Abstract
Pooled human platelet lysate (pHPL) has been used to expand adipose-derived stromal cells (ASCs) and can be formulated using fresh or expired buffy coats (BCs) which are then resuspended in either plasma or an additive solution. Not much is known about the effects that expired products and additive solutions have on ASC expansion, and the need for quality control and release criteria has been expressed. This pilot study compared proliferation, cell size, morphology and immunophenotype of ASCs expanded in the different pHPL alternatives versus foetal bovine serum (FBS). Quality control criteria were assessed prior to and during the manufacture of the pHPL alternatives. ASCs were then expanded in 1%, 2.5%, 5% or 10% of the different pHPL alternatives or in 10% FBS. Cell size, morphology, cell number and immunophenotype were measured using microscopy and flow cytometry. The majority of the pHPL alternatives were within the recommended ranges for the quality control criteria. ASCs expanded in the pHPL alternatives were smaller in size, displayed a tighter spindle-shaped morphology, increased cell growth and had a similar immunophenotype (with the exception of CD34 and CD36) when compared to ASCs expanded in FBS. Here we report on the effects that expired BC products and additive solutions have on ASC expansion. When taken together, our findings indicate that all of the pHPL alternatives can be considered to be suitable replacements for FBS for ASC expansion, and that expired BC products can be used as an alternative to fresh BC products.
Introduction
Adipose-derived stromal cells (ASCs) are multipotent cells with immunomodulatory properties that can be isolated from adipose tissue (Citation1). These properties have resulted in the use of ASCs for cellular therapies, as evidenced by the fact that their therapeutic effect is being assessed in over 180 clinical trials (Citation2,Citation3). Of those clinical trials that have listed their expansion conditions and media preparation (Citation4), the majority make use of foetal bovine serum (FBS), which has had adverse reactions on patients receiving the cells (Citation4). While FBS is essential for ASC attachment, expansion and maintenance, it does harbour the potential for xenoimmunisation and the transmission of zoonotic contaminants (Citation5). In an attempt to circumvent this, many research groups have moved away from FBS as the traditional serum supplementation in ASC expansion. Furthermore, the preparation of ASCs for cellular therapies needs to be compliant with good manufacturing practices (GMP). This can be achieved, in part, by changing to chemically defined, xeno-free or human blood-derived alternatives (Citation4,Citation6). These human blood-derived alternatives include human serum, platelet-rich plasma, platelet-poor plasma and platelet lysate (Citation7–Citation10). Studies comparing these alternatives make use of guidelines set out by the International Society of Cellular Therapy (ISCT) and International Fat Applied Technology Society for the characterisation of ASCs (Citation11,Citation12). These guidelines propose a set of criteria that need to be met when expanding ASCs which include their ability to adhere to plastic, express a predefined surface marker profile (immunophenotype) and have the ability to differentiate into adipose tissue, bone and cartilage (Citation11,Citation12). In vitro studies that have used human alternatives for ASC expansion have described faster proliferation, better morphology and a comparable immunophenotype for certain surface markers (Citation7–Citation9,Citation13,Citation14). In contrast, these studies have also reported differing trilineage differentiation capacities. It has therefore been suggested that the choice of human alternative should be determined on the basis of the proposed downstream application of the ASCs (Citation8,Citation15–Citation17). Human platelet lysate (HPL) has been used in the past few years as an alternative to FBS for GMP-compliant ASC expansion and is being used in several clinical trials (Citation3,Citation15,Citation18,Citation19). Both autologous (recipient-derived) and allogeneic (donor-derived) HPL have been investigated for ASC expansion with favourable outcomes (Citation20,Citation21). While autologous platelet lysate negates the need for testing for disease-causing agents, it is not always possible to produce sufficient volumes for expansion of ASCs. As an alternative, allogeneic platelet lysate can be pooled (pooled human platelet lysate, pHPL) in order to reduce batch-to-batch variation and to create larger quantities for ASC expansion (Citation9,Citation22). HPL is produced from platelet concentrates (PCs) which have been subjected to a series of freeze–thaw cycles and centrifugation, which leads to the release of bioactive molecules. One PC unit is comprised of four buffy coat (BC) units (the white blood cell and platelet fraction obtained following centrifugation), and is resuspended in either one unit of platelet-rich plasma or another additive solution (Citation22,Citation23). Expired PCs, which are usually discarded by blood banks after 4 or 5 days, can also be used to manufacture HPL, although there are very few reports on the use of expired PC products for the expansion of ASCs (Citation19,Citation24). In this study, we have investigated the effects that different pHPL alternatives (fresh versus expired BCs resuspended in either platelet-rich plasma or an additive solution), produced using quality controlled manufacturing protocols, have on ASC proliferation, morphology and immunophenotype.
Materials and methods
pHPL manufacture
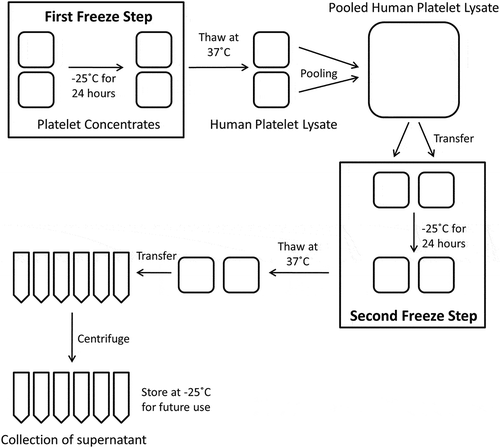
Four different PC formulations were used in this study and are summarised in . Two PCs for each of the alternatives () were obtained from the South African National Blood Service (SANBS). The expired PCs were stored on a platelet shaker at 20–24°C for 5 days after collection, whereafter they were considered expired. The fresh PCs were manufactured directly after collection. The pHPL alternatives were manufactured () as previously described (Citation22). The bags were frozen at −25°C for 24 hours after which the PCs were thawed in a protective cover (SteriZip™, OriGen Biomedical, Texas, USA) using a water bath (EcoBath, Labotec, South Africa) at 37°C. Once completely thawed, the two PC bags were pooled in a double pooling bag (Sterile 3 litre waste bag, Haemonetics Corporation, Massachusetts, USA) using a sterile connection device (TCD® Sterile Tube Welder with wafers, Genesis, New Jersey, USA) and sealed using a sterile tube sealer (Rapid Seal II™ SE540, Genesis, New Jersey, USA). The contents were mixed thoroughly and an aliquot was taken into a small Baxter bag (PEDI-PAK® Single 75 mL Transfer Bags, Genesis, New Jersey, USA) for sterility and pH testing. Roughly 250 mL of the pooled PCs was transferred from the double pooling bag into 600 mL Baxter bags (Platelet Transfer and Storage Bags, Macopharma, Mouvaux, France) and frozen at −25°C for 24 hours. The bags were thawed again and the contents of the bags were then transferred, under sterile conditions in a laminar flow cabinet, into 50 mL conical tubes (Corning, NY, USA). The 50 mL tubes were centrifuged at 4000g at 4°C for 15 min after which the remaining supernatant (pHPL) was transferred to new 50 mL tubes and stored at −25°C for future use.
Table I. Different types of PCs, their composition and their designated name.
Figure 1. A schematic illustration of the pHPL manufacturing process. Adapted from Schallmoser and Strunk (2013) (Citation22).
Quality control criteria for the pHPL alternatives
Quality control criteria () were assessed on the PCs prior to and during the manufacture of the different pHPL alternatives (Citation22). The criteria that were measured included platelet content, platelet volume, sterility, the presence of residual leukocytes and pH.
Table II. Quality control criteria, requirements and their outcomes in the pHPL alternatives.
Platelet count
Before the first freeze step, an aliquot was taken from each PC bag and used to determine the initial platelet count using a Gallios flow cytometer (Beckman Coulter, Miami, USA). The absolute number of platelets was obtained by adding 20 µL of the pHPL alternatives, 20 µL Flow-Count™ fluorospheres (Beckman Coulter, Miami, USA) and either 5 µL mouse anti-human CD42a-FITC or 5 µL mouse anti-human CD61-PE monoclonal antibodies to separate flow cytometry tubes (Beckman Coulter, Miami, USA). The cells were incubated for 10 min in the dark at room temperature (RT), whereafter they were analysed on a Gallios flow cytometer (Beckman Coulter, Miami, USA). Flow cytometry data were analysed post-acquisition using the Kaluza Post-Acquisition Flow Cytometry Analysis software (Version 1.3; Beckman Coulter, Miami, USA). The flow cytometric protocols and gating strategies (Figure S1) are summarised in the supplementary data.
Volume
Prior to the manufacture of the pHPL alternatives, each PC bag was weighed and the volume was calculated using the formula V = m/SG, where V is the volume, m is the mass and SG is the specific gravity (1.0.32, as used by the SANBS).
Sterility test
Sterility tests were performed on the PCs by the SANBS by making use of the Bactec9120 and the BactAlert 3D blood culture systems using their in-house protocols.
Residual leukocytes
Residual leukocytes were measured using the DNA Prep kit (Beckman Coulter, Miami, USA). Briefly, 100 µL cell suspension from each pHPL alternative and 100 µL LPR lysing buffer were added to a flow tube, vortexed and incubated at RT for 5 min. A 500 µL DNA Prep stain containing 50 μL/mg propidium iodide was added and incubated in the dark at RT. After 10 min, 100 µL Flow-Count™ fluorospheres was added to the tube and analysed on a Gallios flow cytometer. The flow cytometry protocols and gating strategies (Figure S2) are summarised in the supplementary data section.
pH
pH was measured in the aliquots taken during the pooling step of the pHPL manufacturing process. Aliquots were stored at 4°C and after 5 days (the clinically accepted shelf-life of PCs) and pH was measured using a CRISON micropH 2001 pH metre at 22°C (Crison Instruments, Barcelona, Spain).
Preparation of media
Media containing four concentrations (1%, 2.5%, 5% and 10%) of each of the pHPL alternatives was prepared as previously described with minor modifications (Citation22). Prior to the preparation of the media, the pHPL alternatives were heated to 37°C. The following reagents were added to alpha Modified Eagle’s Medium (αMEM) (GIBCO, Life Technologies™, New York, USA): 2% (v/v) penicillin [10 000 U/mL]-streptomycin [10 000 µg/mL] (p/s; GIBCO, Life Technologies™, New York, USA) and preservative-free Heparin ([2 U/mL]; Biochrom, Merck Millipore, Berlin, Germany). Thereafter, different volumes of pHPL and FBS were added to the media to produce the different concentrations of pHPL (1%, 2.5%, 5% or 10%), and FBS (10%) (Biochrom, Merck Millipore, Berlin, Germany). After the addition of pHPL or FBS, the medium was filtered through 0.22 µm sterile filtration units (Corning, New York, USA) and stored at 4°C until future use.
Cell culture and expansion
Three previously isolated (Citation25) cryopreserved ASC samples were thawed by adding pre-warmed (37°C) αMEM containing 10% (v/v) FBS and 2% (v/v) p/s to a cryopreservation tube. The liquid portion containing the cellular fraction was then transferred to a 15 mL conical tube (Corning, New York, USA). The above-mentioned step was repeated until the ASC sample was completely thawed. Once thawed, the cell suspension was centrifuged at 184g for 5 min. The pellet was resuspended, seeded into an 80 cm2 culture flask (NUNC™, Roskilde Site, Kamstrupvej, Denmark) and cultured in 10% FBS supplemented medium at 37°C in a 5% CO2 incubator (Thermo Forma CO2 water jacketed incubator, 3111TF). When the cells reached 70–80% confluence, they were dissociated using trypLE (Life Technologies™, New York, USA) and counted in a Gallios flow cytometer. The ASCs were seeded in duplicate at a density of 5000 cells/cm2 into six well plates for cell counts and microscopy, and seeded into 80 cm2 culture flasks for immunophenotyping, after which the respective media were added to the wells and flasks. The ASCs in the six well plates were incubated for 7 days, whereas the cells in the 80 cm2 culture flasks were cultured to confluence at 37°C in a 5% CO2 incubator. Fresh medium was added to the wells and flasks every second day.
ASC morphology and size
Fluorescence microscopy and adherent cell size
During the 7-day culture period, the size and morphology of the adherent ASCs was assessed using fluorescent staining techniques. At roughly 40–50% confluence, the medium was removed and the ASCs were washed twice with PBS. Thereafter, the cytoplasm and nuclei were stained by adding 2 mL of PBS, 20 µL carboxyfluorescein succinimidyl ester (Cell Trace® CSFE, Thermo Fischer, Waltham, Massachusetts, USA) and 2 µL Vybrant® DyeCycle™ Violet (VDC violet; Invitrogen/Molecular Probes®, Life Technologies, Eugene, Oregon, USA) to each well followed by incubation for 15 minutes at 37°C in a 5% CO2 incubator. After the incubation, the ASCs were washed twice with PBS and resuspended in 1 mL PBS. Immediately thereafter, micrographs (five per condition) were captured using a 10× magnification objective lens in an AxioVert A1 inverted fluorescence microscope (Carl Zeiss, Gottingen, Germany) equipped with an AxioCam Cm1 camera (Carl Zeiss, Gottingen, Germany). Each image represents an overlay converted from two single channel images, where the first channel (yellow-green fluorescence; filter set 9; excitation: bandpass filter 450–490; emission: longpass filter 515) captured the CSFE staining (visualisation of cytoplasm) and the second channel (DAPI fluorescence; filter set 49; excitation: green 365; emission: bandpass filter 445/50) captured VDC Violet staining (visualisation of nuclei). Images were captured using AxioVision software (Version 4.8.2, Carl Zeiss, Gottingen, Germany) and analysed post-acquisition using Image J imaging software (Version 1.49; Schneider et al., 2012) (Citation26). Images were enhanced for contrast and brightness. Thereafter, the images and the ASC sizes were analysed in CellProfiler (Citation27) using the Human Cells pipeline. Pixels were converted to micrometre using a conversion factor of 1 pixel = 0.212 µm2 as per the microscope specifications.
Light microscopy
After 7 days, the morphology of the adherent ASCs was assessed using a Zeiss AxioVertA1 microscope. Micrographs were taken at 5× magnification using a Zeiss Axiocam digital camera. Images were captured using AxioVision and analysed post-acquisition using Image J imaging software. Images were enhanced for contrast and brightness.
ASC absolute cell number
Absolute ASC number was assessed using flow cytometry after 7 days. Cells were dissociated using trypLE, and cells from each well were prepared for counting by preparing two duplicate flow cytometry tubes containing 50 µL of the cell suspension and 0.5 µL Vybrant® DyeCycle™ Ruby (VDC Ruby; Invitrogen/Molecular Probes®, Life Technologies, Eugene, Oregon, USA). After 5 min incubation in the dark at RT, 50 µL Flow-Count™ fluorospheres and 500 µL PBS were added to each tube. Each of the flow cytometry tubes was then analysed twice (technical replicates) on a Gallios flow cytometer. The flow cytometric protocols and gating strategies (Figure S3) are summarised in the supplementary data section.
Immunophenotype
ASC immunophenotype was measured using a panel of seven fluorochrome-conjugated monoclonal antibodies. The panel consisted of the following antibodies: CD73-FITC (eBiosciences, San Diego, USA); CD105-PE (BioLegend, San Diego, USA); CD34 PE-Cy7 (BioLegend, San Diego, USA); CD36 APC (BioLegend, San Diego, USA); CD44 APC/Cy7 (BioLegend, San Diego, USA); CD90 BV421 (BD Biosciences, San Jose, USA) and CD45 BV510 (BD Biosciences, San Jose, USA). At confluence, the ASCs were dissociated and 100 µL of cell suspension was simultaneously stained with all seven monoclonal antibodies after adding 5 µL of each antibody. The tubes were incubated for 15 minutes in the dark, whereafter 1 mL PBS was added to the tubes and centrifuged at 184g for 5 min. The PBS was aspirated and 700 µL PBS was added to the tube and analysed on a Gallios flow cytometer. Flow cytometry data were analysed post-acquisition using Kaluza Post-Acquisition Flow Cytometry Analysis software (Version 1.3). The intact ASC population was identified using forward scatter vs side scatter two parameter plots. All subsequent flow cytometric plots were gated on the intact ASC population. The positive expression of the different surface markers was identified using a one parameter log histogram plot, and the co-expression profiles were obtained using tree plots.
Statistical analysis
Data are expressed as mean ± SEM. To compare the means between FBS and pHPL expanded ASCs, a Kruskal–Wallis test, followed by a Dunn’s post hoc multiple comparisons test with a Bonferroni correction was employed. The significance level for all statistical analyses was set at α = 0.05 and a value of p < 0.05 was considered to be significant. Data and statistical analysis were performed in RStudio (Version 3.2.3) (Citation28).
Results
Quality control criteria for the pHPL alternatives
Quality control criteria were assessed for all of the alternatives and are summarised in . The platelet content for the Exp BCs + TSOL group (76.45 × 109 ± 7.47 × 109 platelets) was below the recommended range (>240 × 109 platelets). The remaining groups were above the recommended range, and the BCs + plasma group had the highest number of platelets (1179.64 × 109 ± 167.53 × 109) in the smallest volume (290.12 ± 56.29 mL). All the groups had a volume higher than the recommended volume (>40 mL per 60 × 109 platelets). The freshly isolated BC groups (BCs + Plasma and BCs + TSOL) had a greater platelet concentration (platelets/mL, 2.03 × 109 ± 0.29 × 109 and 0.91 × 109 ± 0.05 × 109, respectively) when compared to the Exp BC (Exp BCs + Plasma and Exp BCs + TSOL) groups (0.41 × 109 ± 0.005 × 109 and 0.19 × 109 ± 0.15 × 109, respectively). No bacterial contamination was found in any of the PCs which were confirmed to be negative by the sterility tests. A few residual leukocytes were detected in each of the pHPL alternatives; however, their number was below the recommended range. The pH for the BCs + Plasma, BCs + TSOL and Exp BCs + TSOL groups was 6.4–6.6, respectively and the Exp BCs + Plasma group was 7.5; however, they were all within the recommended range (>6.4).
ASC morphology and adherent cell size
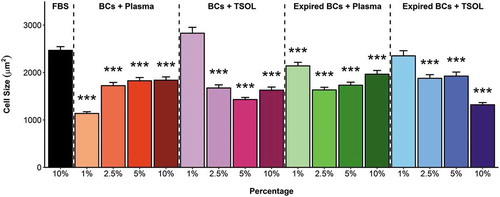
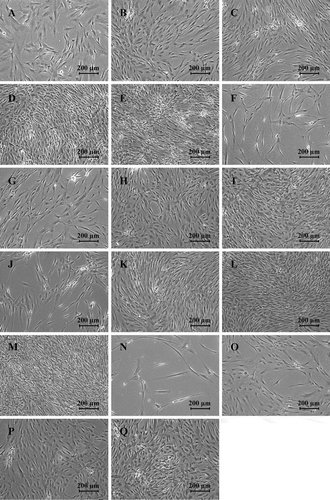
ASCs expanded in the pHPL alternatives displayed a smaller, tighter elongated shape (–)) when compared to ASCs expanded in FBS ()). When the cell size was quantitatively assessed, all the pHPL alternatives, except for the 1% BCs + TSOL and 1% Exp BCs + TSOL, were significantly smaller in size when compared to ASCs expanded in FBS (). There was no significant difference in cell size between 2.5%, 5% and 10% concentrations in the BCs + TSOL expanded ASCs, Exp BCs + Plasma expanded ASCs and the Exp BCs + TSOL expanded ASCs, where the opposite is true for BCs + Plasma. The 1% concentrations were significantly larger in size when compared to the 10% concentrations in all the pHPL alternatives (Table SI). The pHPL alternatives that were resuspended in plasma had the greatest effect on cell size; whereas the alternatives contacting TSOL had the least effect on cell size (Table SI).
Figure 2. Fluorescence micrographs of ASCs expanded in FBS and the pHPL alternatives at different concentrations. ASCs expanded in (A) 10% FBS; (B) 1% BCs + Plasma; (C) 2.5% BCs + Plasma; (D) 5% BCs + Plasma; (E) 10% BCs + Plasma; (F) 1% BCs + TSOL; (G) 2.5% BCs + TSOL; (H) 5% BCs + TSOL; (I) 10% BCs + TSOL; (J) 1% Expired BCs + Plasma; (K) 2.5% Expired BCs + Plasma; (L) 5% Expired BCs + Plasma; (M) 10% Expired BCs + Plasma; (N) 1% Expired BCs + TSOL; (O) 2.5% Expired BCs + TSOL; (P) 5% Expired BCs + TSOL; (Q) 10% Expired BCs + TSOL; captured at 10× magnification. The cytoplasm was stained with CSFE and the nuclei were stained with VDC Violet. Scale bars represent 100 μm.
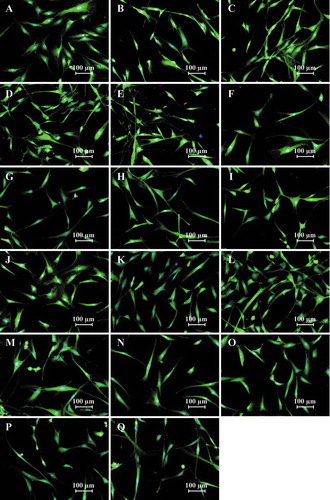
Figure 3. Size (µm2) of adherent ASCs expanded in FBS and pHPL alternatives. Bars represent mean ± SEM. Data are derived from three ASC biological replicates (n = 3) and statistical significance is represented by p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***). Statistical significance is only indicated for groups that were significantly different to FBS.
ASC absolute cell number
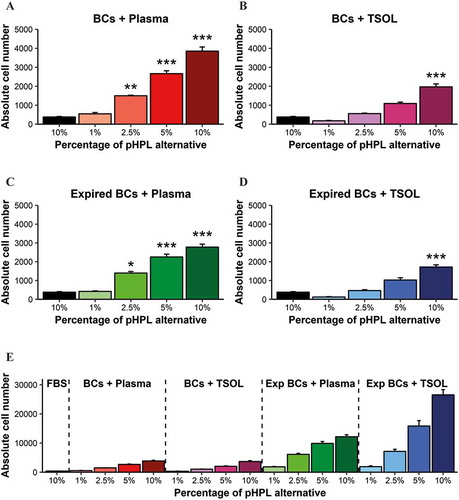
Of the 4 pHPL alternatives, the BCs + Plasma and the Exp BCs + Plasma group had the greatest effect on cell number () & (C)). Cell numbers in the 2.5%, 5% and 10% concentrations for both the BCs + Plasma and the Exp BCs + Plasma were significantly higher than in ASCs expanded in FBS. In contrast, only the 10% concentrations of the BCs + TSOL and Exp BCs + TSOL groups showed a significant increase in cell number when compared to ASC number in FBS () & ()).
Figure 4. Absolute number of ASCs (cells/µL) expanded in FBS and the pHPL alternatives at different concentrations. ASCs expanded in (A) BCs + Plasma; (B) BCs + TSOL; (C) Expired BCs + Plasma and (D) Expired BCs + TSOL. Bars represent mean ± standard error. Data are derived from three ASC biological replicates (n = 3) and statistical significance is represented by p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***). Statistical significance is only indicated for the groups that were significantly different to FBS. (E) The relative effect on absolute cell count when normalised to the starting platelet material of BCs + Plasma (significance is not indicated on the graph).
When examined under a light microscope, ASCs expanded in 10% pHPL alternatives (), (), () & ()) as well as the 5% BCs + Plasma and Exp BCs + Plasma groups () & ()) were almost 100% confluent after 7 days in culture, whereas the remaining ASC expanded in pHPL alternatives and the ASCs expanded in FBS ()) had not reached confluency by day seven.
Figure 5. Light micrographs of ASCs expanded in FBS and the pHPL alternatives at different concentrations after 7 days in culture. ASCs expanded in (A) 10% FBS; (B) 1% BCs + Plasma; (C) 2.5% BCs + Plasma; (D) 5% BCs + Plasma; (E) 10% BCs + Plasma; (F) 1% BCs + TSOL; (G) 2.5% BCs + TSOL; (H) 5% BCs + TSOL; (I) 10% BCs + TSOL; (J) 1% Expired BCs + Plasma; (K) 2.5% Expired BCs + Plasma; (L) 5% Expired BCs + Plasma; (M) 10% Expired BCs + Plasma; (N) 1% Expired BCs + TSOL; (O) 2.5% Expired BCs + TSOL; (P) 5% Expired BCs + TSOL; (Q) 10% Expired BCs + TSOL; captured at 5× magnification. Scale bars represent 200 μm.
Immunophenotype
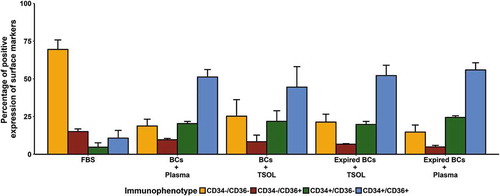
Changes in the co-expression profiles of extracellular cell surface markers (immunophenotype) were assessed using flow cytometry. The immunophenotype was analysed at confluence for ASCs expanded in FBS and the pHPL alternatives. Only the 10% concentrations were used to monitor changes in the immunophenotypic profiles (). ASCs expanded in FBS and the different pHPL conditions had similar immunophenotypic profiles (CD44+CD45−CD73+CD90+CD105+). The only surface markers affected by the exposure to the different pHPL alternatives were CD34 and CD36. The ASCs expanded in FBS displayed the classic immunophenotypic profile where 69.5 ± 6.3% of the ASCs were CD34−CD36− (). In contrast, approximately 50% of the ASCs expanded in the different pHPL alternatives were CD34+CD36+. Although the different subpopulations varied slightly between the different pHPL alternatives, they all shared a similar immunophenotypic profile. No significant differences in the immunophenotype were found between the freshly isolated BCs + Plasma and BCs + TSOL expanded ASCs when compared to the Exp BCs + Plasma and Exp BCs + TSOL expanded ASCs.
Figure 6. Changes in CD34 and CD36 immunophenotypic expression (percentage positive cell surface marker expression) in ASCs expanded in FBS and the pHPL alternatives. Data are displayed as the percentage of CD44+CD45−CD73+CD90+CD105+ ASCs that are either negative for both CD34 and CD36 (CD34−CD36−; yellow bar); negative for CD34 and positive for CD36 (CD34−CD36+; red bar); positive for CD34 and negative for CD36 (CD34+CD36−; green bar); or positive for both CD34 and CD36 (CD34+CD36+; blue bar). No significant differences were found in the subpopulations between ASCs expanded in the pHPL alternatives and ASCs expanded in FBS. Bars represent mean ± SEM. Data are derived from three ASC biological replicates (n = 3).
Discussion
The use of GMPs is essential for providing safe and regulated cell therapy products to patients. Therefore, the process of obtaining ASCs and any downstream manipulation or modification of these cells (expansion and differentiation) needs to adhere to GMP guidelines (Citation6). In the research setting, FBS is the standard serum supplement used for tissue culture and the in vitro expansion of cells. However, FBS has many disadvantages, such as batch-to-batch variation, xenoimmunisation and zoonotic transmission of infectious agents, and may therefore affect the outcome of experiments and ultimately render the cell product unacceptable for clinical use from a safety perspective (Citation1,Citation5,Citation9,Citation29–Citation34). Before ASCs can be used in the clinical setting, GMP-compliant standardised operating procedures as well as isolation and expansion protocols need to be in place (Citation9,Citation24). One of the key adjustments that need to be made during the transition from the research to the clinical setting is the replacement of FBS with alternatives that adhere to GMP standards, such as human-derived alternatives.
pHPL is currently used for the expansion of ASCs in clinical trials (Citation3,Citation4,Citation18). When compared to FBS, platelet-rich plasma and human serum, pHPL has been shown to produce ASCs with greater spindle-like morphology, proliferation and differentiation capacity, while the cells remain untransformed and retain their immunophenotype (Citation35,Citation36).
PCs are manufactured by blood donation services worldwide and are routinely used to treat patients that suffer from conditions associated with defective and/or decreased platelet function or count. Currently, the recommended shelf-life of PCs is 5 days, whereafter the expired PCs are routinely discarded. Expired PCs are therefore readily available and represent an attractive alternative for ASC expansion when compared to fresh PCs, which may be more expensive and difficult to obtain (Citation19,Citation37,Citation38).
In this study, the expansion of ASCs was compared in four different pHPL alternatives (BCs + plasma, BCs + TSOL, expired BCs + plasma and expired BCs + TSOL), at four different concentrations (10%, 5%, 2.5% and 1%) as well as to 10% FBS. Each of these alternatives was manufactured using the same protocol and subjected to the same quality control tests (Citation22). Cryopreserved ASCs were used to investigate the effect of the different pHPL alternatives and FBS supplemented media on ASC cell number, morphology, cell size and immunophenotype. The expansion of the cells in FBS prior to cryopreservation is not expected to affect the results, as it has been shown that ASCs initially expanded in FBS experience no priming effect when subsequently expanded in pHPL (Citation9). We acknowledge however that prior expansion of ASCs in FBS contradicts the need for homologous products, and freshly isolated ASCs expanded in the different alternatives from isolation would be ideal.
It has been suggested by Astori et al. that certain release criteria should be considered prior to using pHPL (Citation19). These criteria include the quality, composition, endotoxin levels and viral and bacterial safety of the pHPL product. In this study, this was achieved in part by using the quality control criteria () as set out by the by Schallmoser & Strunk in their pHPL protocol (Citation22). All of the pHPL alternatives used adhered to the above-mentioned quality control criteria. Although the total platelet content of the pHPL alternatives fell within the recommended ranges, we observed that the platelet concentrations (platelets/mL) were lower in the expired BC groups (Exp BCs + Plasma and Exp BCs + TSOL) compared to the fresh BC groups (BCs + Plasma and BCs + TSOL). This might be due to degradation of the platelets during storage and may explain why the proliferation of ASCs was slightly less in the expired BC groups when compared to their fresh BC group counterparts (). Furthermore, a platelet concentration below 1.5 × 109 platelets/mL has been shown to negatively impact the proliferation of ASCs (Citation16,Citation39). In this study, the platelet concentrations were approximately 2.03 × 109, 0.91 × 109, 0.41 × 109 and 0.19 × 109 for BCs + Plasma, BCs + TSOL, Exp BCs + Plasma and Exp BCs + TSOL. This major difference in platelet concentration would best explain the disparity in the expansion of the ASCs in the different alternatives. In addition, it has been suggested that platelet additive solutions may affect ASC expansion due to the presence or the lack of plasma components (Citation38). In future, to compare the fresh and expired alternatives more accurately, and to truly distinguish the effect of the fresh vs expired PCs and TSOL vs Plasma resuspension, the concentration of platelets (platelets/mL) should be the same in the starting PC prior to pHPL manufacture. Nonetheless, robust expansion of ASCs was achieved when the Exp BC alternatives were used, suggesting that Exp BC products might be an attractive alternative to freshly prepared BC products.
ASCs have a classic spindle-like morphology similar to that of fibroblasts (Citation40,Citation41). Differences in morphology between FBS and pHPL have been noted: ASCs expanded in FBS lose their spindle-like shape and change into a flattened, spread out shape, whereas ASCs expanded in pHPL display a tighter spindle-like shape and are smaller in size (Citation9,Citation40). Various studies have measured the size of non-adherent ASCs using flow cytometric techniques (Supplementary data) such as mean fluorescent intensities (Citation9,Citation40). We measured cell size in both adherent () and non-adherent (Figures S4 and S5) ASCs, and found that the size of adherent ASCs did not correlate with the size of non-adherent ASCs. Adherent ASCs expanded in FBS were significantly larger in size when compared to all of the pHPL alternatives except for the 1% BCs + TSOL and 1% Exp BCs + TSOL groups. These two groups had a similar morphology to that of FBS (large and spread out) which could be due to the cells becoming senescent (Citation42). It is well known that various components of serum and plasma promote spreading of adherent cells on tissue-culture plastic (Citation43,Citation44). TSOL is a chemical formulation (sodium citrate-acetate-chloride) and does not contain any of the biochemical compounds present in plasma (Citation45); the absence of these compounds may contribute to the decreased spreading observed when the cells were treated with the plasma-free additive, TSOL.
It has been well established that ASCs expanded in pHPL proliferate faster than ASCs expanded in FBS (Citation9,Citation14,Citation15). Although most studies have made use of pHPL that was manufactured from fresh BCs and resuspended in platelet-rich plasma, very little is known about the effects that expired BCs and TSOL have on ASC expansion (Citation19). Here we have shown that ASCs expanded in either of the 10% pHPL alternatives proliferate significantly faster than ASCs expanded in FBS. BCs + Plasma and Exp BCs + Plasma had the greatest effect on cell number ((A) and (C)). Although not investigated in this study, the difference in proliferation could be due to the presence of growth factors and other bioactive molecules present in plasma that are known to effect cell proliferation (Citation19). Previous studies have shown that ASCs expanded in either 10% or 5% pHPL display a greater proliferation and growth capacity compared to 10% FBS; however, no difference was found between 10% and 5% pHPL (Citation22,Citation46,Citation47). Contrary to the above-mentioned observations, we found significant differences between 10% and 5% within each of the pHPL alternatives (Supplementary data, Table S2). Because the platelet content of starting material of the different pHPL alternatives was different, comparison is limited. In an attempt to more accurately compare the alternatives, ASC counts were normalised to the platelet count of BCs + Plasma. Here we found that the weakest of the alternatives (Exp BCs + TSOL) would have had the greatest effect on ASC expansion in comparison to the rest ()). However; in future studies the starting material would need to be the same in all of the alternatives in order to be able to truly compare results between different alternatives.
We also recognise that ASC proliferation is not just a measure of cell number. Therefore in future studies, not only will absolute cell counting assays include cell count, but also apoptotic and cell cycle measurements undertaken at more regular intervals. In addition, in order to be able to truly detect changes in proliferation rates, the cells would need to be expanded and measured for a period longer than 7 days. As cells proliferate in vitro they undergo three prominent phases of growth, namely a lag/latent phase, an exponential phase and a plateau phase (Citation7,Citation48–Citation51). Depending on how rapidly the cells grow, 7 days be enough to capture all the cells in the exponential and plateau phase.
ASC immunophenotype is a widely studied and controversial topic. Studies have found that the immunophenotypic profiles differ significantly between ASCs derived from different donors, between ASCs obtained from different tissue sources, between ASC populations at different passages and to a large extent between individual cells within a given ASC population (Citation52–Citation56). To date, no immunophenotypic marker has been identified that is specific to ASCs. Numerous immunophenotypic panels have been proposed; however, no consensus exists as to which of these is ideal for the characterisation of ASCs in vitro (Citation12,Citation53,Citation55,Citation56). According to the ISCT, the classic ASC immunophenotype includes the surface markers CD36 (weakly positive), CD44, CD73, CD90, CD105 while CD34 and 45 should be negative (Citation11,Citation12). Studies comparing the classic immunophenotype (CD34−CD44+CD45−CD73+CD90+CD105+) of ASCs expanded in pHPL and FBS have shown that there is no significant difference between the surface markers investigated (Citation8,Citation9,Citation38,Citation57) or between the percentages of pHPL that were used for tissue culture (Citation15). In our study, we obtained similar findings, namely that ASCs expanded in FBS and the different pHPL alternatives had a CD44+CD45−CD73+CD90+CD105+ phenotype. CD36 is a multi-functional class B scavenger receptor present on various cell types including ASCs, adipocytes, phagocytes, platelets and others. It has been reported to act as a negative regulator of angiogenesis and is a known fatty acid translocase, due to its role in the binding and transportation of long chain fatty acids into cells (Citation58). CD36 is one of the few markers suggested by the ISCT that allows investigators to distinguish between bone marrow derived mesenchymal stem/stromal cells (MSCs) and ASCs (Citation12). Bone marrow-derived MSCs are reported to be negative for CD36, while ASCs express CD36 weakly (Citation12). In a recent study, Durandt et al. showed that a subpopulation of ASCs expanded in FBS inherently expresses higher levels of CD36 in vitro (Citation59). In the current study, we observed that the subpopulation of ASCs with higher levels of CD36 increases when ASCs are expanded in any of the pHPL alternatives (). The physiological relevance of the observed increase in CD36 expression was not studied and requires further investigation. Interestingly, immunophenotyping of ASCs expanded in the different pHPL alternatives revealed two additional subpopulations that were strongly positive for CD34. It is well known that all freshly isolated ASCs have increased CD34 expression which is lost during the expansion and culture process. This has led to several reports indicating that CD34 expression is variable and unstable in ASCs (Citation12,Citation52,Citation53,Citation60). Camilleri et al. suggested in a recent study that ASCs express CD34 weakly and that its expression can be modulated by confluency, whereby increased confluency resulted in increased expression of CD34. In our study, cells grown in the 10% pHPL alternatives were almost 100% confluent ((E), (I), (M) and (Q)) prior to being dissociated, which may explain the strongly positive CD34 expression. These findings are contradictory to a study comparing expired HPL resuspended in platelet additive solutions, in which the CD34 positivity was less than 5% (Citation38). Although the function of CD34 in ASCs is not clear, Suga et al. suggested in their study that it may play a role in the proliferative capacity, angiogenic potential, differentiation capabilities and stemness of these cells (Citation61).
The Schallmoser and Strunk (2013) protocol indicates that either fresh or expired BCs may be used, and these may be resuspended in an additive solution such as TSOL instead of platelet-rich plasma (Citation22). To the best of our knowledge, previous studies using pHPL were performed using fresh BCs + Plasma, or it was not stated which alternative was used. We believe our study is the first to perform a direct comparison between pHPL alternatives – BCs + TSOL, expired BCs + Plasma and expired BCs + TSOL – and FBS. Furthermore, it has been highlighted that there is a lack of data regarding expired pHPL alternatives (Citation19); here we present data that has revealed large differences in ASC proliferation, morphology, cell size and immunophenotype. Our experiments formed part of a pilot study, and we recognise that more in depth studies on these alternatives are needed. This is due to differences in the trilineage differentiation capacity that have been reported between ASCs expanded in different blood products (Citation8) as well as differences in growth factor concentrations between pHPL alternatives (Citation38). This study would also have benefitted from growth factor analysis as platelet-derived growth factors are known to play pivotal roles in cellular proliferation, differentiation and migration (Citation62–Citation64). Furthermore, ASCs expanded in FBS have had adverse immunogenic effects (Citation4,Citation65) when transplanted back into patients. In order to assess the immunomodulatory effects of ASCs expanded in the different pHPL alternatives, functional experiments should be performed both in vitro and in vivo.
In summary, we provide in-house protocols that can aid in establishing release and quality control criteria for pHPL manufacture. We found that 10% pHPL alternatives had the greatest effect on ASC proliferation; however, rapid proliferation and spindle-like morphology is maintained at decreased concentrations. We also found that immunophenotype was comparable between the pHPL alternatives. However, in order to prove the safety and efficacy of these pHPL alternatives, future studies should assess their effect on differentiation capacity, immunomodulation, an investigation of the transcriptome, proteome and secretome, and finally these cells should be studied in appropriate animal models.
Declaration of interest
The authors declare that they have no conflict of or competing interests.
Dessels_et_al.__Supplementary_data.docx
Download MS Word (1.6 MB)Acknowledgements
We would like to thank Prof. P. Coetzee (Head of Plastic Surgery, Steve Biko Academic Hospital) and Dr D. Hoffman (private practice) for their assistance with sample collection, Stephen Marrs and the team at Heamotec (South Africa) for the consumables and donation of their equipment, and SANBS for the blood products provided for the pHPL alternatives. This work was supported by grants from the South African Medical Research Council Flagship Awards Project SAMRC-RFA-UFSP-01-2013/STEM CELLS, the SAMRC Extramural Unit for Stem Cell Research and Therapy and the Institute for Cellular and Molecular Medicine of the University of Pretoria. The National Research Foundation of South Africa also provided funding.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi:10.1089/107632701300062859.
- Gimble JM, Ray SP, Zanata F, Wade J, Khoobehi K, Wu X, Ferreira LM, Bunnell BA. Adipose derived cells and tissues for regenerative medicine. ACS Biomater Sci Eng. 2016;3(8):1477–1482. acsbiomaterials.6b00261.
- Camilleri ET, Gustafson MP, Dudakovic A, Riester SM, Galeano Garces C, Paradise CR, Takai H, Karperien M, Cool S, Im Sampen H-J, et al. Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res Ther. 2016;7:1–16.
- Riis S, Zachar V, Boucher S, Vemuri MC, Pennisi CP, Fink T. Critical steps in the isolation and expansion of adipose-derived stem cells for translational therapy. Expert Rev Mol Med. 2015;17(e11):1–11. doi:10.1017/erm.2015.10.
- Van Der Valk J, Brunner D, De Smet K, Fex Svenningsen A, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, et al. Optimization of chemically defined cell culture media–replacing fetal bovine serum in mammalian in vitro methods. Toxicology In Vitro. 2010 [accessed 2013 May 23];24(4):1053–1063. http://www.ncbi.nlm.nih.gov/pubmed/20362047
- Giancola R, Bonfini T, Iacone A. Cell therapy: cGMP facilities and manufacturing. Muscles, Ligaments Tendons Journal. 2012;2(3):243–247.
- Atashi F, Serre-Beinier V, Nayernia Z, Pittet-Cuénod B, Modarressi A. Platelet rich plasma promotes proliferation of adipose derived mesenchymal stem cells via activation of AKT and Smad2 signaling pathways. J Stem Cell Res Ther. 2015;5(8):1–10.
- Koellensperger E, Bollinger N, Dexheimer V, Gramley F, Germann G, Leimer U. Choosing the right type of serum for different applications of human adipose tissue-derived stem cells: influence on proliferation and differentiation abilities. Cytotherapy. 2014 [accessed 2014 Oct 16];16(6):789–799. http://www.ncbi.nlm.nih.gov/pubmed/24642018. doi:10.1016/j.jcyt.2014.01.007.
- Trojahn Kølle S, Oliveri RS, Glovinski PV, Kirchhoff M, Mathiasen AB, Elberg JJ, Andersen PS, Drzewiecki KT, Fischer-Nielsen A. Pooled human platelet lysate versus fetal bovine serum – investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy. 2013;15(9):1086–1097. doi:10.1016/j.jcyt.2013.01.217.
- Patrikoski M, Sivula J, Huhtala H, Helminen M, Salo F, Mannerström B, Miettinen S. Different culture conditions modulate the immunological properties of adipose stem cells. Stem Cells Transl Med. 2014;3:1220–1230. doi:10.5966/sctm.2013-0201.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006 [accessed 2013 May 21];8(4):315–317. http://www.ncbi.nlm.nih.gov/pubmed/16923606. doi:10.1080/14653240600855905.
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–648. doi:10.1016/j.jcyt.2013.02.006.
- Bogdanova A, Berzins U, Nikulshin S, Skrastina D, Ezerta A, Legzdina D, Kozlovska T. Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J Stem Cells. 2014;9(3):135–148.
- Dessels C, Potgieter M, Pepper MS. Making the switch: alternatives to fetal bovine serum for adipose-derived stromal cell expansion. Front Dev Biol. 2016;4(October):1–10. doi:10.3389/fcell.2016.00115.
- Riis S, Nielsen F, Pennisi C, Zachar V, Fink T. Comparative analysis of media and supplements on initiation and expansion of adipose-derived stem cells. Stem Cells Transl Med. 2016;5(3):314–324. doi:10.5966/sctm.2015-0148.
- Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Desktop V in M. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;223(1):18–26. doi:10.1002/jcp.21081.
- Bernardo ME, Cometa AM, Pagliara D, Vinti L, Rossi F, Cristantielli R, Palumbo G, Locatelli F. Ex vivo expansion of mesenchymal stromal cells. Clin Haematol. 2011 [accessed 2013 Jun 10];24(1):73–81. http://www.ncbi.nlm.nih.gov/pubmed/21396595.
- Trojahn Kølle S-F, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman M-LM, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013 [accessed 2014 Oct 7];382(9898):1113–1120. http://www.ncbi.nlm.nih.gov/pubmed/24075051
- Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schäfer R, Sella S, Rodeghiero F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther. 2016;7(1):1–8. doi:10.1186/s13287-016-0352-x.
- Blande IS, Bassaneze V, Lavini-Ramos C, Fae KC, Kalil J, Miyakawa AA, Schettert IT, Krieger JE. Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion. 2009;49(12):2680–2685. doi:10.1111/j.1537-2995.2009.02346.x.
- Shih DT-B, Chen J-C, Chen W-Y, Kuo Y-P, Su C-Y, Burnouf T. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion. 2011 [accessed 2013 Jun 5];51(4):770–778. http://www.ncbi.nlm.nih.gov/pubmed/21044088. doi:10.1111/j.1537-2995.2010.02915.x.
- Schallmoser K, Strunk D. Generation of a pool of human platelet lysate and efficient use in cell culture. Methods Mol Biol. 2013;946:349–362.
- Iudicone P, Fioravanti D, Bonanno G, Miceli M, Lavorino C, Totta P, Frati L, Nuti M, Pierelli L. The secretion of these growth factors can be enhanced by activating the platelets with thrombin (Doucet et al., 2005; Kocaoemer et al., 2007). J Transl Med. 2014;12(28):1–14.
- Bieback K, Hecker A, Schlechter T, Hofmann I, Brousos N, Redmer T, Besser D, Hklü T, Ammü L, Becker M, et al. Replicative aging and differentiation potential of human adipose tissue-derived mesenchymal stromal cells expanded in pooled human or fetal bovine serum. Cytotherapy. 2012;14(5):570–583. doi:10.3109/14653249.2011.652809.
- Van Vollenstee FA, Jackson C, Hoffmann D, Potgieter M, Durandt C, Pepper MS. Human adipose derived mesenchymal stromal cells transduced with GFP lentiviral vectors: assessment of immunophenotype and differentiation capacity in vitro. Cytotechnology. 2016; 68(5): 2049–2060.
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9(7):671–675. doi:10.1038/nmeth.2089.
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. doi:10.1186/gb-2006-7-10-r100.
- RStudio Team. RStudio: integrated development environment for R. 2016.
- Van Der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, Hellebrekers L, Hyllner J, Jonker FH, Prieto P, et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicology In Vitro. 2004 [accessed 2013 May 21];18(1):1–12. http://linkinghub.elsevier.com/retrieve/pii/S0887233303001590
- Lennon DP, Haynesworth SE, Young RG, Dennis JE, Caplan AI. A chemically defined medium supports in vitro proliferation and maintains the osteochondral potential of rat marrow-derived mesenchymal stem cells. Exp Cell Res. 1995;219(1):211–222. doi:10.1006/excr.1995.1221.
- Lennon DP, Haynesworth SE, Bruder SP, Jaiswal N, Caplan AI. Human and animal mesenchymal progenitor cells from bone marrow: identification of serum for optimal selection and proliferation. In Vitro Cell Dev Biol Anim. 1996;32(10):602–611. doi:10.1007/BF02724045.
- Kyllonen L, Haimi S, Mannerstrom B, Huhtala H, Rajala KM, Skottman H, Sandor GK, Miettinen S, Kyllönen L, Mannerström B, et al. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem Res Ther. 2013;4(1):1–17. doi:10.1186/scrt165.
- Kocaoemer A, Kern S, Klüter H, Bieback K, Kluter H. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25(5):1270–1278. doi:10.1634/stemcells.2006-0627.
- Chieregato K, Castegnaro S, Madeo D, Astori G, Pegoraro M, Rodeghiero F. Epidermal growth factor, basic fibroblast growth factor and platelet-derived growth factor-bb can substitute for fetal bovine serum and compete with human platelet-rich plasma in the ex vivo expansion of mesenchymal stromal cells derived from adipose tissue. Cytotherapy. 2011;13(8):933–943. doi:10.3109/14653249.2011.583232.
- Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, Klüter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009 [accessed 2013 Jun 11];27(9):2331–2341. http://www.ncbi.nlm.nih.gov/pubmed/19544413. doi:10.1002/stem.139.
- Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23(9):1357–1366. doi:10.1634/stemcells.2005-0094.
- Jonsdottir-Buch SM, Lieder R, Sigurjonsson OE. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS ONE. 2013;8(7):1–9. doi:10.1371/journal.pone.0068984.
- Glovinski PV, Herly M, Mathiasen AB, Svalgaard JD, Borup R, Talman M-LM, Elberg JJ, Kølle S-FT, Drzewiecki KT, Fischer-Nielsen A. Overcoming the bottleneck of platelet lysate supply in large-scale clinical expansion of adipose-derived stem cells: a comparison of fresh versus three types of platelet lysates from outdated buffy coat–derived platelet concentrates. Cytotherapy. 2017;19(2):222–234. doi:10.1016/j.jcyt.2016.10.014.
- Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemotherapy. 2013;40(5):326–335. doi:10.1159/000354061.
- Naaijkens BA, Niessen HWM, Prins H-J, Krijnen PAJ, Kokhuis TJA, De Jong N, Van Hinsbergh VWM, Kamp O, Helder MN, Musters RJP, et al. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012;348(1):119–130. doi:10.1007/s00441-012-1360-5.
- Patrikoski M, Juntunen M, Boucher S, Campbell A, Vemuri MC, Mannerström B, Miettinen S. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy compliant human adipose stem cells. Stem Cell Res Ther. 2013 [accessed 2013 Jun 10];4(2):27. http://stemcellres.com/content/4/2/27/abstract. doi:10.1186/scrt175.
- Cholewa D, Stieh T, Schellenberg A, Bokermann G, Joussen S, Koch C, Walenda T, Pallua N, Marciniak-Czochra A, Suschek CV, et al. Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transplant. 2011;20(9):1409–1422.
- Massia S, Hubbell J. Convalent surface immobilization of Arg-Gly-Asp- and Tyr-Ile-Gly-Ser-Arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal Biochem. 1990;187(2):292–301. doi:10.1016/0003-2697(90)90459-M.
- Knox P, Griffiths S. The distribution of cell-spreading activities in sera: a quantitative approach. J Cell Sci. 1980;46:97–112.
- Zhang JG, Carter CJ, Culibrk B, Devine DV, Levin E, Scammell K, Weiss S, Gyongyossy-Issa MIC. Buffy-coat platelet variables and metabolism during storage in additive solutions or plasma. Transfusion. 2008;48(5):847–856. doi:10.1111/j.1537-2995.2008.01645.x.
- Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J, Dietz AB, Terzic A. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20(6):797–812. doi:10.3727/096368910X543376.
- Azouna NB, Jenhani F, Regaya Z, Berrais L, Ben Othman T, Ducroq E, Domenech J, Berraeis L, Othman TB, Ducrocq E. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res Ther. 2012;3(1):6. doi:10.1186/scrt97.
- Alenzi FQB. Links between apoptosis, proliferation and the cell cycle. Br J Biomed Sci. 2004;61(2):99–102. doi:10.1080/09674845.2004.11732652.
- Freshney RI. Basic principles of cell culture. In: Culture of cells for tissue engineering. Hoboken, NJ: John Wiley & Sons, Inc.; 2006. 3–22 p.
- Freshney RI. Culture of animal cells: a manual of basic technique and specialized applications. 6th ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2010. 1–717 p.
- Gérard C, Goldbeter A. The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface Focus. 2014;4(3):20130075. doi:10.1098/rsfs.2013.0075.
- Baer PC, Kuçi S, Krause M, Kuçi Z, Zielen S, Geiger H, Bader P, Schubert R. Comprehensive phenotypic characterization of human adipose-derived stromal/stem cells and their subsets by a high throughput technology. Stem Cells Dev. 2013;22(2):330–339. doi:10.1089/scd.2012.0346.
- Baer PC. Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6(3):256–265. doi:10.4252/wjsc.v6.i3.256.
- Ong WK, Tan CS, Chan KL, Goesantoso GG, Chan XHD, Chan E, Yin J, Yeo CR, Khoo CM, So JBY, et al. Identification of specific cell-surface markers of adipose-derived stem cells from subcutaneous and visceral fat depots. Stem Cell Reports. 2014;2(2):171–179. doi:10.1016/j.stemcr.2014.01.002.
- Donnenberg AD, Meyer EM, Rubin JP, Donnenberg VS. The cell-surface proteome of cultured adipose stromal cells. Cytometry. J Int Soc Anal Cytologyournal Int Soc Anal Cytology. 2015;87:665–674. doi:10.1002/cyto.a.22682.
- Januszyk M, Rennert R, Sorkin M, Maan Z, Wong L, Whittam A, Whitmore A, Duscher D, Gurtner G. Evaluating the effect of cell culture on gene expression in primary tissue samples using microfluidic-based single cell transcriptional analysis. Microarrays. 2015;4(4):540–550. doi:10.3390/microarrays4040540.
- Rauch C, Wechselberger J, Feifel E, Gstraunthaler G. Human platelet lysates successfully replace fetal bovine serum in adipose-derived adult stem cell culture. J Advanced Biotechnol Bioeng. 2014;2(2):39–48. doi:10.12970/2311-1755.2014.02.02.1.
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Cell. 2010;2(72):1–16.
- Durandt C, Vollenstee FAV, Dessels C, Kallmeyer K, Villiers DD, Murdoch C, Potgieter M, Pepper MS. Novel flow cytometric approach for the detection of adipocyte sub-populations during adipogenesis. J Lipid Res. 2016;57(4):729–742. doi:10.1194/jlr.D065664.
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi:10.1634/stemcells.2005-0234.
- Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18(8):1201–1210. doi:10.1089/scd.2009.0003.
- Hebert TL, Wu X, Yu G, Goh BC, Halvorsen YC, Moro C, Gimble JM. Culture effects of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) on cryopreserved human adipose- derived stromal/stem cell proliferation and adipogenesis. J Tissue Eng Regen Med. 2010;3(7):553–561. doi:10.1002/term.198.
- Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012;1(11):771–782. doi:10.5966/sctm.2010-0031.
- Chang X, Fan Y, Karyala S, Schwemberger S, Tomlinson CR, Sartor MA, Puga A. Ligand-independent regulation of transforming growth factor 1 expression and cell cycle progression by the aryl hydrocarbon receptor. Mol Cell Biol. 2007;27(17):6127–6139. doi:10.1128/MCB.00323-07.
- Sundin M, Ringdén O, Sundberg B, Nava S, Götherström C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92(9):1208–1215. doi:10.3324/haematol.11446.
