68(4):880–883.
- McSweeney DJ, Chu KC, Dolphin WF, Guinee LN. 1989. North Pacific humpback whale songs: a comparison of southeast Alaskan feeding ground songs with Hawaiian wintering ground songs. Mar Mamm Sci. 5(2):139–148.
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. 2007. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 27(44):12045–12057.
- Mikhalev YA. 1997. Humpback whales Megaptera novaeangliae in the Arabian Sea. Mar Ecol Prog Ser. 149:13–21.
- Nottebohm F, Nottebohm ME, Crane L. 1986. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol. 46(3):445–471.
- Oseen KL, Wassersug RJ. 2002. Environmental factors influencing calling in sympatric anurans. Oecologia. 133(4):616–625.
- Palsbøll PJ, Allen J, Bérube´ M, Clapham PJ, Feddersen TP, Hammond PS, Hudson RR, Jørgensen H, Katona S, Larsen AH, et al. 1997. Genetic tagging of humpback whales. Nature. 388(6644):767–769.
- Paquet D, Haycock C, Whitehead H. 1997. Numbers and seasonal occurrence of humpback whales, Megaptera novaeangliae, off Brier Island, Nova Scotia. Can Field-Nat. 111(4):548–552.
- Parsons ECM, Wright AJ, Gore MA. 2008. The nature of humpback whale (Megaptera novaeangliae) song. J Mar Anim Ecol. 1(1):22–31.
- Payne K, Tyack PL, Payne R. 1983. Progressive changes in the songs of humpback whales (Megaptera novaeangliae): a detailed analysis of two seasons in Hawaii. In: Payne R, editor. Communication and behavior of whales. Boulder (CO): Westview Press; p. 9–57.
- Payne RS, McVay S. 1971. Songs of humpback whales. Science. 173(3997):585–597.
- Perret M, Aujard F. 2001. Regulation by photoperiod of seasonal changes in body mass and reproductive function in gray mouse lemurs (Microcebus murinus): differential responses by sex. Int J Primatol. 22(1):5–24.
- Pomilla C, Amaral AR, Collins T, Minton G, Findlay K, Leslie MS, Ponnampalam L, Baldwin R, Rosenbaum H. 2014. The world’s most isolated and distinct whale population? Humpback whales of the Arabian Sea. PLoS ONE. 9(12):e114162.
- Rekdahl M, Tisch C, Cerchio S, Rosenbaum H. 2017. Common nonsong social calls of humpback whales (Megaptera novaeangliae) recorded off northern Angola, southern Africa. Mar Mamm Sci. 33(1):365–375.
- Rendell LE, Whitehead H. 2001. Culture in whales and dolphins. Behav Brain Sci. 24(2):309–324.
- Rojansky N, Brzezinski A, Schenker JG. 1992. Seasonality in human reproduction: an update. Hum Reprod. 7(6):735–745.
- Rosa HJD, Bryant MJ. 2003. Seasonality of reproduction in sheep. Small Rumin Res. 48(3):155–171.
- Schneider JN, Mercado III E. 2019. Characterizing the rhythm and tempo of sound production by singing whales. Bioacoustics. 28(3):239–256.
- Sharp PJ. 1993. Photoperiodic control of reproduction in the domestic hen. Poult Sci. 72(5):897–905.
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. 1997. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 17(15):6001–6010.
- Smith JN, Goldizen AW, Dunlop RA, Noad MJ. 2008. Songs of male humpback whales, Megaptera novaeangliae, are involved in intersexual interactions. Anim Behav. 76:467–477.
- Smith TD, Allen J, Clapham PJ, Hammond PS, Katona S, Larsen F, Lien J, Mattila D, Palsbøll PJ, Sigurjónsson J, et al. 1999. An ocean-basin-wide mark-recapture study of the North Atlantic humpback whale (Megaptera novaeangliae). Mar Mamm Sci. 15(1):1–32.
- Stanistreet JE, Risch D, Van Parijs SM. 2013. Passive acoustic tracking of singing humpback whales (Megaptera novaeangliae) on a Northwest Atlantic feeding ground. PLOS One. 8:4.
- Stevick PT, Allen J, Clapham PJ, Katona SK, Larsen F, Lien J, Mattila DK, Palsbøll PJ, Sears R, Sigurjonsson J. 2006. Population spatial structuring on the feeding grounds in North Atlantic humpback whales (Megaptera novaeangliae). J Zool. 270(2):244–255.
- Stevick PT, Bouveret L, Gandilhon N, Rinaldi C, Rinaldi R, Broms F, Wenzel F. 2018. Migratory destinations and timing of humpback whales in the southeastern Caribbean differ from those off the Dominican Republic. J Cetacean Res Manag. 18:127–133.
- Szesciorka AR, Ballance LT, Širović A, Rice A, Ohman MD, Hildebrand JA, Franks PJ. 2020. Timing is everything: drivers of interannual variability in blue whale migration. Sci Rep. 10(1):1–9.
- Thomisch K, Boebel O, Zitterbart DP, Samaran F, Van Parijs SM, Van Opzeeland I. 2015. Effects of subsampling of passive acoustic recordings on acoustic metrics. J Acoust Soc Am. 138:267–278.
- Tyack PL. 1981. Interactions between singing Hawaiian humpback whales and conspecifics nearby. Behav Ecol Sociobiol. 8(2):105–116.
- Tyack PL, Whitehead H. 1983. Male competition in large groups of wintering humpback whales. Behaviour. 83(1/2):132–154.
- Videsen SKA, Bejder L, Johnson M, Madsen PT. 2017. High suckling rates and acoustic crypsis of humpback whale neonates maximise potential for mother–calf energy transfer. Funct Ecol. 31(8):1561–1573.
- Vu ET, Risch D, Clark CW, Gaylord S, Hatch LT, Thompson MA, Wiley DN, Van Parijs SM. 2012. Humpback whale (Megaptera novaeangliae) song occurs extensively on feeding grounds in the Northwest Atlantic Ocean. Aquat Biol. 14(2):175–183.
- Whitehead H, Moore MJ. 1982. Distribution and movements of West Indian humpback whales in winter. Can J Zool. 60(9):2203–2211.
- Winn HE, Winn LK. 1978. The song of the humpback whale Megaptera novaeangliae in the West Indies. Mar Biol. 47(2):97–114.
- Zoidis AM, Smultea MA, Frankel AS, Hopkins JL, Day A, McFarland AS, Whitt AD, Fertl D. 2008. Vocalizations produced by humpback whale (Megaptera novaeangliae) calves recorded in Hawaii. J Acoust Soc Am. 123(3):1737–1746.
Appendix
Table A1. First day of full song, first day when full song became regular, the number of days between the two for each recording station and year, and the average for both year combined as well as 2015–16 and 2016–17 separately
Table A2. Average day of onset of full songs and singing for 2015 and 2016 considered independently and together
