Abstract
Light transmission aggregation (LTA) is the gold standard for the diagnosis of platelet function disorders (PFDs), but it is time-consuming and limited to specialized laboratories. Whole-blood impedance aggregometry (Multiplate) and platelet function analyzer (PFA) may be used as rapid screening tools to exclude PFDs. The aim of this study is to assess the diagnostic performance of Multiplate and PFA for PFDs, as detected by LTA.Data from preoperative patients, patients referred to the hematologist for bleeding evaluation, and patients with a diagnosed bleeding disorder were used. PFDs were defined as ≥2 abnormal LTA curves. Diagnostic performance of Multiplate and PFA for detecting PFDs was expressed as sensitivity and specificity. The ability of Multiplate agonists and PFA kits to detect corresponding LTA curve abnormalities was expressed as area under the receiver operating characteristic curve.
Prevalence of PFDs was 16/335 (4.8%) in preoperative patients, 10/54 (18.5%) in referred patients, and 3/25 (12%) in patients with a diagnosed bleeding disorder. In preoperative and referred patients, the sensitivity of Multiplate and PFA for detecting mild PFDs varied between 0% and 40% and AUCs for detecting corresponding LTA curve abnormalities were close to 0.50. In patients with a diagnosed bleeding disorder, both assays could detect Glanzmann thrombasthenia (GT) with sensitivity of 100% and AUCs of 0.70–1.00.
Multiplate and PFA cannot discriminate between preoperative and referred patients with and without mild PFDs, meaning that they cannot be used as screening tests to rule out mild PFDs in these populations. Both Multiplate and PFA can detect GT in previously diagnosed patients.
Introduction
Platelets are critically involved in normal hemostasis and pathological bleeding [Citation1,Citation2] and platelet function testing is commonly performed in the diagnostic workup of patients with a bleeding tendency, or patients at risk of interventional bleeding. Many clinical laboratories evaluate platelet function by light transmission aggregometry (LTA), which is considered the gold standard for the assessment of platelet function [Citation3,Citation4]. Using a panel of agonists that activate platelets via different receptors, LTA can detect many well-characterized platelet function disorders (PFDs). LTA primarily tests for platelet aggregation defects (e.g. Glanzmann thrombasthenia (GT)), but curve interpretation also gives information about platelet adhesion and secretion (second wave), and certain findings are characteristic for platelet adhesion defects (Bernard–Soulier syndrome) or highly suggest platelet secretion defects. However, LTA is a time-consuming technique and is affected by different pre-analytical conditions (e.g. lipemic plasma, low platelet count) as well as by different procedural conditions (e.g. platelet-rich plasma (PRP) preparation, different agonist concentrations) [Citation5]. A high degree of experience and expertise is necessary to perform and interpret this test, limiting its use to specialized laboratories [Citation1,Citation2,Citation6].
To overcome these limitations, other less-laborious whole-blood platelet function tests and primary hemostatic function tests have been developed and used, such as impedance aggregometry (Multiplate) and platelet function analyzer (PFA). The procedures of these assays are easier and faster, they have disposable cartridges or cups, are less influenced by (pre-)analytical procedures, [Citation7–Citation9] and do not require a specialized laboratory [Citation1]. The use of anticoagulated whole blood eliminates the need for time-consuming centrifugation steps and less blood is needed.
Since Multiplate and PFA do not provide the same extent of information as LTA (characteristics such as the shape change of platelets upon stimulation and the reversibility of platelet aggregation cannot be assessed [Citation1]), these whole-blood assays cannot be used to diagnose specific PFDs [Citation10]. However, these assays may be used to exclude PFDs in a screening setting, as they are rapid and easy to use. Previous studies evaluated the screening value of PFA for PFDs (as detected by the LTA) and found variable results depending on the severity of PFDs [Citation6, Citation8, Citation11]. The screening value of Multiplate for PFDs has not been studied. Yet, both tests are often used to screen for PFDs in clinical practice. A worldwide survey, in which 202 laboratories from 37 countries participated, demonstrated that PFA and Multiplate are used to screen for PFDs in 53% and 12% of the laboratories, respectively [Citation12]. Therefore, we aimed to establish the utility of Multiplate and PFA as screening tests for PFDs, as detected by the gold standard LTA. PFDs were defined as having ≥2 LTA curve abnormalities [Citation13]. In addition, we assessed the ability of individual Multiplate agonists and PFA kits to correctly identify the corresponding LTA curve abnormalities. We used data from three different patient cohorts, in which we expected differences in prevalence and severity of PFDs, including preoperative patients, patients referred to the hematologist for bleeding evaluation, and patients with a diagnosed bleeding disorder.
Methods
Study design
Data from three observational studies, which evaluated the diagnostic value of established and new hemostatic tests in patients with a (suspected) bleeding tendency, were used for this analysis. These studies included adult subjects from September 2013 until September 2016 and performed laboratory tests in preoperative patients (PANE study), patients referred to the hematologist for bleeding evaluation (ProBe-AHP study), and patients with a diagnosed bleeding disorder of any kind (BEPA study). This last group was included to assess whether the tests could discriminate between patients with and without severe PFDs. Detailed study designs are presented in Supplemental File 1. Subjects with platelet count below 100 × 109/L and/or hematocrit below 0.25 L/L, with active bleeding or use of specific medication (e.g. antiplatelet therapy, anticoagulants, nonsteroidal anti-inflammatory drugs (NSAIDs)) were excluded to avoid interference with test results on LTA, Multiplate, and PFA [Citation1,Citation2,Citation6,Citation8,Citation14]. Patients with von Willebrand Factor (vWF) levels below 50% were excluded for the detection of PFDs with PFA, because these patients can have abnormal PFA results (due to von Willebrand Disease, vWD) without having a PFD.
To assess the performance of Multiplate and PFA as screening tests for PFDs, Multiplate and PFA results were compared to the results of LTA as reference test for PFDs, within the three study groups. First, the diagnostic value of Multiplate and PFA to detect PFDs using previously defined cutoff values was assessed. Second, we assessed the ability of the individual Multiplate agonists and PFA kits to correctly identify the corresponding abnormal LTA agonist curve (e.g. Multiplate ASPItest should detect LTA AA curve abnormality and not LTA thrombin receptor activating peptide (TRAP) curve abnormality).
Informed consent was obtained from all patients. The studies were approved by the MUMC medical ethical committee.
Blood sampling
Patients were asked to avoid fat-containing food 4 hours before blood withdrawal, as plasma lipids might interfere with LTA measurements. Patients had to stop any interfering medication (e.g. NSAIDs) 7 days before blood withdrawal. Blood was drawn from the antecubital vein by puncture using a 21G needle. Complete blood count was measured in EDTA blood (1.8mg/mL, BD Vacutainer, Plymouth), citrated blood was used for the LTA and PFA, and Hirudin blood was used for the Multiplate. LTA and Multiplate were performed within 3–4 hours; PFA was performed 1 h after blood withdrawal. For the preparation of PRP, citrated blood was centrifuged at 170g for 10 min at 18°C. For the preparation of platelet-free plasma (PFP), citrated blood was centrifuged at 2500g for 5 min at 18°C. The plasma was transferred to another tube and centrifuged at 10000g for 10 min at 18°C.
Performance of LTA was largely based on Clinical Laboratory Standards Institute (CLSI) and Scientific and Standardization Committee of the International Society of Haemostasis and Thrombosis (ISTH-SSC) guidelines [Citation15,Citation16]. In the ProBe-AHP and BEPA study, platelet count in PRP was adjusted with autologous platelet-poor plasma (PPP) to 250 × 109/L. In the PANE study, the platelet count was not adjusted, unless PRP platelet count was >600 × 109/L (adjusted to 500 × 109/L). Samples from healthy controls were run every 1–2 weeks as an internal quality control for LTA, Multiplate, and PFA.
Platelet function tests
The results of all platelet function tests were interpreted without knowledge of other test results.
Light transmission aggregometry (LTA)
Blood was collected in 3.2% sodium citrate Vacuette tubes (9 mL; Greiner Bio-One, Frickenhausen, Germany). Chronolog (Chrono-log Corporation, Havertown, PA, USA) was used for the Probe-AHP study and the BEPA study, PAR-4 Platelet Aggregometer (Hart Biologicals, Hartlepool, UK) was used for the PANE study. Platelet aggregation in PRP was initiated using adenosine diphosphate (ADP) (chronology CH384) 5 µM, ADP 10 µM, collagen (COL; Chrono-par Ref385) 1 µg/mL and 4 µg/mL, epinephrine (EPI; Chrono-log CH393) 10 µM, arachidonic acid (AA; Bio/Data) 1 mM, Ristocetine (RIST; Chrono-log Stago Ref396) 1.5 mg/ml, and TRAP (Boom H8105) 15 µM. The agonist concentrations were based upon the CLSI guidelines and published literature [Citation3,Citation15,Citation17]. Local within-run coefficient of variation for both the Chrono-log and PAR-4 was between 2.2% and 3.25%.
Multiplate
Blood was collected in Hirudin Blood Tubes (3 mL, Double Wall; Roche, Rotkreuz, Switzerland). Platelet aggregation was measured on the Multiplate Analyzer (Roche, Rotkreuz, Switzerland) and initiated using AA 0.5 mM (ASPItest), TRAP 32 µM (TRAPtest), collagen 3.2 µg/ml (COLtest), and ADP 6.4 µM (ADPtest). The agonist concentrations were according to the manufacturer leaflet. Test results were expressed as area under the curve (AUC). Reference values were established in our laboratory by analyzing samples of 25 healthy volunteers with EP evaluator software (Data Innovations, Brussels, Belgium) [Citation18]. Reference values for AUC were 33–108 for ADP, 50–119 for AA, 70–130 for TRAP, and 45–125 for COL. Local within-run coefficient of variation for all Multiplate agonists was between 8.4% and 9.9%.
PFA
Blood was collected in 3.2% sodium citrate tubes (Greiner Bio-One). PFA-200 (Siemens Healthineers, The Hague, The Netherlands) was used. Closure times (CTs) were recorded with collagen and epinephrine (C-EPI) or collagen and ADP (C-ADP) kits. Reference intervals for PFA-200 were provided by the manufacturer and verified in our laboratory by analyzing the test results from 23 healthy volunteers with EP evaluator software. Local within-run coefficient of variation for both PFA kits was 9.5%.
Definition of PFDs
A platelet function defect was considered present in case of deviant LTA curves with ≥2 agonists or when aggregation with ristocetin or collagen 4 µg/mL was the only abnormal curve (). A deviant LTA curve was defined in case of maximal aggregation below 60%; reversibility to below 50% of the maximal aggregation; or if maximal aggregation was not reached within 3 min (visually interpreted as delayed). A delayed aggregation with epinephrine was not considered abnormal, as this is very often present in healthy subjects [Citation2,Citation6,Citation16]. The cutoff point of <60% for abnormal maximal aggregation was verified in our laboratory by analyzing maximal aggregation in 40 healthy volunteers, calculating the 2.5 percentile values. For all agonists, the maximal aggregation was above 60% (between 62.0% and 75.3%). All LTA results were visually interpreted and discussed in a multidisciplinary team with a senior technician, a hematologist, and a clinical chemist.
Table I. Definitions of platelet function disorders (LTA) and Multiplate/PFA abnormalities.
Statistical analysis
Continuous variables are expressed as mean with standard deviation (SD) for normally distributed variables or median with interquartile range (IQR) otherwise. Categorical variables are expressed as counts and percentages. To assess whether Multiplate and PFA can detect PFDs (≥2 abnormal LTA agonists), the diagnostic performance of Multiplate and PFA was assessed by calculating sensitivity and specificity, negative predictive value (NPV), and positive predictive value (PPV) with 95% confidence intervals, using previously proposed cutoff values. Multiplate was considered ‘abnormal’ when ≥1 agonist showed low AUC (AUC <33 for ADP, <50 for AA, <70 for TRAP, and <45 for COL), while PFA was considered ‘abnormal’ when ≥1 kits showed prolonged CT (>160 s for C-EPI, >118 s for C-ADP) (). Diagnostic performance of individual Multiplate agonists and PFA kits was assessed by construction of receiver operating characteristic (ROC) curves, which visualize the trade-off between sensitivity and specificity over a range of cutoff points for positive test results. Areas under the ROC curves (AUC) with 95% confidence intervals were calculated. Statistical analyses were performed with IBM SPSS statistics version 24.0; significance was assumed at p < 0.05.
Results
Study population
Data were used from 414 patients: 335 preoperative patients, 54 patients referred to the hematologist for bleeding evaluation, and 25 patients previously diagnosed with a bleeding disorder or hemostatic defect. This latter group included three (12%) patients with Glanzmann thrombasthenia (GT), a severe platelet function disorder (10, 17, 18). The remaining patients had other diagnoses, such as vWD type 1 (3), vWD type 2 (5), hemophilia A (7) or hemophilia B (1), carriers of hemophilia A (2) or hemophilia B (2), factor XI deficiency (1), and factor XII deficiency (1). shows baseline characteristics of all patients.
Table II. Patient characteristics.
LTA detected a PFD in 16 (4.8%) preoperative patients, and in 10 (18.5%) patients referred for bleeding evaluation. All PFDs that were identified were ‘thrombopathy not otherwise specified’ (NOS), usually classified as mild PFDs [Citation10,Citation19,Citation20]. In the group of patients with a previously diagnosed bleeding disorder, LTA abnormalities specific for GT were identified in all three patients with GT (LTA detection rate of 100%). In the other patients, as suspected, no LTA abnormalities were found. For details on PFDs as detected by the LTA, see Supplemental File 2.
No adverse events from performing the tests were reported. Not all tests were successfully accomplished in all patients, due to partially failed blood withdrawal or laboratory errors. The number of platelet function test results that were available for the analyses are shown in Supplemental File 3.
Test performance of Multiplate and PFA for the detection of PFDs
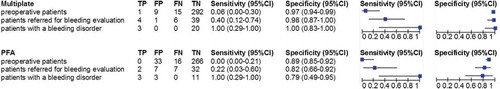
To assess whether Multiplate and PFA can detect PFDs (≥2 abnormal LTA agonists), the sensitivity and specificity of Multiplate and PFA for PFDs were calculated, shown in . Two-by-two tables for preoperative patients and patients referred for bleeding evaluation are shown in (two-by-two tables for patients with a diagnosed bleeding disorder are shown in Supplemental File 3.) In preoperative patients and patients referred for bleeding evaluation, sensitivity for the detection of PFDs with Multiplate was 6% and 40%, respectively, and for PFA 0% and 22%, respectively. Specificity was 97% and 98% for Multiplate in preoperative patients and referred patients respectively, and for PFA it was 89% and 82%, respectively. The NPV of Multiplate was 95% and 87%, respectively, and that of PFA 94% and 82%, respectively. In patients with a diagnosed bleeding disorder, both Multiplate and PFA showed abnormalities compatible with GT, meaning that the sensitivity was 100%. Specificity was 100% for Multiplate and 79% for PFA.
Table III. Multiplate and PFA results regarding the detection of PFDs on LTA.
Figure 1. Diagnostic performance of the Multiplate and PFA for detection of PFDs. Legend: Sensitivity and specificity of the Multiplate and PFA when using predefined cutoff levels in three patient groups, using the presence of PFDs as detected by the LTA as reference. For these calculations, the Multiplate was considered abnormal in case of ≥1 agonist abnormalities, and the PFA was considered abnormal in case of one or two prolonged PFA CTs (see Table 1). Abbreviations: FN, false negative. FP, false positive. PFA, platelet function analyzer. TN, true negative. TP, true positive.
Test performance of multiplate agonists and PFA kits for detection of LTA curve abnormalities
To assess whether individual Multiplate agonists and PFA kits can detect corresponding LTA curve abnormalities, ROC curve analyses were performed. These results are shown in and V (ROC curves and numbers of abnormal LTA curves are shown in Supplemental File 5). In preoperative patients and patients referred for bleeding evaluation, the AUCs of all Multiplate agonists and PFA kits varied around 0.50 (between 0.36 and 0.63), except for Multiplate ASPI with and AUC of 0.71 (95%CI 0.49–0.92) in preoperative patients and Multiplate TRAP with an AUC of 0.72 (95%CI 0.45–0.99) in referred patients. In patients with a previously diagnosed bleeding disorder, AUCs of 0.80 and higher, which significantly exceeded the nondiagnostic value of 0.50, were found for all Multiplate agonists and PFA kits, except for Multiplate COL with an AUC of 0.70 (95%CI 0.38–1.00) and PFA-EPI with an AUC of 0.74 (95%CI 0.33–1.00).
Table IV. Performance of Multiplate agonists compared to corresponding LTA agonists as reference test.
Table V. Performance of PFA kits compared to corresponding LTA agonists as reference test.
Discussion
We evaluated the diagnostic performance of Multiplate and PFA as screening tests for PFDs detected by LTA and their ability to detect corresponding LTA curve abnormalities in different patient populations. In both preoperative patients and patients referred for bleeding evaluation, only mild PFDs were found. The sensitivity for detection of mild PFDs was low for both tests at the predefined (conventionally used) cutoff values. In addition, the individual Multiplate agonists and PFA kits were not, or only poorly, able to identify corresponding LTA agonist abnormalities. However, for the detection of GT, which was present in the patient group with previously diagnosed bleeding disorders, the sensitivity of both Multiplate and PFA was high and both tests discriminated well between patients with and without GT. This suggests that Multiplate and PFA may be used to rule out severe PFDs like GT, but cannot be used as screening tests to rule out mild PFDs. The high NPVs for both tests are misleading. As we have many true negative test results, since most patients do not have a PFD, the NPV is high, but the few people with a PFD are mostly ‘missed’ by the Multiplate and PFA. This is demonstrated by the low sensitivity and implies that both Multiplate and PFA cannot detect the few patients with a mild PFD and therefore are not adequate screening tests.
This is the first study which directly evaluates the clinical utility of Multiplate as a screening test for PFDs in preoperative patients and patients referred for bleeding evaluation. Two previous studies compared LTA results with Multiplate in a total of 24 patients with GT. In these studies, all patients with GT were detected by Multiplate [Citation7,Citation21].
A small number of prospective studies investigated the value of PFA as a screening test for mild PFDs in patients referred for bleeding evaluation. These studies show that the overall sensitivity of the PFA for mild PFDs is poor [Citation22–Citation24]. In addition, many reviews as well as a recent guideline from the Platelet Physiology Subcommittee of the ISTH [Citation25] do not recommended PFA as a screening tool for PFDs [Citation2,Citation6,Citation9,Citation10,Citation19,Citation26–Citation28]. In the preoperative setting, to the best of our knowledge, no previous studies consistently related PFA outcomes to PFDs, as detected with LTA. In patients with diagnosed bleeding disorders, the value of PFA was assessed by several studies. These studies predominantly included patients with severe PFDs. In agreement with our findings, these studies reported a sensitivity of 80% and higher [Citation29–Citation31].
Although the survey by Gresele et al. showed that Multiplate and PFA are often used in clinical practice to screen for PFDs [Citation12], our results suggest that this practice is obsolete. Multiplate and PFA are not able to exclude the presence of mild PFDs (low sensitivity) and LTA curve abnormalities, but they might be able to exclude more severe PFDs. However, severe PFDs are unlikely to be encountered in preoperative patients and adult patients referred for bleeding evaluation, as demonstrated by our findings. The main reason for this is that patients with a severe PFD often undergo elaborate hemostatic diagnostic tests in childhood, including LTA, as these patients present early in life with a severe bleeding phenotype or are family members of a proband. However, if LTA testing is not possible, or when there is only a small amount of blood available (e.g. in children), Multiplate and PFA might be attractive alternatives for LTA to exclude GT and possibly other severe PFDs.
Our study has some limitations. Although we included a total of 414 patients, only three patients with severe PFDs were included; therefore, we cannot draw firm conclusions on the value of these tests to detect severe PFDs.
Furthermore, different LTA procedures regarding platelet count adjustment were used for different studies. The LTA measurements of the ProBe-AHP and BEPA studies were performed in our clinical laboratory, for which the procedure is to adjust the platelet count in PRP (CLSI guideline [Citation15]). The LTA measurements of the PANE study were performed in our research laboratory where non-adjustment of the platelet count in PRP is the procedure (ISTH-SCC guideline [Citation16]). Results of a study by Castilloux et al. [Citation32] showed that there is no difference in clinical diagnosis when adjusting is compared to non-adjusting.
The reference intervals of the LTA were not established according to recent guidelines, [Citation33] and the cutoff point of <60% used for abnormal aggregation might be too low for some agonists, according to the values in our healthy volunteers. However, the LTA curves were always visually checked for large variability between maximal aggregation of the agonists and specific curve abnormalities and all LTA results were discussed in a multidisciplinary team. Furthermore, the number of healthy volunteers used to calculate the reference intervals of the Multiplate was lower than the minimum number advised in the CLSI guideline [Citation15].
Finally, we recognize that the LTA may not be the ultimate gold standard test for PFDs. Tests like flow cytometry, transmission electron microscopy, or genetic tests might detect a PFD in patients with a normal LTA [Citation25]. In addition, these tests may have been able to diagnose the exact platelet function defect in some of our patients with ‘thrombopathy not otherwise specified’.
Conclusion
In preoperative patients and patients referred for bleeding evaluation, Multiplate and PFA were not able to discriminate between patients with and without PFDs and aggregation curve abnormalities as detected by LTA. This means that they cannot be used as screening tests to rule out mild PFDs in these populations. Both Multiplate and PFA can detect Glanzmann Thrombastenia, a severe PFD, in previously diagnosed patients.
Declaration of interest
Funding for this research was received from BAYER B.V., Siemens Healthineers Nederland and Roche Nederland B.V.
Supplemental_File_1.pdf
Download PDF (57.6 KB)Supplemental_File_2.pdf
Download PDF (15.5 KB)Supplemental_File_3.pdf
Download PDF (86.4 KB)Supplemental_File_4.pdf
Download PDF (47.8 KB)Supplemental_File_5.pdf
Download PDF (375 KB)References
- Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: A comparative review. Vasc Health Risk Manag 2015;11:133–148.
- Hayward CP. Diagnostic approach to platelet function disorders. Transfus Apher Sci 2008;38:65–76.
- Dawood BB, Lowe GC, Lordkipanidze M, Bem D, Daly ME, Makris M, et al. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood 2012;120:5041–5049.
- Cattaneo M, Hayward CP, Moffat KA, Pugliano MT, Liu Y, Michelson AD Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: A report from the platelet physiology subcommittee of the SSC of the ISTH. J Thromb Haemost 2009;7:1029.
- Cattaneo M. Light transmission aggregometry and ATP release for the diagnostic assessment of platelet function. Semin Thromb Hemost 2009;35:158–167.
- Harrison P, Mackie I, Mumford A, Briggs C, Liesner R, Winter M, et al. Guidelines for the laboratory investigation of heritable disorders of platelet function. Bjh 2011;155:30–44.
- Albanyan A, Al-Musa A, AlNounou R, Al Zahrani H, Nasr R, AlJefri A, et al. Diagnosis of Glanzmann thrombasthenia by whole blood impedance analyzer (MEA) vs. light transmission aggregometry. Int J Lab Hematol 2015;37:503–508.
- Görlinger K, Hanke A, Dirkmann D, Adamzik M, Hartmann M, Rahe-Meyer N. Perioperative coagulation management and control of platelet transfusion by point-of-care platelet function analysis. Transfus Med Hemother 2007;34:396–411.
- Harrison P. The role of PFA-100 testing in the investigation and management of haemostatic defects in children and adults. Bjh 2005;130:3–10.
- Podda G, Femia EA, Cattaneo M. Current and emerging approaches for evaluating platelet disorders. Int J Lab Hematol 2016;38:50–58.
- Orsini S, Noris P, Bury L, Heller PG, Santoro C, Kadir RA, et al. Bleeding risk of surgery and its prevention in patients with inherited platelet disorders. The Surgery in Platelet disorders And Therapeutic Approach (SPATA) study. Haematologica 2017 April 6. doi:10.3324/haematol.2016.160754. [ Epub ahead of print].
- Gresele P, Harrison P, Bury L, Falcinelli E, Gachet C, Hayward CP, et al. Diagnosis of suspected inherited platelet function disorders: Results of a worldwide survey. J Thromb Haemost 2014;12:1562–1569.
- Hayward CP, Moffat KA, Raby A, Israels S, Plumhoff E, Flynn G, et al. Development of North American consensus guidelines for medical laboratories that perform and interpret platelet function testing using light transmission aggregometry. Am J Clin Pathol 2010;134:955–963.
- Kuiper GJ, Houben R, Wetzels RJ, Verhezen PW, Van Oerle R, Ten CH, et al. The use of regression analysis in determining reference intervals for low hematocrit and thrombocyte count in multiple electrode aggregometry and platelet function analyzer 100 testing of platelet function. Platelets 2017 January 9. doi:10.1080/09537104.2016.1257782. [ Epub ahead of print].
- Clinical and Laboratory Standards Institute (CLSI). Platelet function testing by aggregometry; approved guideline. CLSI document H58-A (ISBN 1-56238-683-2). 950 West Valley Road, Suite 2500, Wayne, Pensylvannia 19087, USA: Clinical and Laboratory Standards Institute; 2008.
- Cattaneo M, C C, Harrison P, Hayward CP, Kenny D, Nugent D, et al. Recommendations for the standardization of light transmission aggregometry: A consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J Thromb Haemost 2013 April 10. doi:10.1111/jth.12231. [ Epub ahead of print].
- Moffat K, Ledford-Kraemer M, Nichols W, Hayward C. Variability in clinical laboratory practice in testing for disorders of platelet function. Results of two surveys of the North American Specialized Coagulation Laboratory. Thromb Haemost 2005;93:549–553.
- EP Evaluator product information available on: http://datainnovations.com/ep-evaluator, access date: 23-03-2017.
- Norman JE, Westbury SK, Jones ML, Mumford AD. How should we test for nonsevere heritable platelet function disorders? Int J Lab Hematol 2014;36:326–333.
- Nurden AT, Nurden P. Congenital platelet disorders and understanding of platelet function. Bjh 2014;165:165–178.
- Awidi A, Maqablah A, Dweik M, Bsoul N, Abu-Khader A. Comparison of platelet aggregation using light transmission and multiple electrode aggregometry in Glanzmann thrombasthenia. Platelets 2009;20:297–301.
- Quiroga T, Goycoolea M, Munoz B, Morales M, Aranda E, Panes O, et al. Template bleeding time and PFA-100 have low sensitivity to screen patients with hereditary mucocutaneous hemorrhages: comparative study in 148 patients. J Thromb Haemost 2004;2:892–898.
- Cattaneo M. Are the bleeding time and PFA-100 useful in the initial screening of patients with mucocutaneous bleedings of hereditary nature? J Thromb Haemost 2004;2:890–891.
- Podda GM, Bucciarelli P, Lussana F, Lecchi A, Cattaneo M. Usefulness of PFA-100 testing in the diagnostic screening of patients with suspected abnormalities of hemostasis: Comparison with the bleeding time. J Thromb Haemost 2007;5:2393–2398.
- Gresele P. Subcommittee on Platelet Physiology of the International Society on Thrombosis and Hemostasis. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost 2015;13:314–322.
- Hayward CP, Harrison P, Cattaneo M, Ortel TL, Rao AK. Platelet Physiology Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Platelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet function. J Thromb Haemost 2006;4:312–319.
- Hayward CP. Diagnostic evaluation of platelet function disorders. Blood Rev 2011;25:169–173.
- Favaloro EJ. Clinical utility of the PFA-100. Semin Thromb Hemost 2008;34:709–733.
- Cattaneo M, Federici AB, Lecchi A, Agati B, Lombardi R, Stabile F, et al. Evaluation of the PFA-100 system in the diagnosis and therapeutic monitoring of patients with von Willebrand disease. Thromb Haemost 1999;82:35–39.
- Posan E, McBane RD, Grill DE, Motsko CL, Nichols WL. Comparison of PFA-100 testing and bleeding time for detecting platelet hypofunction and von Willebrand disease in clinical practice. Thromb Haemost 2003;90:483–490.
- Mammen EF, Comp PC, Gosselin R, Greenberg C, Hoots WK, Kessler CM, et al. PFA-100 system: A new method for assessment of platelet dysfunction. Semin Thromb Hemost 1998;24:195–202.
- Castilloux JF, Moffat KA, Liu Y, Seecharan J, Pai M, Hayward CP. A prospective cohort study of light transmission platelet aggregometry for bleeding disorders: Is testing native platelet-rich plasma non-inferior to testing platelet count adjusted samples? Thromb Haemost 2011;106:675–682.
- Cp H, Ka M, Pai M, Liu Y, Seecharan J, McKay H, et al. An evaluation of methods for determining reference intervals for light transmission platelet aggregation tests on samples with normal or reduced platelet counts. Thromb Haemost 2008;100:134–145.
