To the Editor
These days the application range of antiplatelet therapy is expanding, because activated platelets are implicated in various clinical conditions. We recently reported that the serum levels of soluble P-selectin and thromboxane (TX) B2 were elevated in lung cancer patients treated with gefitinib and that low-dose aspirin improved the associated skin eruption, a common adverse effect of gefitinib Citation[1]. Although we were unable to fully clarify the mechanisms involved, we suggested that epithelial growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) may activate platelets and contribute to several related adverse effects. On the other hand, erlotinib is the new approved EGFR-TKI and is administered as an oral medicine. Erlotinib has been reported to improve the survival rate of patients with non-small cell lung cancer (NSCLC) after first- or second-line chemotherapy Citation[2] and to significantly improve the patients’ quality of life Citation[3]. However, skin rash and diarrhea are common adverse effects of EGFR-TKIs including erlotinib Citation[3]. Here, we describe two NSCLC patients who received erlotinib therapy and developed a skin rash, and were measured for their serum levels of TXB2. We finally used low-dose aspirin to treat the skin rash in both patinets. To the best of our knowledge, this is the first report that aspirin is effective for skin rash after erlotinib therapy.
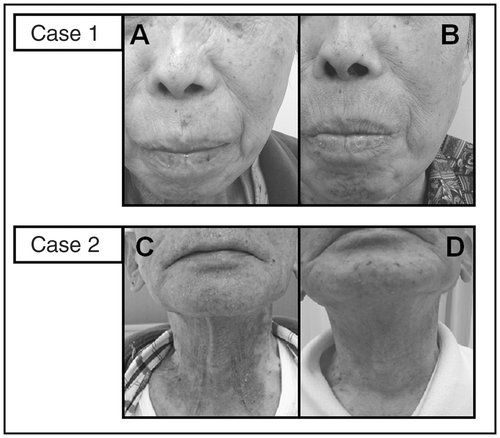
Case 1 was a 69-year-old Japanese female who was diagnosed with adenocarcinoma (stage VI). Although conventional chemotherapy (carboplatin and paclitaxel) was performed, tumor markers increased again. Therefore, erlotinib (150 mg/day) was started. At first, skin lesions below grade 1 with acne developed. However, the skin lesions progressed to grade 2 () with severe itching. Low-dose aspirin (100 mg/day) was started with the patient's consent without dose reduction of erlotinib. The skin lesions were ameliorated () with improved itching. Case 2 was a 77-year-old Japanese male and was diagnosed with squamous cell carcinoma (stage IIIb) without metastasis. He also did not respond to conventional chemotherapy (docetaxel hydrate). After 1 week of treatment with erlotinib, a grade 2 skin rash developed () with severe itching. This case also observed the improvement of skin rash after low-dose aspirin treatment (). In both cases, TXB2 was elevated after erlotinib treatment, and low-dose aspirin finally exhibited the decrease of TXB2. Our results suggest that the mechanism of the skin rash development after erlotinib treatment involves platelet activation.
Figure 1. Case 1, (A) the skin lesions progressed to grade 2 with severe itching. (B) The skin lesions were ameliorated with improved itching after low-dose aspirin was started without dose reduction of erlotinib. Case 2, (C) After 1 week of treatment with erlotinib, a grade 2 skin rash developed with severe itching. (D) The skin lesions were ameliorate with improved itching after low-dose aspirin was started without dose reduction of erlotinib.
Cyclooxygenase (COX) must play an important role in platelet activation by erlotinib because TXA2 is synthesized by platelets through COX. Low-dose aspirin mainly inhibits COX-1, and has a weak inhibitor effect on COX-2. Therefore, we propose that inhibition of COX-1 is very important as a preventative measure against skin rash development after erlotinib administration. Our only concern is that low-dose aspirin may have a negative association with the therapeutic effect of erlotinib for lung cancer. If platelet activation is related to the anti-cancer effect the use of aspirin would decrease the effect of the EGFR-TKI. However, our previous study with gefitinib suggested that the effects of aspirin through inhibition of platelet COX-1 reduced the adverse effects of gefitinib but not its anti-cancer effects [Citation[4–6]].
The anti-cancer effects of EGFR-TKIs differ among races Citation[7]. In addition, their effects on platelet functions may differ among races. The anti-cancer effects of gefitinib at less than its maximum tolerated dose also differ among races. One of the awkward diverse effects of EGFR-TKIs in Japanese patients is interstitial pneumonitis Citation[8]. Recently Nomura et al. Citation[9] reported that platelets were activated in scleroderma patients with interstitial pneumonitis. This observation suggests that platelet activity is one of the factors for the interstitial pneumonitis associated with EGFR-TKI therapy. We hope that low-dose aspirin prevents the adverse effects of erlotinib and that we will be able to use EGFR-TKIs more safely in cancer patients. Additional randomized studies are required to determine the usefulness of our new therapy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kanazawa S, Yamaguchi K, Kinoshita Y, Muramatsu M, Komiyama Y, Nomura S. Aspirin reduces adverse effects of gefitinib. Anticancer Drugs 2006; 17: 423–427
- Shepherd FA, Pereira JR, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, et al. Erlotinib in previously treated non small cell lung cancer. N Eng J Med 2005; 353: 123–132
- Bezjak A, Tu D, Seymour L, Clark G, Trajkovic A, Zukin M, Ayoub J, Lago S, de Albuquerque Riberiro R, Gerogianni A, et al. Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2006; 24: 3831–3837
- Kanazawa S, Yamaguchi K, Kinoshita Y, Muramatsu M, Komiyama Y, Nomura S. Gefitinib affects functions of platelets and blood vessels via changes in prostanoids balance. Clin Appl Thrombosis/Hemostasis 2005; 11: 429–434
- Kanazawa S, Yamaguchi K, Kinoshita Y, Komiyama Y, Muramatsu M, Nomura S. Elevation of soluble interleukin-2 receptor in patients with non-small cell lung cancer treated with gefitinib. J Cancer Res Clin Oncol 2006; 132: 719–725
- Kanazawa S, Muramatsu M, Kinoshita Y, Yamaguchi K, Nomura S. Gefitinib has the potential of activating cell immunity against malignant cells. J Clin Oncol 2005; 23: 3865–3866
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Dauillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, et al. Multi institutional randomized phase trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 2003; 21: 2237–2246
- Okamoto I, Fujii K, Matsumoto M, Terasaki Y, Kihara N, Kohrogi H, Suga M. Diffuse alveolar damage after ZD1839 therapy in a patient with non-small cell lung cancer. Lung Cancer 2003; 40: 339–342
- Nomura S, Inami N, Ozaki Y, Kagawa H, Fukuhara S. Significance of microparticles in progressive systemic sclerosis with interstitial pneumonia. Platelets 2008; 19: 192–198
