Abstract
Systemic inflammation and hypoxia frequently occur simultaneously in critically ill patients, and are both associated with platelet activation and coagulopathy. However, human in vivo data on the effects of hypoxia on platelet function and plasmatic coagulation under systemic inflammatory conditions are lacking. In the present study, 20 healthy male volunteers were randomized to either 3.5 h of hypoxia (peripheral saturation 80–85%) or normoxia (room air), and systemic inflammation was elicited by intravenous administration of 2 ng/kg endotoxin. Various parameters of platelet function and plasmatic coagulation were determined serially. Endotoxemia resulted in increased circulating platelet–monocyte complexes and enhanced platelet reactivity, effects which were attenuated by hypoxia. Furthermore, endotoxin administration resulted in decreased plasma levels of platelet factor-4 levels and increased concentrations of von Willebrand factor. These endotoxemia-induced effects were not influenced by hypoxia. Neither endotoxemia nor hypoxia affected thrombin generation. In conclusion, our data reveal that hypoxia attenuates the endotoxemia-induced increases in platelet–monocyte formation and platelet reactivity, while leaving parameters of plasmatic coagulation unaffected.
Introduction
Systemic inflammation is a commonly observed phenomenon in critically ill patients, for instance, in sepsis or following trauma. Systemic inflammation induces the activation of platelets and coagulation, ultimately resulting in coagulopathy, organ dysfunction, and worse outcome [Citation1]. Hypoxia is also frequently encountered in these patients, possibly due to the fact that tissue hypoxia can be one of the consequences of systemic inflammation, and is associated with adverse outcome as well. Hypoxia has also implied to directly activate coagulation. For instance, it has been associated with an increased risk of thrombotic events [Citation2], and was shown to increase platelet reactivity in rats [Citation3] and to enhance procoagulant activity of human endothelial cells in vitro [Citation4]. Therefore, next to systemic inflammation, hypoxia may also contribute to altered platelet function and coagulopathy in critically ill patients, thereby increasing their risks for organ dysfunction. Human in vivo data on the effects of hypoxia on platelet function and plasmatic coagulation during systemic inflammation are currently lacking. In the present study, we describe the effects of hypoxia on platelet function and plasmatic coagulation during experimental human endotoxemia, an in vivo model of systemic inflammation.
Methods
Study Design, Population, and Procedures
Data were obtained from healthy male volunteers participating in a randomized intervention study (registered at ClinicalTrials.gov: #NCT01978158) aimed at investigating the effects of oxygenation during systemic inflammation on immunologic (primary outcome) and many other parameters (provided at https://clinicaltrials.gov/ct2/show/NCT01978158). The primary outcomes have been published elsewhere and are freely accessible [Citation5]. The trial was approved by the local ethics committee and carried out according to GCP standards and the Declaration of Helsinki, including current revisions. Detailed methods are provided elsewhere [Citation5]. Briefly, after providing written informed consent, subjects were randomized to normoxia (room air) or hypoxia (titration of fraction of inspired oxygen to a peripheral oxygen saturation of 80–85%) for 3.5 h using an airtight respiratory helmet. One hour after initiation of hypoxia or normoxia, at time point t = 0, 2 ng/kg E. coli-derived endotoxin was administered intravenously. Blood was collected in 3.2% citrate-containing vacutainers at several time points.
Assays
Platelet counts were determined using an automated hematology cell counter. Platelet monocyte complexes (PMCs) were flow cytometrically analyzed after incubating whole blood with PE-labeled anti-CD14 (BD Biosciences) and FITC-labeled anti-CD42b (Bio-Legend). PMCs were defined as CD14+ cells positive for CD42b. Platelet reactivity was determined by measuring membrane expression of CD62P (P-selectin) in unstimulated whole blood and after ex vivo stimulation with seven increasing concentrations of adenosine diphosphate (ADP; Sigma-Aldrich) for 20 min. Antibodies used were PE-labeled anti-CD62P (Bio-Legend) and PC7-labeled anti-CD61 (Beckman Coulter). The mean fluorescent intensity of CD62P on CD61-postive platelets was determined by flow cytometry for each ADP concentration, and the area under the ADP-response curve was used as a measure for platelet reactivity. Thrombin generation was determined by means of calibrated automated thrombography, as described elsewhere [Citation6]. Plasma concentrations of von Willebrand factor (vWF), platelet factor-4 (PF4), and thrombin–antithrombin (TAT) complexes were measured using ELISA, as described previously [Citation7].
Statistical Analysis
Within-group changes over time were analyzed using one-way analysis of variance, and p-values are reported in the figure legends. Between-group differences over time were analyzed using general linear mixed models, and p-values are indicated in the figures. Data were analyzed with SPSS version 22 (IBM). A p-value of <0.05 was considered significant.
Results
As published elsewhere [Citation5], endotoxin administration resulted in transient flu-like symptoms (i.e., fever, headache, muscle ache, nausea) and increased plasma cytokines levels in all subjects. Hypoxia enhanced plasma concentrations of the anti-inflammatory cytokine interleukin-10 by 230%, whereas levels of pro-inflammatory mediators – tumor necrosis factor-α, interleukin (IL)-6 and IL-8 – were attenuated by 41%, 39%, and 37%, respectively [Citation5].
Platelet-Associated Parameters
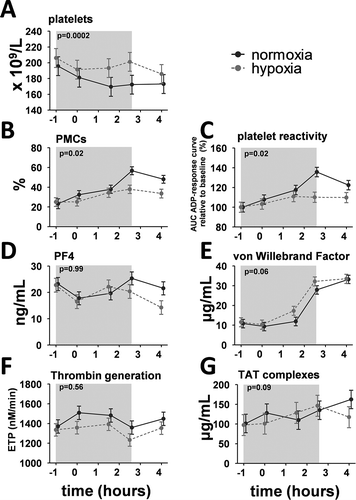
Platelet counts decreased slightly, but significantly, in normoxic endotoxemia subjects, an effect which was less pronounced in hypoxic subjects (). Percentages of circulating PMCs increased following endotoxin administration in both groups, but to significantly lesser extent in hypoxic subjects (). In both groups, platelet reactivity showed a very similar pattern as PMCs (). Plasma levels of PF4 in normoxic subjects tended to decrease and normalize afterward, and hypoxia did not modulate this effect ().
Figure 1. Time course of platelet function and coagulation parameters during experimental endotoxemia in normoxic and hypoxic healthy volunteers. (a) Whole blood platelet counts. Changes over time: normoxia p < 0.0001, hypoxia p < 0.0001. (b) Percentage of platelet–monocyte complexes (PMCs). Changes over time: normoxia p = 0.0006, hypoxia p = 0.03. (c) Platelet reactivity expressed as the change from baseline of the area under the adenosine diphosphate dose-response curve. Changes over time: normoxia p < 0.0001, hypoxia p = 0.41. (d) Plasma concentrations of platelet factor-4 (PF4). Changes over time: normoxia p = 0.08, hypoxia p = 0.001. (e) Plasma concentrations of von Willebrand Factor. Changes over time: normoxia p < 0.0001, hypoxia p < 0.0001. (f) Endogenous thrombin generation. Changes over time: normoxia: p = 0.08, hypoxia p = 0.10. (g) Plasma concentrations of thrombin–antithrombin (TAT) complexes. Changes over time: normoxia p = 0.03, hypoxia p = 0.007.
The gray box indicates the period during which subjects were exposed to hypoxia. Endotoxin was administered intravenously at t = 0 h. Data are expressed as the estimated mean with error obtained from the mixed linear model, and p-values reported in the panels express the difference between the normoxic and hypoxic endotoxemia model.
vWF Levels and Plasmatic Coagulation
Endotoxemia resulted in increased plasma concentrations of the endothelial activation marker vWF, with a trend toward slightly higher levels in the hypoxia group (). Plasmatic coagulation was evaluated by measuring thrombin generation and TAT complexes. Thrombin generation was unaffected following endotoxin administration (), while TAT complex concentrations gradually increased (). Both measures of plasmatic coagulation were not influenced by hypoxia.
Discussion
In the present study, we demonstrate that hypoxia mitigates the effects of systemic inflammation on platelet-associated parameters, as endotoxemia-induced decreases in platelet counts, and increases in PMCs and platelet reactivity were attenuated in hypoxic subjects. Hypoxia did not significantly influence the endotoxemia-induced increase in plasma levels of vWF and TAT complexes. Furthermore, concentrations of the platelet degranulation marker PF4 and ex vivo thrombin generation, a measure of plasmatic coagulation, were also unaffected by hypoxia.
In accordance with our results, previous work has consistently demonstrated that experimental human endotoxemia results in decreased platelet counts [Citation8], and increased PMCs [Citation9], platelet reactivity [Citation10], and plasma levels of vWF [Citation8,Citation10] and TAT complexes [Citation11]. Correspondingly, increases in platelet activation markers are observed in sepsis patients [Citation12]. Thrombin generation has not been previously assessed during endotoxemia, and our data reveal that it is not relevantly influenced by either systemic inflammation or hypoxia. The discrepancy between TAT complexes and thrombin generation may indicate that endotoxemia induces enhanced in vivo thrombin production, but that this does not result in a functional tendency toward hypercoagulation [Citation13].
The few human studies that have investigated the influence of hypoxia on platelets under noninflammatory conditions reported no effects of either 8 h of mild hypoxia [Citation14] or 7 min of deep hypoxia [Citation15] on soluble P-selectin levels, PMCs, and platelet reactivity. In contrast, deeply hypoxic rats displayed increased platelet activation and aggregation [Citation3]. Previous studies on the effects of hypoxia on plasmatic coagulation have yielded conflicting results. Some have reported increased concentrations of prothrombin fragments 1 + 2 (F1+2) and TAT complexes (both markers of thrombin formation) during hypoxia [Citation16], whereas others found no effects on either F1+2, TAT complexes, endogenous thrombin-generating potential, or several other measures of plasmatic coagulation [Citation14,Citation15,Citation17]. Taken together, most studies have not demonstrated procoagulant effects of hypoxia in humans in vivo, which is consistent with our current findings. Therefore, the assumption that hypoxia per se results in a procoagulant state is not supported by experimental data.
Several potential mechanisms may explain the effects of hypoxia on the endotoxemia-induced changes in platelet counts and coagulation parameters observed. Although platelets express Toll-like Receptor-4, the archetypal receptor for endotoxin, it is debated whether it can be directly activated by endotoxin [Citation12]. Therefore, it is plausible that the endotoxemia-induced effects on platelets are mediated by secondary mechanisms, for example, through the release of cytokines. In this context, it is important to emphasize that the hypoxic subjects in this study exhibited approximately 40% lower circulating levels of pro-inflammatory mediators [Citation5], which may explain the attenuated effects on platelets. Clearly, our work is limited by the fact that the experimental human endotoxemia model does not fully represent the inflammatory response observed in critically ill patients. Nevertheless, given the paucity of human in vivo data on this subject, our study does provide valuable insights into the complex interactions between inflammation, hypoxia, platelets, and coagulation. We report that hypoxia does not augment, but rather attenuates systemic inflammation-induced increases in PMC formation and platelet reactivity in humans in vivo, whereas it does not affect parameters of plasmatic coagulation.
Statement of contribution
DK, PP, QdM, and MK designed the experiments. DK, RT, and RB performed the experiments. GJS, PGG, RTU, and AJV supervised the experiments. DK drafted the manuscript. PP, QdM, and MK supervised the experiments and critically revised the manuscript.
Disclosure Statement
This work was supported by a PhD grant from the Radboud Centre for Infectious Diseases (RCI) and a young investigator Grant from the Dutch Society of Anesthesiology (to MK).
Additional information
Funding
References
- Levi M, Schultz M, Van Der Poll T. Sepsis and thrombosis. Semin Thromb Hemost 2013;39(5):559–566. doi:10.1055/s-00000077.
- Liak C, Fitzpatrick M, Dabsm F. Coagulability in obstructive sleep apnea. Can Respir J 2011;18(6):338–348. doi:10.1155/2011/924629.
- Tyagi T, Ahmad S, Gupta N, Sahu A, Ahmad Y, Nair V, Chatterjee T, Bajaj N, Sengupta S, Ganju L, et al. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood 2014;123(8):1250–1260. doi:10.1182/blood-2013-05-501924.
- Gertler J, Weibe D, Ocasio V, Abbott W. Hypoxia induces procoagulant activity in cultured human venous endothelium. J Vasc Surg 1991;13:428–433. doi:10.1067/mva.1991.25767.
- Kiers D, Wielockx B, Peters E, Eijk LT Van, Gerretsen J, John A, Janssen E, Groeneveld R, Peters M, Damen L, et al. Short-term hypoxia dampens inflammation in vivo via enhanced adenosine release and adenosine 2B receptor stimulation. EBioMedicine [Internet]. 2018;33:144–156. doi:10.1016/j.ebiom.2018.06.021.
- Hemker HC, Giesen P, Al Dieri R, Regnault V, De Smedt E, Wagenvoord R, Lecompte T, Béguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb 2003;33(1):4–15. doi:10.1159/000071636.
- Snoep JD, Roest M, Barendrecht AD, De Groot PG, Rosendaal FR, Van Der Bom JG. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J. Thromb. Haemost. [Internet]. 2010;8(5):906–913. Available: http://www.ncbi.nlm.nih.gov/pubmed/20128867
- Li N, Soop A, Sollevi A, Hjemdahl P. Multi-cellular activation in vivo by endotoxin in humans - limited protection by adenosine infusion. Thromb Haemost 2000;84(3):381–387. doi:10.1055/s-0037-1614032.
- Kälsch T, Elmas E, Nguyen XD, Suvajac N, Klüter H, Borggrefe M, Dempfle CE. Endotoxin-induced effects on platelets and monocytes in an in vivo model of inflammation. Basic Res Cardiol [Internet] 2007 Sep;102(5):460–466. [cited 2016 Feb 9]. Available: http://www.ncbi.nlm.nih.gov/pubmed/17624488
- Reitsma PH, Branger J, Van Den Blink B, Weijer S, Van Der Poll T, Meijers JCM. Procoagulant protein levels are differentially increased during human endotoxemia. J Thromb Haemost 2003;1(5):1019–1023. doi:10.1046/j.1538-7836.2003.00237.x.
- Derhaschnig U, Schweeger-Exeli I, Marsik C, Cardona F, Minuz P, Jilma B. Effects of aspirin and NO-aspirin (NCX 4016) on platelet function and coagulation in human endotoxemia. Platelets [Internet] 2010 Jan;21(5):320–328. [cited 2016 Jan 13]. Available: http://www.ncbi.nlm.nih.gov/pubmed/20608787
- de Stoppelaar SF, van ’T Veer C, van der Poll T. The role of platelets in sepsis. Thromb Haemost 2014;112(4):666–677. doi:10.1160/TH14-02-0126.
- Al Dieri R, De Laat B, Hemker HC, Thrombin generation: what have we learned? Blood Rev [Internet]. 2012;26(5):197–203. doi:10.1016/j.blre.2012.06.001.
- Toff WD, Jones CI, Ford I, Pearse RJ, Watson HG, Watt SJ, Ross JA, Gradwell DP, Batchelor AJ, Abrams, KR, et al. Effect of hypobaric hypoxia simulating conditions during long-haul air travel, on coagulation, fibrinolysis, platelet function and endothelial activation. JAMA 2015;295(19):2251–2262. doi:10.1001/jama.295.19.2251.
- Mäntysaari M, Joutsi-Korhonen L, Siimes MA, Siitonen S, Parkkola K, Lemponen M, Lassila R. Unaltered blood coagulation and platelet function in healthy subjects exposed to acute hypoxia. Aviat Sp Environ Med 2011;82(7):699–703. doi:10.3357/ASEM.3012.2011.
- Bendz B, Rostrup M, Sevre K, Andersen TO, Sandset PM. Association between acute hypobaric hypoxia and activation of coagulation in human beings. Lancet [Internet]. 2000;356(9242):1657–1658. Available: http://www.ncbi.nlm.nih.gov/pubmed/11089830
- Hodkinson P, Hunt B, Parmar K, Ernsting J. Is mild normobaric hypoxia a risk factor for venous thromboembolism? J Thromb Haemost 2003;1:2131–2133. doi:10.1046/j.1538-7836.2003.00407.x.
