ABSTRACT
Signal detection is a fundamental requirement for any communicative interaction. Acoustic signals, however, experience amplitude losses during their transmission through the environment, reducing their detection range. Displaying from sites that increase the amplitude of the sound produced, such as cavities or some reflective surfaces, can improve the detectability of signals by distant receivers. We measured the effect of leaf calling sites on the calls of an arboreal (Hyalinobatrachium fleischmanni) and a leaf-litter specialist (Silverstoneia flotator) frog species. We collected the leaves where males of both species were observed calling, and conducted playback experiments to measure their effect on the amplitude of frog calls. Overall, the leaves used by H. fleischmanni and S. flotator were of similar dimensions, and amplified the calls of each species by about 5.0 and 2.5 dB, respectively. The degree of call amplification was unrelated to leaf dimensions or the position of the frogs on the leaves, but explained by the different frequency content of the calls of each species. We suggest that amplification of frogs calls by leaves could represent either a benefit or impose costs for arboreal and terrestrial species, which may depend on the spatial location of intended and unintended receivers.
Introduction
In animals, communication by means of sounds is widespread and crucially involved in activities such as mate choice, rival competition and parent-offspring interactions. Communication comprises three interacting components: a sender that produces the signal, the environment through which it propagates, and a receiver that perceives the sound (Bradbury and Vehrencamp Citation2011). Under this framework, the transmission environment is generally considered to impose constraints to communication. Propagating signals will experience a number of changes, including the modification of their acoustic structure (i.e. changes in their temporal and spectral attributes) and the loss of amplitude (Morton Citation1975; Wiley and Richards Citation1978; Ryan and Kime Citation2003). Spectro-temporal alterations can jeopardise the ability of receivers to recognise the signal and respond accordingly (Slabbekoorn Citation2004), but the loss of amplitude can have even more profound consequences for communication. This is because the ability of receivers to detect a signal is tightly linked to its amplitude, and signal detection is a fundamental step in any communicative interaction. If receivers fail to detect the presence of a signal, then communication will be completely hindered irrespective of other possible alterations in the sound structure.
The range over which a signal can be detected is mainly determined by the interplay of four variables: the amplitude of the signal at the source, the degree of environmental attenuation, the noise level at the receiver’s position, and the hearing sensitivity and tuning of the receiver (Marten and Marler Citation1977; Brumm and Slabbekoorn Citation2005). Environmental attenuation of acoustic signals is a well-described phenomenon. The amplitude of a sound decreases with increasing distance from the source, even in free-field conditions where there are no objects between the sender and receiver (Wiley and Richards Citation1978; Forrest Citation1994). However, the presence of sound reflective and absorptive elements (e.g. vegetation) is a typical feature of natural environments. These objects will induce additional amplitude losses to propagating sound, especially in the high frequency range (Wiley and Richards Citation1978). Additionally, sounds produced close to the ground often experience particularly high attenuation rates due to refractions caused by temperature and pressure layers (Wiley and Richards Citation1978; Arak and Eiriksson Citation1992; Mathevon et al. Citation1996; Bradbury and Vehrencamp Citation2011; Schwartz et al. Citation2016). Animals could, thus, reduce signal attenuation and improve detection distances simply by producing louder sounds, producing them at lower frequencies, or from elevated display sites.
Louder signals are not only more likely to be detected, but can also influence mate choice and male-male interactions. Acoustic signals presented at higher amplitude are preferred by females in a number of taxa (e.g. Gerhardt Citation1987; McKibben and Bass Citation1998; Ritschard et al. Citation2010). Importantly, females are known to discriminate relatively small amplitude differences. For example, females of many frog species are estimated to discriminate amplitude differences of 2–5 dB between two signals (see Bee et al. Citation2012 for a summary). In some frogs, the vocal responses of males are stronger to louder signals (e.g. Penna et al. Citation2017). However, high amplitude signals can also be detected and preferred by unintended receivers (e.g. Tuttle and Ryan Citation1981; Gomes et al. Citation2017). Acoustically-guided predators and parasites will impose costs on the senders and thus are expected to counterbalance some of the mating benefits of high amplitude signalling.
While habitat-dependent attenuation can limit signal detection, other environmental features can enhance sound broadcast. In some species, signallers can use, build, or modify display sites that result in louder signals. For example, the burrows occupied by some frogs and crickets enhance the amplitude of their songs (e.g. Bennet-Clark Citation1987; Muñoz and Penna Citation2016). While these cavities seem rather complex display sites, other apparently simpler structures have the potential to generate similar effects. The surface of leaves, for example, are used by many insects and frogs for sound broadcast and could act as sound reflectors. Indeed, recent studies show that leaves have relevant acoustic properties across a surprisingly wide range of ecological contexts. Singing tree-crickets, for example, produce louder songs by creating so-called baffles on leaves (Mhatre et al. Citation2017), whereas gleaning bats rely on ultrasonic reflections to detect motionless prey resting on leaves (Geipel et al. Citation2019).
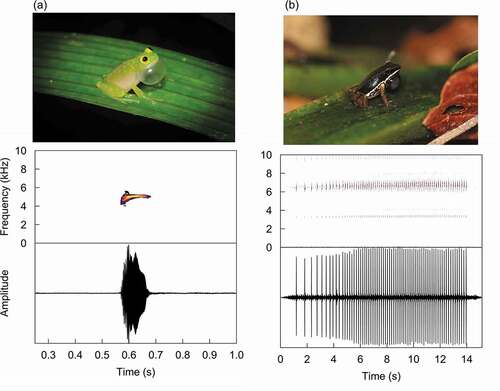
Male frogs advertise their presence to potential mates and rivals using vocalisations. Different species broadcast these vocalisations from specific substrates, such as from the water, cavities, or the branches and leaves of trees. Here, we studied the effect of leaf calling sites on the call amplitude of two tropical species: a glass frog (Hyalinobatrachium fleischmanni) and a rocket frog (Silverstoneia flotator). Hyalinobatrachium fleischmanni belongs to the family Centrolenidae, and males vocalise during the night from leaves on trees and bushes next to streams (). Males can either call on the top or the underside of leaves (Delia et al. Citation2010). On the other hand, S. flotator belongs to the family Dendrobatidae, and males call during the morning and afternoon while sitting on top of leaves in the leaf litter (). Besides different activity periods and calling heights, males of both species share a number of reproductive behaviours, like the defence of territories where calling, mating, oviposition and paternal care take place (Savage Citation2002). The males of these frog species often come back to the same calling leaves during consecutive days, and thus are suitable candidates to study the acoustic consequences of calling from leaves.
Materials and methods
Study area and leaf collection
The territories of male H. fleischmanni (N = 25) and S. flotator (N = 22) were surveyed between June and July 2019 in Barro Colorado Island (BCI), Panamá. Males were photographed at their calling sites and placed on a nearby leaf. Then, we collected the leaves used for calling and noted the exact position of males using a permanent marker. Leaves were transported to the facilities of the Smithsonian Tropical Research Institute in BCI for measurements. To reduce possible changes in the shape of leaves or other relevant properties, all the measurements were done within a few hours after collection.
Leaf dimensions and frog calling position
Each leaf was photographed next to a reference scale, and using ImageJ (Schneider et al. Citation2012) we measured three variables related to their dimensions: maximum leaf width (cm), maximum leaf length (cm), and leaf area (cm2). First, we rotated the photographs to vertically align the apex and base of the leaf. The maximum widths and lengths were obtained from measuring the sides of a rectangle encompassing the apex, the base, and both sides of the rotated leaf image. The centre of the leaf was defined as the geometric centre of this rectangle. To measure leaf area, photographs were transformed to black and white and the colour threshold adjusted until the leaf area was discriminated from the background. Finally, we also measured two variables related to the position of the calling males on the leaves: distance to leaf centre (cm), and distance to nearest leaf edge (cm). These two measures were obtained by drawing a circle centred on the frog position and measuring its radius until reaching the centre and the nearest edge of the leaf.
Acoustic stimuli creation
We assessed the acoustic properties of leaves using pure tones and natural advertisement calls. A sequence of 0.25 s duration tones from 2.0 to 15.0 kHz in 0.5 kHz steps was created using Audacity 2.2.2. The relative amplitude of each tone was equalised to correct for the frequency response of the speaker used for the playbacks.
Advertisement calls of H. fleischmanni (N = 10 individuals) and S. flotator (N = 10 individuals) were recorded along the trails and streams of BCI. Vocalisations were recorded at a distance of 50 cm from either the front or side of the males using a microphone (G.R.A.S. 1/2 inch 46AE, flat frequency response between 3.15 Hz to 20 kHz) connected to a digital recorder (Zoom H6). For each recorded H. fleischmanni individual we randomly selected 10 calls which were included in the audio file used for acoustic testing (). Because the call of S. flotator consists of long sequences of rapidly repeated short pulses, a single vocalisation of each recorded individual was included in the audio file (). The peak frequency of H. fleischmanni (N = 100 calls from 10 individuals) and S. flotator (N = 10 calls from 10 individuals) calls was (mean±SD) 5.19 ± 0.16 and 6.46 ± 0.33, respectively.
Acoustic properties of leaves
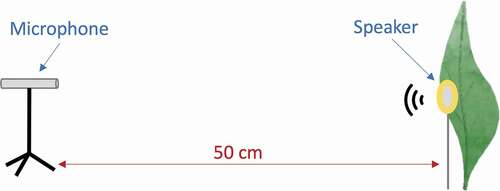
To measure the acoustic properties of leaves we employed a disk-shaped piezoelectric speaker (diameter: 35 mm, KEPO FT-35T-2.6A1-475, see the speaker frequency response in Figure S1) connected to a mini audio amplified (PAM8610) and a laptop computer (Apple Inc.). The piezoelectric was mounted on a thin vertical wire and placed at 50 cm from the G.R.A.S microphone (). We covered the wall behind the piezoelectric, and the ground path between the microphone and the piezoelectric with acoustic absorbing foam to reduce unwanted sound reflections.
In each trial, a leaf was placed behind the speaker at a distance of about 0.5 cm from its back surface (), mimicking the position of a frog sitting on the leaf surface. The piezoelectric was centred at the exact position where the frog was observed calling, and care was taken not to alter the position of the piezoelectric relative to the microphone while placing and removing the leaf. Pure tones and frog calls were broadcast with the leaf in this position and recorded with the microphone. To compare the amplitude of tones and calls in the absence of the leaf, we obtained a baseline recording of these sounds before placing and also after removing the leaves from the set up. Leaves were tested only with the calls of the species observed calling on them, and the leaves used by H. fleischmanni were tested on the same side (upper or underside) where males were observed calling.
Acoustic analyses
We measured the root-mean-square (RMS) amplitude of tones and calls using RavenPro 1.5 (Center for Conservation Bioacoustics Citation2014). For tones, recordings were band-stop filtered between 0 and 1.0 kHz to reduce the presence of low frequency sounds, and a 0.15 seconds segment from the middle of each tone was selected for RMS amplitude measurements. For the H. fleischmanni and S. flotator calls we measured the RMS amplitude in the 4.0–6.0 kHz and 5.0–8.0 kHz frequency bands, respectively. For each leaf we computed the amplitude gain of tones and calls using the following equation: Amplitude gain (dB) = 20*log10(RMSleaf/RMSno leaf). Where RMSleaf corresponds to RMS amplitude measurements obtained with the tested leaf placed behind the piezoelectric buzzer, and RMSno leaf corresponds to RMS amplitude values measured before placing the leaf. Positive amplitude gains indicate amplification due to the presence of the leaf, while negative values indicate attenuation. The variation between the two baseline measurements was minimal, and thus we only report results obtained using the values obtained at the beginning of each trial.
To validate our experimental approach and acoustic analyses, we performed acoustic tests using an optimal sound reflector (i.e. a 13 cm diameter polystyrene disk). These tests showed that amplitude gains are largely independent of the frequency response of the speaker, and that relative (i.e. obtained using an uncalibrated microphone) and absolute (i.e. obtained with an SPL-meter) amplitude measurements are equivalent approaches to compute amplitude gains (see Supplementary Material).
Statistical analyses
All the statistical analyses were performed in R (version 3.6.3, R Core Team Citation2020). The five measures of leaf dimensions and frog position were compared between the two studied species using unpaired two-sample t-tests, or Wilcoxon rank sum tests if the normality assumption was not met. Additionally, to reduce the number of leaf dimension and frog position variables for further analyses, we used principal component analysis on the correlation matrix using the base function prcomp(). The five leaf measures were log10-transformed, centred and scaled for this analysis. Only principal components with eigenvalues >1 were considered.
The amplification of pure tones was analysed using generalised additive models (GAM) with the library ‘mgcv’ (version 1.8–33, Wood Citation2011). We fitted a GAM model with gaussian distribution and identity link function. We included the amplitude gain (dB) as dependent variable and the tone frequency (kHz) as a by-species smooth predictor. Leaf identity was included as a random smooth spline in the models to account for the repeated measures obtained on a same leaf. Model estimates and 95% confidence intervals were plotted using the library ‘mgcViz’ (version 0.1.6, Fasiolo et al. Citation2018), and used to visually evaluate significant amplification or attenuation of specific frequencies. Additionally, to compare the profile of tone amplification between both species we used spectral cross-correlation implemented in the R package ‘seewave’ (version 2.1.4, Sueur et al. Citation2008). Spectral cross-correlation is often used to compare the frequency spectra of two sounds, and here we used it to compare the mean tone amplification profiles of glass frog and rocket frog leaves. Cross-correlation coefficients go from −1 to +1, and are interpreted in a similar way as regular correlation coefficient. A cross-correlation coefficient of +1 indicates that the two spectra are identical.
To evaluate whether the amplitude gains of advertisement calls differed from 0 dB (i.e. no effect of leaves on call amplitude) we used one-sample t-tests, or one-sample Wilcoxon signed rank test if normality assumption was not met.
To evaluate differences in call amplification between both species we used two-sample Wilcoxon rank sum test. Male H. fleischmanni called from either the top or the underside of leaves. We compared the amplitude gains of leaves where males called on top and the underside using two-sample Wilcoxon rank sum test.
We evaluated associations between the leaf dimensions and call amplification levels using ANCOVA. We fitted two ANCOVA models, one for each principal component obtained from the leaf dimensions analysis. In both models we included amplitude gain as the dependent variable, and the species and principal component (either PC1 or PC2) as independent variables. We initially included the statistical interaction between species and principal component as independent variable, but these terms were not significant and were dropped from the final models reported. For all the linear models fitted (GAMs and ANCOVAs) we visually inspected the residuals to evaluate deviations from normality.
Mean and standard deviation amplitude gain values of tones and calls were obtained using the meandB() and sddB() functions in the R package ‘seewave’ (version 2.1.4, Sueur et al. Citation2008).
Results
Leaf dimensions and frog calling positions
Male H. flotator called from leaves with a larger area and wider than male H. fleischmanni (). In contrast, leaves used by both species had similar lengths, and males of both species were equally distanced from the centre of leaves (). Male S. flotator called further away from the edges of leaves relative to male H. fleischmanni ().
Table 1. Dimensions of the leaves used male H. fleischmanni (N = 25) and S. flotator (N = 22) for calling. Results of statistical tests used to compare the dimensions of leaves used by both species are also shown. Values in the table correspond to mean ± SD
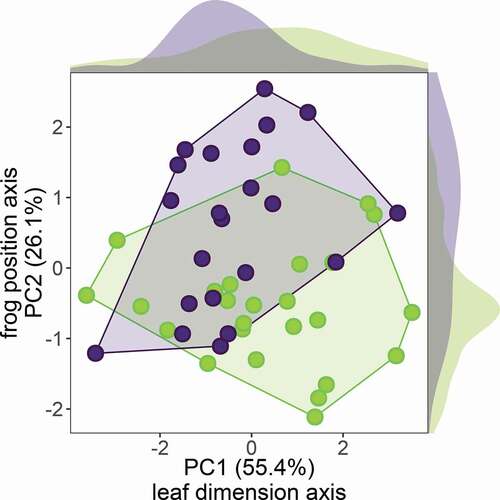
The first two principal components explained 81.5% of the variation in leaf dimensions and frog calling position, and were the only components with eigenvalues >1 ( and ). The PC1 was inversely correlated with leaf width, length, area, and the distance of the frog to the centre of the leaves (). The PC2 was positively correlated with the distance of males to the edge, and moderately and inversely correlated with the distance of males to the centre of the leaves (). Overall, there was large overlap between the PC scores of both species, although glass frogs that called from small leaves (i.e. large PC1 scores) tended to do it closer to the leaf edges (i.e. small PC2 scores) as compared to the rocket frogs ().
Table 2. Principal component analysis of leaf dimensions and frog calling position measured for H. fleischmanni and S. flotator.
Amplification of pure tones
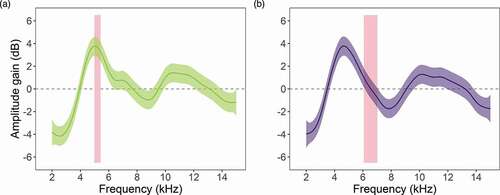
For both species, tones in the 4.0–6.0 kHz frequency range experienced larger amplification than other frequencies, and deviated from 0 dB as judged by the estimated 95% confidence intervals. This frequency band overlaps with the peak frequency range of H. fleischmanni calls ()), but not the calls of S. flotator (). The leaves of both species showed similar pure tone amplification profiles, as indicated by a large and significant spectral cross-correlation coefficient between them (Spearman’s rho = 0.748, P < 0.001, frequency offset = 0 kHz).
Figure 4. Amplitude gain of tones measured for leaves used by (a) H. fleischmanni and (b) S. flotator. Solid lines and shaded areas represent fitted amplitude gains and 95% confidence intervals estimated from the generalised additive model. The red vertical bars represent the range of call peak frequencies of each species
Amplification of calls
The calls of H. fleischmanni (N = 100 calls from 10 individuals) were amplified by (mean±SD) 4.93 ± 1.39 dB, and the calls of S. flotator (N = 10 calls from 10 individuals) by 2.44 ± 1.53 dB. For both species, call amplitude gains significantly deviated from 0 dB (H. fleischmanni: one-sample Wilcoxon signed rank test, W = 324, P < 0.001; S. flotator: one-sample t-test, t = 6.44, d.f. = 21, P < 0.001), and thus leaves effectively amplified the calls. The comparison between both species showed that H. fleischmanni calls are amplified to a larger extent than S. flotator vocalisations (Two-sample Wilcoxon rank sum test, W = 505, P < 0.001).
For H. fleischmanni, in 48% (12/25) of the leaves collected males called from the top, and in the rest (13/25) they called from the underside. The amplitude gains measured for the calls were not affected by the side of the leaf used by males (Two-sample Wilcoxon rank sum test, W = 52, P = 0.277).
Relationship between leaf dimension, frog position and call amplification
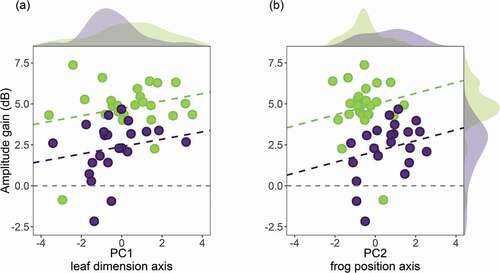
Not leaf dimensions (i.e. PC1) nor male positions (i.e. PC2) explained the amplitude gain measured for the calls of both species ( and ). Male H. fleischmanni calls were amplified to a larger extent than S. flotator vocalisations, even when accounting for the variation in leaf dimensions or male position between both species ( and ).
Table 3. Summary of ANCOVA analyses used to evaluate the effect of leaf dimension and frog position on call amplitude gains
Discussion
In the present study, we measured the effect of leaf calling sites on the call amplitude of two tropical frogs, an arboreal glass frog and a terrestrial rocket frog species. Overall, there was large overlap between the dimensions of the calling leaves used by both species, although there were some differences between the position of males on their surface. The leaves used by male H. fleischmanni amplified the calls of this species by about 5.0 dB, while S. flotator leaves moderately amplified their calls by about 2.5 dB. The playback of pure tones showed for the leaves used by both species a frequency band of increased amplification between 4–6 kHz. This band matches the frequency content of H. fleischmanni calls better than that of S. flotator’s. Thus, our results indicate that the different amplitude gains measured for the two species is related to differences in the frequency of their calls, and not in the acoustic properties on the leaves they call from.
Our experimental setup simulated the effect of leaves on the amplitude of calls as perceived by a receiver located above of the calling frog (i.e. located along the vertical plane). Because rocket frogs are typically associated to the leaf-litter, amplification above a calling male could facilitate the detection by unintended receiver located above the ground. For example, some birds or parasitic flies could detect these males more easily if their calls are amplified above them, although bird predation on rocket frogs has been reported to be extremely low at the studied locality (Poulin et al. Citation2001).
Unlike other colourful dendrobatids, the brown dorsal colouration of rocket frogs matches well with the surrounding leaf litter, and this species lacks the skin toxins characteristic of other representatives in the family (Mebs et al. Citation2018). On the contrary, the ventral surface and vocal sac of rocket frogs are lightly coloured and conspicuous on the horizontal plane (i.e. when viewed from the forest floor), where other conspecific males and females are located. Altogether these traits suggest that being undetectable to aerial eavesdroppers is advantageous for this terrestrial species, and therefore the leaf amplification effect we measured could impose costs for calling males.
Calling from leaves that amplify predominantly on the horizontal than the vertical plane would favour mate attraction over attracting unintended receivers in terrestrial species. Given that the attenuation of high-frequency sounds can be severe close to the ground (Wiley and Richards Citation1978), such an effect would be highly beneficial for calling males. Whether leaves also improve sound beaming to the frogs’ horizontal plane is unknown, although there is some evidence of this effect for frogs calling from leaves (Narins and Hurley Citation1982; Wells and Schwartz Citation1982) and singing crickets (Erregger and Schmidt Citation2018). Precisely measuring (e.g. Erregger and Schmidt Citation2018) or modelling (e.g. Mhatre et al. Citation2017) the effect of leaves on call radiation along multiple planes would help to elucidate the adaptive role of signalling from these substrates.
Unlike rocket frogs, the arboreal habits of glass frogs indicate that females and unwanted eavesdroppers can be found almost anywhere in the environment around calling males. Frogs calling from the underside will radiate more energy towards the ground, while frogs calling from the top will radiate more energy on the direction of the canopy (Wells and Schwartz Citation1982, this study). Indeed, Wells and Schwartz (Citation1982) measured the amplitude of glass frog calls recorded at both sides of calling leaves (i.e. the side where the calling frog was sitting and the opposite unoccupied side). In agreement with our playback experiments, they found that calls are about 6 dB louder on the male side relative to the opposite side (Wells and Schwartz Citation1982). Although the spatial location of females attending to glass frog choruses is unclear, it has been reported that males calling from higher positions achieve higher reproductive success than males close to the ground (Greer and Wells Citation1980). This increased success could be explained by favourable above-ground sound propagation (Wiley and Richards Citation1978), but could also indicate that females approach calling males from the canopy. Therefore, by calling from elevated positions and from the top of leaves male glass frogs could improve detectability by females, although they may be also more exposed to predators and parasites.
Bats are the most likely acoustic eavesdroppers of glass frogs, although katydids are also known to predate on them (Delia et al. Citation2017). The fringe-lipped bat (Trachops cirrhosus) predates on calling frogs, and is attracted by the calls of H. fleischmanni (Tuttle and Ryan Citation1981). Thus, leaf sound reflections that enhance the detection by females may also increase the risk of predation by bats. Male glass frogs calling from the underside of leaves are less exposed to bat attacks. Indeed, geographic variation in the proportion of glass frog calling from the underside is related to the presence of T. cirrhosus (Delia et al. Citation2010). Thus, by flexibly changing the side of the leaf used for calling, male glass frogs can reduce the risk of predation, but also radiate more energy towards the ground, which could decrease the probability of detection by females. Monitoring the effects of males flexibly moving between different sides of calling leaves, and measuring their survival as well as reproductive output would contribute to understand how these frogs balance the attraction of potential mates and predators.
The dimensions of the leaves used by both frog species did not affect the amplification of their calls. Although initially puzzling, as we expected larger leaves to reflect more, we find support for this result in the measurements done by Wells and Schwartz (Citation1982) on glass frogs (see discussion above). In agreement with our measurements, the amplitude difference they measured between both leaf sides (about 6 dB) was the same for small and large leaves (Wells and Schwartz Citation1982). It is possible, however, that leaf dimensions are relevant for other organisms and bioacoustic phenomena. Some tree crickets, for example, build acoustic baffles by chewing a hole at the centre of leaves (Prozesky-Schulze et al. Citation1975). Males call while sitting at this hole, where the leaf physically separates the front and back surfaces of the stridulating wings, therefore reducing destructive interferences between soundwaves travelling forward and backwards (Mhatre et al. Citation2017). Larger leaves reduce destructive interferences to a larger extent, and therefore are better at improving sound radiation (Mhatre et al. Citation2017). Also, leaf properties (e.g. rugosity or furriness) could potentially affect the detection capacities of some gleaning bats, which rely on leaf reflections to detect silent and motionless preys sitting on them (Geipel et al. Citation2019). Altogether, these examples indicate that leaf properties may have unprecedented consequences for the attraction of mates, communication with rivals, and even prey detection by echolocating predators.
The scale at which animals interact with their environment for effective communication needs to be investigated at many different levels. Here we show that the leaves used by a terrestrial and an arboreal frog species amplify their calls, but argue that this effect needs to be considered in relation to the broad-scale environment, and also the ecological singularities of different species. Animals can display from diverse sites in the environment, yet they often select specific locations, and the adaptive significance of these choices are just beginning to be understood.
Supplemental Material
Download PDF (214.4 KB)Acknowledgements
We are especially grateful to the Smithsonian Tropical Research Institute staff on Barro Colorado Island for logistical support and warm attitude during data collection. We thank one anonymous reviewer for commenting on a previous version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Supporting data are available at https://doi.org/10.6084/m9.figshare.16529163.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Arak A, Eiriksson T. 1992. Choice of singing sites by male bushcrickets (Tettigonia viridissima) in relation to signal propagation. Behav Ecol Sociobiol. 30:365–372. doi:https://doi.org/10.1007/BF00176170.
- Bee MA, Vélez A, Forester JD. 2012. Sound level discrimination by gray treefrogs in the presence and absence of chorus-shaped noise. J Acoust Soc Am. 131:4188–4195. doi:https://doi.org/10.1121/1.3699271.
- Bennet-Clark HC. 1987. The tuned singing burrow of mole crickets. J Exp Biol. 128:383–409. doi:https://doi.org/10.1242/jeb.128.1.383.
- Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication. 2nd ed. Sunderland (MA): Sinauer Associates.
- Brumm H, Slabbekoorn H. 2005. Acoustic communication in noise. Adv Study Behav. 35:151–209.
- Center for Conservation Bioacoustics. 2014. RavenPro: interactive sound analysis software [computer software]. Version 1.5. Ithaca (NY): The Cornell Lab of Ornithology. Available from http://ravensoundsoftware.com/
- Delia J, Bravo-Valencia L, Warkentin KM. 2017. Patterns of parental care in Neotropical glassfrogs: fieldwork alters hypotheses of sex-role evolution. J Evol Biol. 30:898–914.
- Delia J, Cisneros-Heredia DF, Whitney J, Murrieta-Galindo R. 2010. Observations on the reproductive behavior of a Neotropical glassfrog, Hyalinobatrachium fleischmanni (Anura: Centrolenidae). South Am J Herpetol. 5:1–12. doi:https://doi.org/10.2994/057.005.0101.
- Erregger B, Schmidt AKD. 2018. Anthropogenic calling sites boost the sound amplitude of advertisement calls produced by a tropical cricket. Anim Behav. 142:31–38. doi:https://doi.org/10.1016/j.anbehav.2018.05.021.
- Fasiolo M, Nedellec R, Goude Y, Wood SN. 2018. Scalable visualization methods for modern generalized additive models. ArXiv preprint arXiv:1809.10632.
- Forrest TG. 1994. From sender to receiver: propagation and environmental effects on acoustic signals. Am Zool. 34:644–654. doi:https://doi.org/10.1093/icb/34.6.644.
- Geipel I, Steckel J, Tschapka M, Vanderelst D, Schnitzler HU, Kalko EKV, Peremans H, Simon R. 2019. Bats actively use leaves as specular reflectors to detect acoustically camouflaged prey. Curr Biol. 29:2731–2736.e3. doi:https://doi.org/10.1016/j.cub.2019.06.076.
- Gerhardt HC. 1987. Evolutionary and neurobiological implications of selective phonotaxis in the green treefrog, Hyla cinerea. Anim Behav. 35:1479–1489. doi:https://doi.org/10.1016/S0003-3472(87)80020-9.
- Gomes DGE, Halfwerk W, Taylor RC, Ryan MJ, Page RA. 2017. Multimodal weighting differences by bats and their prey: probing natural selection pressures on sexually selected traits. Anim Behav. 134:99–102. doi:https://doi.org/10.1016/j.anbehav.2017.10.011.
- Greer BJ, Wells KD. 1980. Territorial and reproductive behavior of the tropical American frog Centrolenella fleischmanni. Herpetologica. 36:318–326.
- Marten K, Marler P. 1977. Sound transmission and its significance for animal vocalization - I. Temperate habitats. Behav Ecol Sociobiol. 2:271–290. doi:https://doi.org/10.1007/BF00299740.
- Mathevon N, Aubin T, Dabelsteen T. 1996. Song degradation during propagation: importance of song post for the wren Troglodytes troglodytes. Ethology. 102:397–412. doi:https://doi.org/10.1111/j.1439-0310.1996.tb01135.x.
- McKibben JR, Bass AH. 1998. Behavioral assessment of acoustic parameters relevant to signal recognition and preference in a vocal fish. J Acoust Soc Am. 104:3520–3533. doi:https://doi.org/10.1121/1.423938.
- Mebs D, Yotsu-Yamashita M, Pogoda W, Vargas Alvarez J, Ernst R, Köhler G, Toennes SW. 2018. Lack of alkaloids and tetrodotoxin in the neotropical frogs Allobates spp. (Aromobatidae) and Silverstoneia flotator (Dendrobatidae). Toxicon. 152:103–105. doi:https://doi.org/10.1016/j.toxicon.2018.07.027.
- Mhatre N, Malkin R, Deb R, Balakrishnan R, Robert D. 2017. Tree crickets optimize the acoustics of baffles to exaggerate their mate-attraction signal. Elife. 6:e32763. doi:https://doi.org/10.7554/eLife.32763.
- Morton ES. 1975. Ecological sources of selection on avian sounds. Am Nat. 109:17–34. doi:https://doi.org/10.1086/282971.
- Muñoz MI, Penna M. 2016. Extended amplification of acoustic signals by amphibian burrows. J Comp Physiol A. 202:473–487. doi:https://doi.org/10.1007/s00359-016-1093-0.
- Narins PM, Hurley DD. 1982. The relationship between call intensity and function in the Puerto Rican Coqui (Anura: Leptodactylidae). Herpetologica. 38:287–295.
- Penna M, Moreno-Gómez FN, Muñoz MI, Cisternas J. 2017. Vocal responses of austral forest frogs to amplitude degradation patterns of advertisement calls. Behav Processes. 140:190–201. doi:https://doi.org/10.1016/j.beproc.2017.05.008.
- Poulin B, Lefebvre G, Ibáñez R, Jaramillo C, Hernández C, Rand SA. 2001. Avian predation upon lizards and frogs in a neotropical forest understory. J Trop Ecol. 17:21–40. doi:https://doi.org/10.1017/S026646740100102X.
- Prozesky-Schulze L, Prozesky OPM, Anderson F, Van Der Merwe GJJ. 1975. Use of a self-made sound baffle by a tree cricket. Nature. 255:142–143. doi:https://doi.org/10.1038/255142a0.
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. https://www.R-project.org/ .
- Ritschard M, Riebel K, Brumm H. 2010. Female zebra finches prefer high-amplitude song. Anim Behav. 79:877–883. doi:https://doi.org/10.1016/j.anbehav.2009.12.038.
- Ryan MJ, Kime NM. 2003. Selection on long-distance acoustic signals. In: Simmons AM, Fay RR, Popper AN, editors. Acoustic communication. Springer handbook of auditory research. Vol. 16. New York: Springer; p. 225–274.
- Savage JM. 2002. The amphibians and reptiles of Costa Rica: a herpetofauna between two continents, between two seas. Chicago and London: University of Chicago Press.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9:671–675. doi:https://doi.org/10.1038/nmeth.2089.
- Schwartz JJ, Hunce R, Lentine B, Powers K. 2016. Calling site choice and its impact on call degradation and call attractiveness in the gray treefrog, Hyla versicolor. Behav Ecol Sociobiol. 70:1–19. doi:https://doi.org/10.1007/s00265-015-2016-8.
- Slabbekoorn H. 2004. Singing in the wild: the ecology of birdsong. In: Marler P, Slabbekoorn H, editors. Nature’s music: the science of birdsong. San Diego (CA): Elsevier Academic Press; p. 178–205.
- Sueur J, Aubin T, Simonis C. 2008. Equipment review: seewave, a free modular tool for sound analysis and synthesis. Bioacoustics. 18:213–226. doi:https://doi.org/10.1080/09524622.2008.9753600.
- Tuttle MD, Ryan MJ. 1981. Bat predation and the evolution of frog vocalizations in the Neotropics. Science. 214:677–678. doi:https://doi.org/10.1126/science.214.4521.677.
- Wells KD, Schwartz JJ. 1982. The effect of vegetation on the propagation of calls in the Neotropical frog Centrolenella fleischmanni. Herpetologica. 38:449–455.
- Wiley RH, Richards DG. 1978. Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav Ecol Sociobiol. 3:69–94. doi:https://doi.org/10.1007/BF00300047.
- Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B Stat Methodol. 73:3–36. doi:https://doi.org/10.1111/j.1467-9868.2010.00749.x.