Abstract
Patients on P2Y12 inhibitors may still develop thrombosis or bleeding complications. Tailored antiplatelet therapy, based on platelet reactivity testing, might reduce these complications. Several tests have been used, but failed to show a benefit of tailored antiplatelet therapy. This could be due to the narrowness of current platelet reactivity tests, which are limited to analysis of platelet aggregation after stimulation of the adenosine diphosphate (ADP)-pathway. However, the response to ADP does not necessarily reflect the effect of P2Y12 inhibition on platelet function in vivo. Therefore, we investigated whether measuring platelet reactivity toward other physiologically relevant agonists could provide more insight in the efficacy of P2Y12 inhibitors.
The effect of in vitro and in vivo P2Y12 inhibition on αIIbβ3-activation, P-selectin and CD63-expression, aggregate formation, release of alpha, and dense granules content was assessed after stimulation of different platelet activation pathways. Platelet reactivity measured with flow cytometry in 72 patients on P2Y12 inhibitors was compared to VerifyNow results.
P2Y12 inhibitors caused strongly attenuated platelet fibrinogen binding after stimulation with peptide agonists for protease activated receptor (PAR)-1 and -4, or glycoprotein VI ligand crosslinked collagen-related peptide (CRP-xl), while aggregation was normal at high agonist concentration. P2Y12 inhibitors decreased PAR-agonist and CRP-induced dense granule secretion, but not alpha granule secretion. A proportion of P2Y12-inhibitor responsive patients according to VerifyNow, displayed normal fibrinogen binding assessed with flow cytometry after stimulation with PAR-agonists or CRP despite full inhibition of the response to ADP, indicating suboptimal platelet inhibition.
Concluding, measurement of platelet fibrinogen binding with flow cytometry after stimulation of thrombin- or collagen receptors in addition to ADP response identifies different patients as nonresponders to P2Y12 inhibitors, compared to only ADP-induced aggregation-based assays. Future studies should investigate the value of both assays for monitoring on-treatment platelet reactivity.
Introduction
Platelets are the first to respond when the integrity of the vasculature is breached. They quickly adhere to sites of vascular damage, where they aggregate and form a platelet plug that prevents further blood loss. During platelet activation, intracellular alpha and dense granules fuse with the platelet membrane, leading to P-selectin expression and the release of multiple pro-inflammatory and vasoactive substances into the local environment. This causes secondary platelet activation and promotes interaction between the activated platelets and the endothelium or white blood cells [Citation1–Citation3]. Although the formation of a platelet plug is essential for the prevention of bleeding, an excessive platelet response can lead to vascular occlusion and downstream tissue ischemia.
For this reason, patients with severe atherosclerosis are treated with different classes of platelet inhibitors, ranging from relatively mild antiplatelet drugs (COX-1 inhibitors) to a combination of COX-1 inhibitors and P2Y12 inhibitors, which induces stronger platelet inhibition. Clopidogrel is the mildest and most commonly prescribed P2Y12 inhibitor, whereas prasugrel, ticagrelor, and cangrelor are prescribed when stronger platelet inhibition is required. Currently, there is no evidence-based approach for choosing the best P2Y12 inhibitor in individual patients.
Although large-scale clinical studies have shown that P2Y12 inhibitors reduce thrombosis incidence in severe atherosclerotic patients, some patients are still insufficiently protected and develop thromboembolic events, while other patients on P2Y12 inhibitors may develop bleeding complications [Citation4, Citation5]. This suggests that there is a therapeutic window for platelet reactivity with insufficient platelet inhibition at one end of the spectrum and excessive platelet inhibition at the other end. Monitoring of platelet reactivity in patients on platelet inhibitors may prevent future thromboembolic and bleeding complications, although level I evidence from large randomized controlled trials is lacking.
The most widely used platelet reactivity test in clinical trials and daily practice is the VerifyNow P2Y12 assay [Citation6, Citation7]. This assay specifically addresses the effect of treatment with P2Y12 inhibitors on the platelets adenosine diphosphate (ADP) pathway through two separate aggregation measurements: (1) ADP/prostaglandin E1-induced aggregation to determine the effect of P2Y12 inhibition on platelet function and (2) thrombin receptor-activating peptide (TRAP)-induced platelet aggregation to determine baseline P2Y12 inhibitor-free platelet activation [Citation8]. These two measurements are then used to calculate the percentage of platelet inhibition by clopidogrel. The premise of this system is that TRAP-induced platelet activation is unaffected by P2Y12 inhibitors. However, multiple studies have shown inhibitory effects of these drugs on PAR-1 and PAR-4-mediated platelet aggregation, both in platelet-rich plasma (PRP) and in whole blood [Citation8–Citation11].
So far, there is no convincing evidence that tailoring antiplatelet therapy based on platelet reactivity tests is effective [Citation12–Citation14]. This is not surprising, as the effect of P2Y12 blockade on ADP-induced platelet aggregation does not necessarily reflect the platelet response toward either collagen or thrombin, which are physiologically essential during platelet plug formation. Loss of thrombin activity greatly inhibits the formation of the thrombus core region and thus attenuates full platelet activation [Citation15].
Here, we investigated whether assessment of the platelet response to thrombin or collagen-mimetics allows identification of poor responders to P2Y12 inhibitors. To this end, we compared the effects of P2Y12 inhibition on several activation pathways and validated our findings in a cohort of patients receiving antiplatelet therapy.
Methods
Reagents
Adenosine 5′-diphosphate (ADP), apyrase, and phosphoenolpyruvate (PEP) were from Sigma-Aldrich (A2754-1G, A6132-1KU, and 10108294001, Zwijndrecht, the Netherlands). Pyruvate kinase (PK) was from Roche (10128155001, Almere, the Netherlands). PAR-1 agonist SFLLRN was from Bachem (H-2936, Weil am Rhein, Germany) and PAR-4 agonist AYPGKF was synthesized at the Dutch Cancer Institute (NKI-AVL, Amsterdam, the Netherlands). Cross-linked collagen-related peptide (CRP-xl) was a generous gift from Professor Richard Farndale (Cambridge, United Kingdom). HORM collagen was obtained from Hormon Chemie (10500, Boom BV, Meppel, the Netherlands). Phycoerythrin (PE)-labeled mouse antihuman P-selectin (AK-4) and Alexa Fluor 647 (Alexa647)-labeled mouse antihuman CD63 (H5C6) were from BD Pharmingen™ (55524 and 561983 Franklin Lakes, NJ, USA). Fluorescein isothiocyanate (FITC)-labeled antihuman fibrinogen antibodies were obtained from Dako (F0111, Glostrup, Denmark). P2Y12 antagonist AR-C69931MX (cangrelor) was kindly provided by AstraZeneca (Loughborough, United Kingdom).
Optilyse-B was from Beckman Coulter (IM1400, Woerden, the Netherlands). Antibodies against human platelet factor 4 (PF4), β-thromboglobulin (β-TG), chemokine ligand 5 (RANTES/CCL5), and platelet-derived growth factor (PDGF) A/B were from R&D systems (AF795, BAF393, AB-278-NA, and DY222, Minneapolis, MN, USA). Prostacyclin was from Cayman Chemicals (18220, MI, USA).
Patients
Blood of 72 consecutive patients on P2Y12 inhibitors visiting the vascular surgery department of the University Medical Center Utrecht was collected in 0.105 M tri-sodium citrate tubes (454321, Greiner Bio-One Vacuette) via venipuncture. Both VerifyNow responses and platelet reactivity as determined with flow cytometry were measured in the same sample. The type of P2Y12 inhibitor and indication for monitoring was recorded. Data were processed anonymously. Similar measurements for flow cytometry were performed for 43 consecutive healthy controls. Flow cytometry measurements of the 72 patients were normalized to the results of the 43 healthy controls. In the absence of an evidence-based cutoff value for the ideal platelet inhibition measured by VerifyNow, we arbitrarily adopted P2Y12 reactive units (PRU) < 208 for clopidogrel as indicators of good response to clopidogrel based on previous studies [Citation16–Citation18].
Blood from the 43 healthy controls, who claimed not to have taken antiplatelet therapy, was obtained through venipuncture and collected in 0.105 M tri-sodium citrate (367714, Becton Dickinson, Temse, Belgium) vacuum tubes.
Approval for this study was obtained from the medical ethics review board of the UMC Utrecht. Informed consent requirement for patients on P2Y12 inhibitors was waived by the Institutional Review Board. All healthy donors gave written informed consent in accordance with the declaration of Helsinki.
Assessment of platelet activation with flow cytometry
Blood was diluted 1:10 in a reaction mixture containing a platelet agonist and FITC-conjugated antibodies against fibrinogen (1:100), PE-conjugated antibodies against P-selectin (1:25), and Alexa647-conjugated antibodies against CD63 (1:25). Platelets were activated with ADP, CRP-xl, PAR-1 agonist SFLLRN or PAR-4 agonist AYPGKF in the presence or absence of 1-µM AR-C69931MX or 0.2-U/mL apyrase. After 20 min, samples were fixed in buffer containing 0.148% formaldehyde, 137 mM NaCl, 2.7 mM KCl, 1.12 mM NaH2PO4, 1.15 mM KH2PO4, 10.2 mM NaHPO4, and 4 mM EDTA (pH 6.8) and analyzed on a BD FACS Canto II flow cytometer (BD-biosciences, San Jose, CA, USA) on the same day of processing. Single platelets were gated based on forward and side scatter properties. Fibrinogen binding was used as a measure of αIIbβ3 activation and P-selectin- and CD63 expression as markers of granule release. Data are expressed as median fluorescence intensity (MFI) for dose–response curves or normalized to the median MFI values obtained in 43 healthy controls. The normalized data were used to calculate the percentage inhibition of platelet reactivity in P2Y12 inhibitor users as compared to healthy controls without antiplatelet therapy. We used an in-house cutoff for poor platelet reactivity, set at the 2.5th percentile of the response in 122 healthy donors.
Light transmission aggregometry
PRP was prepared by centrifugation of whole blood at 160 g for 15 min at room temperature. The supernatant was collected in a new tube. The remaining blood was centrifuged for 10 min at 2000 g and this supernatant (plasma) was used to adjust the PRP to a platelet count of approximately 200–250 × 109/L. Platelet aggregation was assessed after addition of ADP (2 or 10 µM), PAR-1 agonist (2 or 10 µM), PAR-4 agonist (100 or 250 µM), and CRP-xl (12.5 or 200 ng/mL) in the presence or absence of 1-μM AR-C69931MX on a Chronolog Optical Lumi-Aggregometer (Havertown, PA, USA). Aggregation was monitored for 15 min at 37°C at 900 rpm.
Release of alpha granule content
Washed platelets were stimulated with 156-µM PAR-1 agonist with or without 1-µM AR-C69931MX for 20 min. Platelets were centrifuged at 4000g for 2 min and the supernatant was collected. ELISA’s were performed on the supernatant for PF-4, β-TG, RANTES/CCL5, and PDGF-A/B as described before [Citation19]. In short, plates were coated with capture antibodies overnight at 4°C and subsequently blocked with 1% BSA in PBS. Samples were diluted and incubated on the ELISA plates. Bound factors were detected with biotin-coupled antibodies raised against PF-4, β-TD, RANTES, or PDGF-A/B in PBS with 1% bovine serum albumin. Plates were washed five times with 0.05% Tween 20 in PBS pH 7.4 between each subsequent incubation step. Biotin-coupled antibodies were detected with Super Signal West Pico Chemiluminescent substrate (Westburg, Leusden, the Netherlands) and read with a Spectramax L Luminometer (Molecular Devices, Sunnyvale, CA, USA). Sample concentrations were deduced from a calibration curve of normal pooled serum with known concentrations.
Platelet ADP/ATP release
Washed platelets from four healthy donors and three patients on P2Y12 inhibitors were stimulated with vehicle, 10-µM PAR-1 agonist or 100-ng/mL CRP-xl, with or without 1-µM AR-C69931MX. After 20 min, reactions were stopped with EDTA (0.5 mM final concentration) and prostacyclin (10 ng/ml), after which platelet suspensions were centrifuged at 500g for 10 min. The supernatant was used for ATP/ADP quantification. Supernatants were divided into two fractions. In the first fraction, ADP was converted into ATP by incubation with 95 µM PEP and 25 µg/mL PK in 0.2 M Tris–Maleate, 10 mM KCl, 15 mM MgSO4, pH 7.4 for 15 min at 37°C. Reactions were stopped by heating the samples at 80°C for 10 min. ATP levels in both fractions were determined with the ATPLite 1 step kit (6016731, Perkin Elmer, Waltham, MA, USA) according to the instructions of the manufacturer. ATP levels were deduced from an ATP calibration curve. ADP levels were calculated by subtracting the ATP levels in the second fraction from those in the first.
Statistical analysis
Differences in final aggregation with and without P2Y12 inhibitor were calculated per agonist concentration with the non-parametric Wilcoxon signed-rank test. Differences in αIIbβ3 activation, P-selectin and CD 63 expression with and without apyrase or AR-C69931MX, assessed with flow cytometry and differences in alpha- and dense granule release by presence and absence of P2Y12 inhibition, were also calculated with the non-parametric Wilcoxon signed-rank test. Differences in αIIbβ3 activation after stimulation with ADP, PAR-1 agonist or CRP-xl between responders and nonresponders were calculated with the Mann–Whitney U test for independent samples. All statistic tests were performed using IBM SPSS Statistics Version 21 (Armonk, NY, USA).
Results
P2Y12 inhibitors strongly inhibit αIIbβ3 activation after PAR-1, PAR-4, and CRP-xl stimulation, despite mild inhibition of platelet aggregation
Full platelet activation requires both activation of the fibrinogen receptor integrin αIIbβ3 and the secretion of alpha and dense granule content into the extracellular environment. To compare the effects of P2Y12 inhibition on ADP-mediated platelet activation with those on thrombin and collagen-mediated platelet activation, we first studied the effects of P2Y12 inhibition on platelet aggregation.
P2Y12 inhibition completely prevented ADP-induced platelet aggregation, but only partially attenuated aggregation induced with low doses of PAR-1 agonist, PAR-4 agonist, or CRP-xl ( and ). P2Y12 inhibition had no effect on platelet aggregation induced with higher concentrations of the same agonists.
Figure 1. Fibrinogen binding after stimulation in the presence of P2Y12 antagonists. (A) Light transmission aggregation was recorded after stimulation with high and low concentrations of ADP, PAR-1 and PAR-4 agonist, and CRP-xl in the presence and absence of cangrelor (AR-C) in four healthy donors. Mean and standard error of mean are shown for the final amplitude of aggregation after 14 min. (B) Representative aggregation traces. (C) Platelets were stimulated with increasing concentrations of ADP, PAR-1, PAR-4 agonist, or CRP-xl in presence of cangrelor (dark gray), apyrase (light gray) or buffer only (black) and fibrinogen binding was analyzed with flow cytometry as a measure of αIIbβ3 activation. Area under the curve of αIIbβ3 activation in presence of cangrelor or apyrase is expressed as a percentage of mock-treated platelets. Mean and standard error of the mean are shown. Measurements were performed in five healthy donors (*p < 0.05).
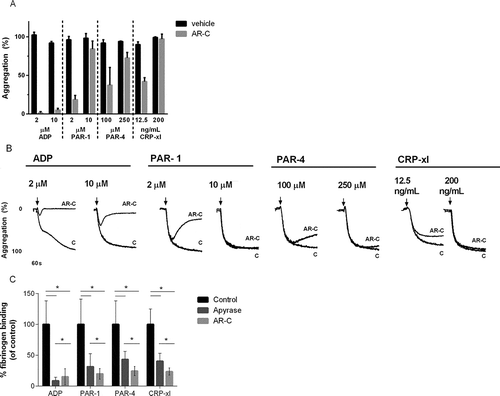
Next, we studied the effect of P2Y12 inhibition on αIIbβ3 activation as measured with flow cytometry. As expected, addition of cangrelor or apyrase to platelets stimulated with ADP almost completely abrogated αIIbβ3 activation (). Addition of cangrelor or apyrase to platelets stimulated with PAR-1 agonist, PAR-4 agonist, or CRP-xl resulted in strong inhibition of αIIbβ3 activation ( and Supplemental ).
Dense granule secretion is more strongly reduced than the release of α-granule content by P2Y12 inhibitors
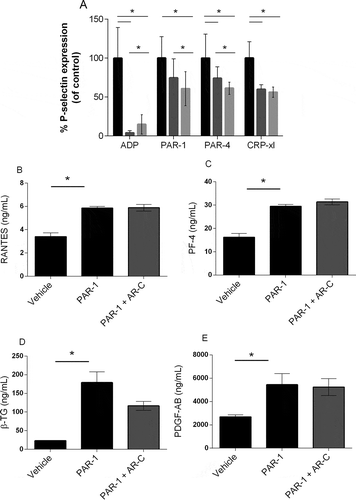
Next, we analyzed the effect of P2Y12 inhibition on platelet granule secretion. Whereas ADP-induced P-selectin expression was completely prevented by addition of cangrelor or apyrase, P-selectin expression was only mildly reduced in platelets stimulated with PAR-1 agonist, PAR-4 agonist, or CRP-xl () as measured with flow cytometry (Supplemental . Dose response curves for all agonists). Since P-selectin is present in both alpha and dense granules, we next investigated the effect of P2Y12 inhibition on PAR-1-mediated release of alpha granule content. PAR-1-induced secretion of RANTES (), PF4 (), β-TG (), or PDGF A/B () was similar between platelets treated with cangrelor and untreated platelets, which suggests that P2Y12 inhibition scarcely influences PAR-1-mediated alpha granule secretion.
Figure 2. Alpha-granule release after stimulation in the presence of P2Y12 antagonists. Platelets were stimulated with increasing concentrations of ADP, PAR-1, PAR-4 agonist, or CRP-xl in presence of cangrelor (AR-C) (dark gray), apyrase (light gray) or buffer only (black), and P-selectin expression was analyzed with flow cytometry as a measure of granule secretion. (A) Area under the curve of P-selectin expression in the presence of cangrelor or apyrase is expressed as a percentage of mock-treated platelets. Mean and standard error of the mean are shown. Measurements were performed in five healthy donors. (B) Platelets were activated with PAR-1 agonist with or without cangrelor and concentrations of RANTES, (C) platelet factor 4 (PF-4), (D) β-thromboglobulin (β-TG), and (E) PDGF-A/B were determined with ELISA. Data are expressed as mean ± SEM (*p < 0.05).
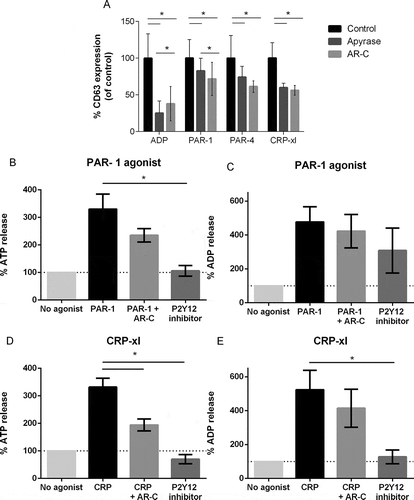
We therefore investigated whether P2Y12 inhibition influences dense granule release. As expected, ADP-induced CD63 expression was completely abolished by addition of cangrelor or apyrase (). However, CD63 expression in platelets stimulated with PAR-1 agonist, PAR-4 agonist, or CRP-xl was mildly reduced, as to a similar extent as P-selectin expression, in platelets treated with P2Y12 inhibitors (Supplemental ). Interestingly, both ATP ( and ) and ADP release ( and ) was significantly reduced in platelets treated with P2Y12 inhibitor after stimulation with PAR-1 agonist ( and ) or CRP-xl ( and ).
Figure 3. Dense granule release after stimulation in the presence of P2Y12 antagonists. Platelets were stimulated with increasing concentrations of ADP, PAR-1, PAR-4 agonist or CRP-xl in presence of cangrelor (dark gray), apyrase (light gray) or buffer only (black), and CD63 expression was analyzed with flow cytometry as a measure of dense granule secretion. (A) Area under the curve of CD63 expression in the presence of cangrelor or apyrase are expressed as a percentage of mock-treated platelets. Mean and standard error of the mean are shown. Measurements were performed in five healthy donors. ATP release (B, D) and ADP release (C, E) was measured in healthy donors (n = 4) in the presence and absence of cangrelor and in blood of three patients on P2Y12 inhibitors after stimulation with PAR-1 agonist (B, C) and CRP-xl (3D, E). Data are expressed as mean ± SEM (*p < 0.05).
Multiple good responders to P2Y12 inhibitors, identified with VerifyNow, show normal αIIbβ3 activation after stimulation with PAR-1 and CRP-xl
Because the response to ADP differed substantially from the response to either PAR-1 agonist, PAR-4 agonist, or CRP-xl in platelets treated with P2Y12 inhibitors in vitro, we determined the platelet response (agonist induced fibrinogen binding and P-selectin expression) during antiplatelet therapy in a cross-sectional cohort of 72 patients that visited the outpatient clinic of the vascular surgery department (–). Antiplatelet therapy is shown in . Platelet responses were normalized on the median response of a group of 43 healthy individuals. The threshold of whether or not adequate platelet inhibition was achieved was set at the 2.5th percentile of the normal response.
Table I. Characteristics and test results of anonymous patients on P2Y12 inhibitors.
Figure 4. Platelet reactivity testing in patients on P2Y12 inhibitors. Inhibition of αIIbβ3 activation and P-selectin expression measured in 72 patients on P2Y12 inhibitors, normalized against the response in 43 healthy donors. Black symbols represent responders to P2Y12 inhibitors according to the VerifyNow P2Y12 assay (based on cut-off PRU < 208) and gray symbols represent nonresponders. (A) Platelets were stimulated with 31.25 µM ADP, (B) 100 µM PAR-1 agonist, or (C) 1000 ng/mL CRP-xl (5c). The dotted lines represent the lowest 2.5th percentile of fibrinogen binding and P-selectin expression of the healthy donors. (D) Percentage comparison of fibrinogen binding after stimulation with ADP, PAR-1 agonist, or CRP-xl between healthy donors (controls), responders to P2Y12 inhibition and nonresponders (*p < 0.05).
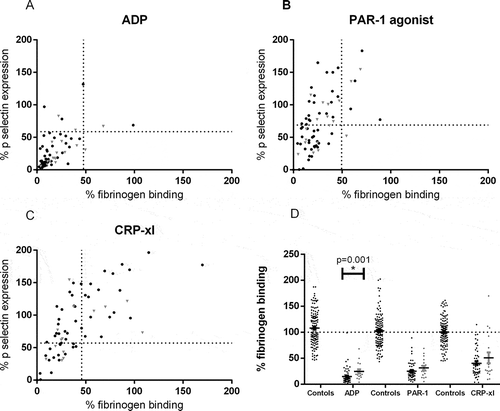
The VerifyNow P2Y12 assay identified 18 patients (25.0%) as nonresponders with the cut-off set at PRU < 208. The majority of patients on P2Y12 inhibitors had a strongly reduced response to ADP (). After ADP stimulation, only four patients (5.6%) showed normal remaining αIIbβ3 activation and seven patients (9.7%) showed normal remaining P-selectin expression. In concordance with our in vitro data, the inhibition of αIIbβ3 activation was stronger than inhibition of granule release. Ten percent of patients had normal fibrinogen binding after stimulation with PAR-1 agonist, but P-selectin expression remained normal in 45.8% of the patients (). Activation of αIIbβ3 after stimulation with CRP-xl was normal in 31.9% of patients and P-selectin expression remained normal in 66.7% of the patients ().
There was little agreement in the identification of responders and nonresponders to P2Y12 inhibitors between VerifyNow and platelet reactivity assessment with flow cytometry. However, fibrinogen binding assessed with flow cytometry was lower in VerifyNow-identified responders than in nonresponders after stimulation with ADP (p = 0.001) (). No difference in fibrinogen binding was found after stimulation with PAR-1 agonist and CRP-xl. P-selectin expression was similar between VerifyNow-responders and nonresponders after stimulation with all agonists (data not shown).
Discussion
The efficacy of antiplatelet therapy with P2Y12 inhibitors for thromboprophylaxis is mostly monitored with tests that measure the platelet response to P2Y12-ligand ADP, ignoring the response to other physiologically relevant platelet agonists. This study shows that P2Y12 inhibitors not only attenuate the response to ADP, but also have a strong inhibitory effect on the response of platelets to collagen and thrombin. Measurement of αIIbβ3 activation after stimulation with collagen or thrombin in addition to the response to ADP provides insight in the inhibitory status of patients receiving antiplatelet therapy and might allow identification of patients with suboptimal platelet inhibition that benefit from more stringent antiplatelet therapy.
There are marked differences in the effects of P2Y12 inhibition on the ADP pathway and those on the collagen or thrombin pathways of platelet activation. P2Y12 inhibitors completely abrogate the capacity to aggregate after ADP stimulation. In contrast, P2Y12 inhibition only mildly attenuates the platelet capacity to aggregate after stimulation with low-dose thrombin-receptor agonist or CRP-XL. Interestingly, platelet aggregation is normal at high concentrations of these agonists, despite a greatly reduced capacity of the platelets to bind fibrinogen. P2Y12 inhibition has been shown to reduce thrombus growth after vascular injury, leaving platelet granule release unaffected [Citation15]. This is in line with the attenuated response toward collagen- and thrombin mimetics after P2Y12 inhibition that we and others observed with flow cytometry [Citation20], but is at odds with the lack of effect of P2Y12 inhibition we observed in aggregation experiments with the same agonists. This disparity between flow cytometric analysis and aggregation-based analysis of platelet reactivity might be explained by a threshold in αIIbβ3 activation above which platelet aggregation can take place. When platelet stimulation results in αIIbβ3 activation exceeding this threshold, aggregation-based assays lose their sensitivity for the effects of platelet inhibiting drugs, whereas flow cytometric quantification of platelet activation can still differentiate between good and poor responses to stimuli. However, currently, LTA is still accepted as gold standard for laboratory platelet reactivity testing: high-platelet reactivity after 5 or 20 µmol ADP increased the risk of MACE 3.25-fold or 2.98-fold,respectively [Citation6]. Sensitivity and specificity of the LTA ADP 5 and 20 µmol for prediction of atherothrombotic events was 54.6–60.2% and 53.1–60.9% [Citation14]
In this study, the influence of differential set-up conditions between flow cytometry and LTA might also play part; whole blood diluted 1:10 was used for flow cytometry, diminishing GPIIbIIIa outside in signaling and potentially the sustained fibrinogen binding. LTA is performed with vigorous stirring of the sample, which may activate platelets, which does not occur in flow cytometry assays. Lastly, the temperature of the LTA (37°C) might have induced full platelet activation, in contrast to room temperature of flow cytometry.
While ADP-induced granule release is fully prevented by P2Y12 inhibitors, granule release after stimulation of the thrombin or collagen pathways is affected to a lesser extent. This is in line with previous studies by other groups, which reported only moderate reduction in P-selectin expression after stimulation of the thrombin pathway [Citation21–Citation23]. Our data might indicate that the reduction in P-selectin expression is mainly due to the inhibition of dense granule release, while alpha granule release remains largely unaffected. Alpha granules are the major transporters of VEGF, PDGF, TNF-α, TGF-β, PF-4, β-TG, RANTES, and thrombospondin through the blood and can release their content at high concentrations at the site of injury. Physiologically, this may be important for the regulation of wound healing and neo vascularization. Our findings that P2Y12 inhibitors have minor impact on the expression of P-selectin indicate that the platelets keep their capacity to regulate interactions between platelets and endothelial cells, white blood cells and progenitor cells via P-selectin-PSGL-1 interaction or CD40L–CD40 interaction [Citation24, Citation25].
Large clinical trials have shown that treatment with P2Y12 inhibitors may significantly reduce the number of secondary cardiovascular events on population level [Citation26–Citation29]. However, on individual patient level there is still recurrence of thrombotic events, while other patients develop bleeding complications [Citation4, Citation5]. A meta-analysis by our group summarized that high on treatment platelet reactivity is an important predictor for secondary thrombotic events [Citation6]. Both the thromboembolic and bleeding complication rate might be further reduced by tailored adjustment of treatment intensity, based on adequate platelet reactivity monitoring of individual patients. However, clinical trials with patients undergoing percutaneous coronary interventions found that tailored treatment adjustment based on VerifyNow platelet reactivity monitoring did not reduce the recurrence rate of arterial thrombosis, when compared to patients who were not monitored [Citation13, Citation30]. Although these first attempt were disappointing, this does not completely rule out the potential benefit of tailored antiplatelet therapy.
Platelet reactivity testing is still in its infancy and the VerifyNow has severe limitations, including a varying sensitivity in studies for the prediction of thrombotic events [Citation31–Citation33]. More importantly, the read-out of the VerifyNow is restricted to the P2Y12 activation pathway and does not quantify the other major physiological platelet activation pathways. Our data show that platelet reactivity measurements based on multiple activation pathways provide more insight in platelet inhibition status than measurements limited to the ADP pathway. In fact, a large percentage of responders according to the VerifyNow retained normal fibrinogen binding after stimulation with PAR-1 agonist and CRP-xl (inducing the strongest primary platelet activation pathways), despite strongly reduced responses to ADP, suggesting poor platelet inhibition. Overall, this study showed little agreement between the results of the VerifyNow P2Y12 assay and the flow cytometric analysis of αIIbβ3 binding in patients receiving P2Y12 inhibitors; although there were differences in fibrinogen binding between VerifyNow responders and nonresponders, these differences were small and there was considerable overlap between groups. It is therefore doubtful that these differences can be used to guide treatment in clinical practice.
A number of groups all over the world are currently developing comprehensive assays for the detection of platelet dysfunction in bleeding disorders. Amongst these are assays based on flow cytometric analysis of microaggregate formation after stimulation with various agonists [Citation34]. This assay requires much lower numbers of platelets than conventional LTA, which makes the assay suitable for thrombocytopenic samples. Another approach is based on the analysis of various stages of thrombus formation after perfusion of whole blood over arrays of 52 microspotted adhesive surface at high shear stress [Citation35]. A third example is the Optimul assay, a 96 well-plate-based assay that allows an appraisal of 7 platelet activation pathways [Citation36]. The assay is based on light absorbance through stirred PRP and resembles the traditional method of the LTA. Yet, the Optimul is faster and requires less blood than LTA.
All of these assays could be used for monitoring of antiplatelet therapy, although none of these assays has been used for this purpose to this date. A Point-of-care platelet reactivity test that is further along in its development is the Multiplate, which detects the increase of electrical impedance resulting from the adhesion and aggregation of platelets on two metal sensor wires. To stimulate the aggregation, multiple agonists can be used to test different platelet activation pathways. An attempt has been made to determine a therapeutic window for APT dosage based on the Multiplate results in patients with coronary artery disease [Citation37]. However, measuring platelet function by global aggregation measure approach is usually less specific to evaluate the effect of specific antiplatelet drug.
One of the limitations of our study is that we were not able to investigate the association between platelet fibrinogen binding after stimulation with ADP, thrombin receptor agonists or collagen mimetics and clinical outcome during follow-up. Future clinical studies should indicate whether assessment of additional platelet activation pathways will improve the predictive value of antiplatelet therapy monitoring on the individual patient level.
In summary, we show that P2Y12 inhibition affects not only the ADP platelet activation pathway, but also the thrombin and collagen pathways. Flow cytometry-based assays are more sensitive for decreases in the platelets response to collagen and thrombin than aggregation-based assays. Additional insight in the inhibitory status of patients receiving antiplatelet therapy can be obtained by measurements of platelet fibrinogen binding after stimulation of thrombin- or collagen receptors. These additional insights can improve identification of patients with suboptimal platelet inhibition, who might benefit from more stringent antiplatelet therapy.
Declaration of interest
The authors report no declarations of interest. This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine (www.ctmm.nl), project CIRCULATING CELLS: [Grant Number 01C-102], and supported by the Netherlands Heart Foundation and the Landsteiner Foundation for Blood Transfusion Research: [Grant Number 0912F to SK].
Leunissen et al Supp Figure
Download PDF (107.1 KB)Acknowledgments
We want to thank Astra Zeneca for providing cangrelor and R. Farndale at Cambridge University for giving CRP-xl.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Stellos K, Gawaz M. Platelet interaction with progenitor cells: potential implications for regenerative medicine. Thromb Haemost 2007;98:922–929.
- Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest 2005;115:3378–3384.
- Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: Linking proinflammatory responses to procoagulant state. Thromb Res 2014;131:191–197.
- Sibbing D, Schulz S, Braun S, Morath T, Stegherr J, Mehilli J, Schömig A, von Beckerath N, Kastrati A. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost 2010;8:250–256.
- Cuisset T, Cayla G, Frere C, Quilici J, Poyet R, Gaborit B, Bali L, Morange PE, Alessi MC, Bonnet JL. Predictive value of post-treatment platelet reactivity for occurrence of post-discharge bleeding after non-ST elevation acute coronary syndrome. Shifting from antiplatelet resistance to bleeding risk assessment? EuroIntervention 2009;5:325–329.
- Wisman PP, Roest M, Asselbergs FW, de Groot PG, Moll FL, van der Graaf Y, de Borst GJ.. Platelet-reactivity tests identify patients at risk of secondary cardiovascular events: a systematic review and meta-analysis. J Thromb Haemost 2014;12:736–747.
- Bouman HJ, Parlak E, van Werkum JW, Breet NJ, ten Cate H, Hackeng CM, ten Berg JM, Taubert D. Which platelet function test is suitable to monitor clopidogrel responsiveness? A pharmacokinetic analysis on the active metabolite of clopidogrel. J Thromb Haemost 2010;8:482–488.
- Lordkipanidze M, Pharand C, Nguyen TA, Schampaert E, Diodati JG. Assessment of VerifyNow P2Y12 assay accuracy in evaluating clopidogrel-induced platelet inhibition. Ther Drug Monit 2008;30:372–378.
- Behan MW, Fox SC, Heptinstall S, Storey RF. Inhibitory effects of P2Y12 receptor antagonists on TRAP-induced platelet aggregation, procoagulant activity, microparticle formation and intracellular calcium responses in patients with acute coronary syndromes. Platelets 2005;16:73–80.
- Badr Eslam R, Lang IM, Koppensteiner R, Calatzis A, Panzer S, Gremmel T. Residual platelet activation through protease-activated receptors (PAR)-1 and -4 in patients on P2Y12 inhibitors. Int J Cardiol 2013;168:403–406.
- Jakubowski JA, Zhou C, Egan B, Wells M, Kotob-Yahfoufi M, Sugidachi A, Dahlen JR. Modification of the VerifyNow(R) P2Y12 test BASE channel to accommodate high levels of P2Y(12) antagonism. Platelets 2011;22:619–625.
- Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097–1105.
- Montalescot G, Range G, Silvain J, Bonnet JL, Boueri Z, Barthélémy O, Cayla G, Belle L, Van Belle E, Cuisset T, et al. High on-treatment platelet reactivity as a risk factor for secondary prevention after coronary stent revascularization: A landmark analysis of the ARCTIC study. Circulation 2014;129:2136–2143.
- Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, Deneer VH, Harmsze AM, van der Heyden JA, Rensing BJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010;303:754–762.
- Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 2013;121:1875–1885.
- Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, Richardt G, Jakubowski JA, Neumann FJ. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol 2012;59:2159–2164.
- Storey RF, Angiolillo DJ, Bonaca MP, Thomas MR, Judge HM, Rollini F, Franchi F, Ahsan AJ, Bhatt DL, Kuder JF, et al. Platelet Inhibition With Ticagrelor 60 mg Versus 90 mg Twice Daily in the PEGASUS-TIMI 54 Trial. J Am Coll Cardiol 2016;67:1145–1154.
- Alexopoulos D, Stavrou K, Koniari I, Gkizas V, Perperis A, Kontoprias K, Vogiatzi C, Bampouri T, Xanthopoulou I. Ticagrelor vs prasugrel one-month maintenance therapy: impact on platelet reactivity and bleeding events. Thromb Haemost 2014;112:551–557.
- van Holten TC, Bleijerveld OB, Wijten P, de Groot PG, Heck AJ, Barendrecht AD, Merkx TH, Scholten A, Roest M. Quantitative proteomics analysis reveals similar release profiles following specific PAR-1 or PAR-4 stimulation of platelets. Cardiovasc Res 2014;103:140–146.
- Nylander S, Mattsson C, Ramstrom S, Lindahl TL. The relative importance of the ADP receptors, P2Y12 and P2Y1, in thrombin-induced platelet activation. Thromb Res 2003;111:65–73.
- Storey RF, Sanderson HM, White AE, May JA, Cameron KE, Heptinstall S. The central role of the P(2T) receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol 2000;110:925–934.
- Storey RF, Newby LJ, Heptinstall S. Effects of P2Y(1) and P2Y(12) receptor antagonists on platelet aggregation induced by different agonists in human whole blood. Platelets 2001;12:443–447.
- Klinkhardt U, Bauersachs R, Adams J, Graff J, Lindhoff-Last E, Harder S. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet-leukocyte aggregates in patients with atherosclerotic vascular disease. Clin Pharmacol Ther 2003;73:232–241.
- Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC, Wijnands E, Goossens P, van Kruchten R, Thevissen L, et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 2010;116:4317–4327.
- Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Müller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998;391:591–594.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502.
- Lee M, Wu YL, Saver JL, Lee HC, Lee JD, Chang KC, Wu CY, Lee TH, Wang HH, Rao NM, et al. Is clopidogrel better than aspirin following breakthrough strokes while on aspirin? A retrospective cohort study. BMJ Open 2014;4:e006672.
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr., Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–425.
- Clark MG, Beavers C, Osborne J. Managing the acute coronary syndrome patient: Evidence based recommendations for anti-platelet therapy. Heart Lung 2015;44:141–149.
- Price MJ, Angiolillo DJ, Teirstein PS, Lillie E, Manoukian SV, Berger PB, Tanguay JF, Cannon CP, Topol EJ. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation 2011;124:1132–1137.
- Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Comparison of methods to evaluate clopidogrel-mediated platelet inhibition after percutaneous intervention with stent implantation. Thromb Haemost 2009;101:333–339.
- Flechtenmacher N, Kammerer F, Dittmer R, Budde U, Michels P, Rother J, Eckert B. Clopidogrel Resistance in Neurovascular Stenting: Correlations between Light Transmission Aggregometry, VerifyNow, and the Multiplate. AJNR Am J Neuroradiol 2015;36:1953–1958.
- Zhang HZ, Kim MH, Jeong YH. Predictive values of post-clopidogrel platelet reactivity assessed by different platelet function tests on ischemic events in East Asian patients treated with PCI. Platelets 2014;25:292–299.
- De Cuyper IM, Meinders M, van de Vijver E, de Korte D, Porcelijn L, de Haas M, Eble JA, Seeger K, Rutella S, Pagliara D, et al. A novel flow cytometry-based platelet aggregation assay. Blood 2013;121:e70–80.
- de Witt SM, Swieringa F, Cavill R, Lamers MM, van Kruchten R, Mastenbroek T, Baaten C, Coort S, Pugh N, Schulz A, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun 2014;5:4257.
- Lordkipanidze M, Lowe GC, Kirkby NS, Chan MV, Lundberg MH, Morgan NV, Bem D, Nisar SP, Leo VC, Jones ML, et al. Characterization of multiple platelet activation pathways in patients with bleeding as a high-throughput screening option: use of 96-well Optimul assay. Blood 2014;123:e11–22.
- Petricevic M, Milicic D, White A, Boban M, Mihaljevic MZ, Piljic D, Rotim A, Buca A, Mihalj M, Biocina B. Development of a concept for a personalized approach in the perioperative antiplatelet therapy administration/discontinuation management based on multiple electrode aggregometry in patients undergoing coronary artery surgery. J Thromb Thrombolysis 2015;40:383–391.
