Abstract
Higher aspirin doses may be inferior in ticagrelor-treated acute coronary syndrome (ACS) patients and reducing bleeding risk whilst maintaining antithrombotic benefits could improve outcomes. We characterized the pharmacodynamics of a novel dual-antiplatelet-therapy regimen consisting of very-low-dose twice-daily (BD) aspirin with standard-dose ticagrelor. A total of 20 ticagrelor-treated ACS patients entered a randomized crossover to take aspirin 20 mg BD (12-hourly) during one 14-day period and 75 mg once-daily (OD) in the other. After 14 days of treatment, serum thromboxane (TX)B2 and light-transmittance aggregometry were assessed pre- and 2 h post-morning-dose, bleeding time was measured post-dose, and TXA2 and prostacyclin stable metabolites were measured in urine collected 2 h post-morning-dose. Data are expressed as mean ± SD. After 14 days treatment, serum TXB2 levels were significantly greater 2 h post-dosing with aspirin 20 mg BD vs. 75 mg OD (3.0 ± 3.6 ng/mL vs. 0.8 ± 1.9 ng/mL; p = 0.018) whereas pre-dosing levels were not significantly different (3.5 ± 4.1 ng/mL vs. 2.5 ± 3.1 ng/mL, p = 0.23). 1-mmol/L arachidonic acid-induced platelet aggregation was similarly inhibited by both regimens pre-dose (8.5 ± 14.3% vs. 5.1 ± 3.6%, p = 0.24) and post-dose (8.7 ± 14.2% vs. 6.6 ± 5.3%; p = 0.41). Post-dose bleeding time was shorter with 20 mg BD (680 ± 306 s vs. 834 ± 386 s, p = 0.02). Urinary prostacyclin and TX metabolite excretion were not significantly different. In conclusion, compared to aspirin 75 mg OD, aspirin 20 mg BD provided consistent inhibition of platelet TXA2 release and aggregation, and improved post-dose hemostasis, in ticagrelor-treated ACS patients. Further studies are warranted to assess whether this regimen improves the balance of clinical efficacy and safety.
Abbreviations
| 8-iso-PGF2α: | = | 8-iso prostaglandin 2α |
| ACS: | = | acute coronary syndrome |
| BD: | = | twice-daily |
| COX: | = | cyclo-oxygenase |
| DAPT: | = | dual antiplatelet therapy |
| MACE: | = | major adverse cardiovascular events |
| OD: | = | once-daily |
| PLATO: | = | PLATelet inhibition and patient Outcomes |
| PGI2: | = | prostacyclin |
| TX: | = | thromboxane |
Introduction
The combination of the irreversible cyclo-oxygenase (COX) inhibitor aspirin (acetylsalicylic acid) 75–100 mg once-daily (OD) and the P2Y12 inhibitor ticagrelor 90 mg twice-daily (BD) represents a standard recommended regimen of dual antiplatelet therapy (DAPT) in acute coronary syndromes (ACS) [Citation1–Citation4]. The PLATelet inhibition and patient Outcomes (PLATO) study showed that DAPT with aspirin and ticagrelor was superior to the previous standard regimen of aspirin and clopidogrel in ACS, reducing the incidence of major adverse cardiovascular events (MACE). Despite such potent antiplatelet therapy, the residual MACE risk remains around 10% at 1 year [Citation5].
Continuing DAPT with aspirin and reduced-dose ticagrelor long-term in stable high-risk individuals further decreases the risk of MACE compared with aspirin alone [Citation6]. However, increasing the potency or duration of DAPT also leads to increased bleeding, which is associated not only with higher mortality but also patient distress, inconvenience, and premature discontinuation, even if events are considered minor or trivial by clinicians [Citation7,Citation8]. Concern about bleeding risk may, therefore, dissuade clinicians from recommending DAPT, particularly in the long-term, to the potential detriment of high-risk patients.
Further subgroup analysis of the PLATO study suggested that doses of aspirin ≥ 300 mg were associated with reduced benefit of ticagrelor over clopidogrel and, as a result, daily aspirin doses >100 mg are not recommended when used in DAPT [Citation9,Citation10].
Aspirin and ticagrelor have additive antithrombotic effects [Citation11]. Whilst ticagrelor inhibits the platelet adenosine diphosphate P2Y12 receptor, aspirin’s action relates to its ability to irreversibly inhibit platelet COX-1, thus reducing prothrombotic eicosanoid TXA2 production. Aspirin can achieve almost-complete (≥95%) inhibition of TXA2 biosynthesis in healthy subjects at repeated daily doses as low as 20–30 mg [Citation12–Citation14]. At doses above 300 mg, aspirin also inhibits COX-2 in humans, a key enzyme in the pathway of constitutive, vascular-protective prostacyclin (PGI2) release by the endothelium [Citation12,Citation15]. Inhibition of COX-2 and PGI2 biosynthesis may be counterproductive in patients with ACS: both traditional and COX-2-selective nonsteroidal anti-inflammatory drugs are associated with an increased risk of MACE [Citation16–Citation18]. There is also evidence from animal studies that ticagrelor may beneficially induce COX-2 and endothelial nitric oxide synthase through an adenosine-dependent mechanism, which may limit myocardial infarct size through synergistic antiplatelet and vasodilatory effects of PGI2, nitric oxide and P2Y12 inhibition [Citation19–Citation21]. Therefore, inhibition of COX-2 by high-dose aspirin may compromise the beneficial effects of ticagrelor on this pathway.
Aspirin dosing frequency may also influence pharmacodynamic efficacy: OD aspirin administration may be insufficient to maintain consistency of platelet inhibition over 24 h in patients with higher platelet turnover, including smokers and those with obesity or diabetes mellitus, or in individuals undergoing procedural intervention [Citation22–Citation27]. Multiple daily-dosing regimens of aspirin have been shown to improve consistency of pharmacodynamic effect, not only in conditions with extreme acceleration of platelet turnover, such as essential thrombocythemia [Citation28–Citation30], but also in patients with ischemic heart disease [Citation25,Citation27,Citation31–Citation33] when administered as single antiplatelet therapy. However, such regimens of aspirin have not been studied in those receiving dual antiplatelet therapy (DAPT) with ticagrelor, which is already given BD, in whom the overall pharmacodynamic profile of DAPT may potentially be improved by BD aspirin dosing.
An ideal regimen of aspirin and ticagrelor would be one that maintains the anti-ischemic benefit of DAPT through effective and steady inhibition of platelet COX-1 and P2Y12, ensures consistency of effect across the dosing interval, avoids inhibition of vasoprotective COX-2-dependent PGI2 biosynthesis and has minimized effects on hemostatic capacity. We hypothesized that ticagrelor administered with a lower-than-standard, multiple-daily dose of aspirin establishes a beneficial hemostatic profile and so we investigated a novel regimen of aspirin 20 mg BD and ticagrelor 90 mg BD in patients treated for ACS.
Methods
In the WILL lOWer dose aspirin be more effective in ACS? (WILLOW ACS) study, 20 patients with a history of recent ACS established on ticagrelor-based DAPT provided informed consent to participate in this study. To proceed to randomization, participants were required to meet the following inclusion criteria: male or female aged greater than 18 years; previous diagnosis of acute coronary syndrome greater than 30 days and less than 10 months before enrolment; and receiving DAPT with aspirin 75 mg OD and ticagrelor 90 mg BD. Participants were excluded if they met any of the following criteria: indication for DAPT other than ischaemic heart disease; percutaneous coronary intervention with drug-eluting or bare-metal stent(s) within 30 days prior to randomization; any history of stent implantation to the left main coronary artery; any history of stent thrombosis during DAPT; further planned coronary revascularization procedure; any planned surgery or other procedure that might require suspension or discontinuation of DAPT expected to occur within 3 months of randomization; prior intention by patient or physician to discontinue aspirin and/or ticagrelor within the study period; doses of aspirin and ticagrelor other than 75 mg OD and 90 mg BD, respectively; treatment or planned treatment with antiplatelet medication apart from aspirin or ticagrelor (eg. clopidogrel, prasugrel, dipyridamole or ticlopidine); currently receiving a diuretic agent (including loop, thiazide or potassium-sparing diuretics) as these may affect prostanoid assays; any ACS event within 30 days prior to randomization; most recent ACS event greater than 10 months prior to randomization; current or planned use of an oral anticoagulant (e.g. warfarin, dabigatran, rivaroxaban, apixaban), parenteral anticoagulant (e.g. unfractionated heparin, low-molecular-weight heparin, bivalirudin), glycoprotein IIb/IIIa inhibitor (e.g. abciximab, tirofiban) or fibrinolytic agent (e.g. tissue plasminogen activator); or requiring, or likely to require, treatment with a nonsteroidal anti-inflammatory drug, including intermittent/as required use; history of acute or chronic liver disease (e.g. cirrhosis), end-stage renal failure requiring dialysis, alcohol or drug abuse, defined as regular use of an illicit substance for recreational purposes or regular consumption of greater than 50 units (males) or 35 units (females) of alcohol per week in the last year; any comorbidity associated with life expectancy less than 1 year; or any other condition deemed by the investigator to affect hemostasis, coagulation, bleeding risk or ability to comply with the study protocol. To prevent interaction with ticagrelor treatment, those receiving a strong inhibitor of cytochrome P450 3A (e.g. ketoconazole, itraconazole, voriconazole, telithromycin, clarithromycin, nefazadone, ritonavir, saquinavir, nelfinavir, indinavir, atanazavir, or over 1 L daily of grapefruit juice), simvastatin or lovastatin at doses higher than 40 mg daily, a cytochrome P450 3A substrate with a narrow therapeutic index (e.g. cyclosporine or quinidine), or a strong inducer of cytochrome P450 3A (e.g. rifampin, rifabutin, phenytoin, carbamazepine, phenobarbital) were also excluded.
Females of child-bearing potential were similarly prevented from proceeding to randomization unless they had a negative pregnancy test at screening and were willing to use protocol-defined effective contraception for the duration of treatment with study medication.
Study Design
After collection of baseline demographic and clinical information, participants were randomized using opaque-sealed envelopes shuffled by an individual outside of the research team, in a 1:1 ratio, to one of two sequences of different regimens of soluble aspirin in an open-label crossover design ():
Aspirin 20 mg BD (12-hourly, morning and evening) for 14 days then aspirin 75 mg OD (in the morning) for 14 days.
Aspirin 75 mg OD (in the morning) for 14 days then aspirin 20 mg BD (12-hourly, morning and evening) for 14 days.
Figure 1. Overall design of the WILL lOWer dose aspirin be more effective in ACS? (WILLOW ACS) study. ACS, acute coronary syndrome; BD, twice daily; mg, milligrams; OD, once daily; R, point of randomization.
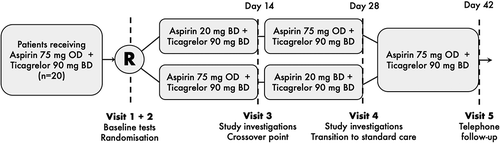
All other usual medications, including ticagrelor 90 mg BD (12-hourly, morning and evening), were continued throughout the study in all participants.
Blood samples for serum thromboxane B2 (TXB2) and light transmittance aggregometry (LTA), urine samples for PGI2 metabolite and TX metabolite, and bleeding time measurement using a standard lancet method were obtained at the following time points:
After 14 days of study medication, pre-aspirin dose (platelet function and serum TXB2), representing trough effect, and 2 h post-dose (platelet function, serum TXB2, urinary prostanoids and bleeding time), representing peak effect.
After 28 days (end of second study medication period) pre-aspirin dose (platelet function and serum TXB2) and 2 h post-dose (platelet function, serum TXB2, urinary prostanoids and bleeding time).
Treatment periods of 14 days ensured that steady-state effects of aspirin were reached, whilst sampling 2 h after aspirin dose meant it could be confidently asserted that the time to maximal plasma concentration had elapsed, and so peak effects on platelet function, which persist beyond this point, were being observed [Citation34,Citation35].
Clinical outcomes were reviewed and adverse events recorded at 14 and 28 days. At the 28-day visit, patients with an indication for ongoing DAPT were transitioned to standard care with aspirin 75 mg OD and arrangements for ongoing supply of this medication were ensured. A telephone contact was made at 14 days after study medication discontinuation to ensure the successful transition to standard care and to record any adverse events for safety monitoring. Vital signs, physical examination findings and concurrent medications were recorded at each timepoint. On the last day of each medication period, participants were asked to take their ticagrelor on waking but delay aspirin until they attended for the study visit.
Drug Supply and Accountability
To ensure accurate titration of aspirin doses, a fully soluble aspirin lysine preparation was used (Aspegic; Sanofi-Aventis, Machelen, Belgium). Each “100 mg” sachet contained 180 mg of aspirin lysine, including 100 mg of acetylsalicylic acid (aspirin). Participants were provided with tuition, illustrated written instructions and dosing equipment. They were asked to dissolve the whole of a 100 mg aspirin sachet in 100 mL of drinking water, measured in a graduated beaker (Sarstedt, Nümbrecht, Germany), stirring for 30 s to ensure full dissolution. To prepare 20 mg, they were asked to withdraw 20 mL of the solution using a marked syringe (Beckton-Dickinson, Franklin Lakes, New Jersey, USA) and ingest that amount, discarding the remainder. To dispense 75 mg they were asked to withdraw 25 mL and ingest the remainder. A new sachet was used for each dose to minimize drug hydrolysis once in solution.
Participants were provided with a medication diary to record times of aspirin and ticagrelor administration during the study, in order to assess compliance with study medication, which was also measured by counting returned aspirin sachets.
Serum Thromboxane B2
Whole blood was collected into 5 mL serum separator tubes (Becton-Dickinson, New Jersey, USA) and immediately placed into a water bath, preheated to 37oC, for 30 min, as previously described [Citation36,Citation37]. Tubes were then centrifuged, serum was drawn off and stored at −80oC until analysis. Levels of the nonenzymatic metabolite of TXA2, TXB2, which in contrast to its precursor is highly stable in serum and therefore more suitable for measurement as a marker of platelet COX-1 activity [Citation37], were estimated using a commercially available enzyme-linked immunosorbent assay (Cayman Chemical, Ann Arbor, USA). Samples were assessed twice and repeated if significant discrepancies were detected between the measurements. Interplate coefficient of variability (CV) for a standard mid-range concentration of TXB2 (62.5 pg/mL) was 14.3% in our laboratory, and mean intraplate CV was 9.5%.
Light Transmittance Aggregometry
A broad assessment of platelet reactivity was made pre- and 2 h post-dose at the end of each treatment period using 6-min LTA with arachidonic acid (0.1, 0.3 and 1.0 mmol/L), collagen (1, 4 and 16 µg/mL) and adenosine diphosphate (20 µmol/L) as agonists (PAP-8 v2.0, Bio/Data Corporation, Horsham, PA, USA; stir speed 1200 rpm). Maximum aggregation response, adjusted for baseline aggregation, was recorded for each agonist. Samples were assessed in duplicate, taking the mean value for analysis, and repeated if a discrepancy of >10% was observed between the readings.
Bleeding Time
Hemostasis was assessed using a standard lancet method (Haemolance Max Flow Plus, HTL-STREFA S.A., Poland). A calibrated sphygmomanometer cuff (Welch Allyn, Buckinghamshire, UK) was inflated around the upper arm to 40 mmHg and three incisions made on the volar aspect of the forearm. Each puncture site was checked for hemostasis every 30 s using filter paper (Whatman Grade 1, GE Healthcare, Little Chalfont, UK) until bleeding stopped or 30 min was reached. A mean of bleeding times was calculated. The method was validated in healthy volunteers receiving no medication (n = 5).
Urinary Prostanoids
Midstream urine was collected around 2 h after the last study medication dose of each treatment period, centrifuged to remove any cellular material and then stored at −80oC prior to analysis. Urine PGI2 metabolite (2,3-dinor-6-keto prostaglandin F1α, PGI2 metabolite) was measured by gas chromatography-mass spectrometry, as previously described [Citation38,Citation39].
Urinary TX metabolite (11-dehydro-TXB2) and 8-iso prostaglandin F2α (8-iso PGF2α), a marker of oxidant stress, were measured by previously validated immunoassays [Citation40]. Urinary prostanoids were corrected for urinary creatinine levels, measured by the Department of Laboratory Medicine, Northern General Hospital, Sheffield, using a clinically accredited automated assay (Roche, Basel, Switzerland).
Fibrin Clot Dynamics
Aspirin is known to affect fibrin clot dynamics [Citation41,Citation42]. To study the effects of the two aspirin regimens on clot formation and lysis, high-throughput turbidimetric analysis was performed as described and validated elsewhere [Citation43–Citation47]. Briefly, plasma samples were mixed with standard lysis and activation mixes to form acellular clots. Serial absorbance was measured using an automated plate reader until lysis was achieved. Variables recorded were maximum absorbance (a representation of fibrin clot turbidity), lag time (time from addition of clot activation mix to the start of clot formation) and lysis time (time taken for turbidity to drop by 50% from maximum as a measure of lysis potential).
Safety Monitoring
Although primarily a pharmacodynamic study, we monitored safety throughout: the primary safety objective was to estimate the incidence of PLATO-defined major plus minor bleeding at 14 days in patients treated with ticagrelor and either one of the two aspirin dosing regimens [Citation5]. Additionally, all adverse events (AEs) occurring between randomization and the telephone call 14 days after completion of study treatment were recorded. Serious adverse events (SAEs) were defined as any that resulted in death, was life-threatening, required hospitalization or prolongation of existing hospitalization, resulted in persistent disability or incapacity or consisted of a congenital abnormality or birth defect. Causality was assessed by the investigators. All AEs were followed up until resolved or stable.
Statistical Analysis
The primary endpoint of the study was 2-h post-dose serum TXB2, compared within patients between the two dosing regimens by a paired t-test. Based on our previous data showing a mean serum TXB2 level of 2.9 ng/mL following ticagrelor 90 mg and aspirin 75 mg maintenance doses [Citation36], we estimated that 20 patients entering the crossover design would have 80% power to detect a difference of 1.7 ng/mL in post-dose serum TXB2 between the regimens with alpha of 0.05. Based on previous data showing a mean circulating TXB2 level in non-aspirin-treated individuals (both healthy volunteers and patients with atherosclerotic disease) of around 45 ng/mL [Citation15,Citation48], we estimated that a difference of 1.7 ng/mL would represent a change of 3.8% in TXB2 inhibition.
Statistical analysis was performed using GraphPad PRISM version 7.03 (GraphPad Software Inc., La Jolla, CA, USA), using an alpha of 0.05 to determine significance. Continuous data are presented as mean ± standard deviation (SD). Where data were significantly skewed (as was the case for TXB2), log transformation was performed prior to analysis. Analysis was by intention to treat.
Study Oversight
Approval to conduct the study was granted by the Sheffield National Research Ethics Service Committee, by the Medicines and Healthcare products Regulatory Authority and by the Health Research Authority prior to commencement of research activities, which were monitored by the Sheffield Teaching Hospitals National Health Service Foundation Trust Clinical Research Office. The study was registered with clinicaltrials.gov (NCT02741817) before commencing any research activity.
Results
Twenty participants (16 males and 4 females) were randomized between July and December 2016, and all completed the study. Baseline characteristics are shown in . Sampling times, compliance and physiological observations were similar between the two treatment regimens ().
Table I. Baseline demographics of the 20 randomized patients.
Table II. Comparison of study medication period duration, sampling times and compliance between the 20 mg BD and 75 mg OD aspirin regimens. Values are shown as mean ± SD and p values were generated by paired t-tests.
Serum TXB2
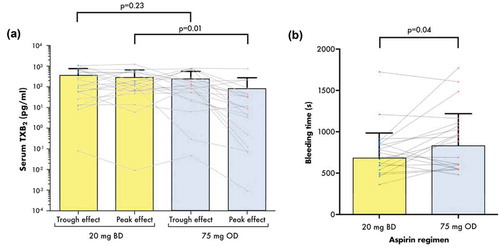
At steady state, mean (± SD) serum TXB2 levels 2 h post-dose were significantly greater when receiving 20 mg BD when compared to 75 mg OD (3.03 ± 3.64 ng/mL vs. 0.83 ± 1.93 ng/mL, p = 0.018) (, ) and there was no significant difference between the regimens at pre-dose, which corresponds to the trough effect (3.51 ± 4.07 ng/mL vs. 2.48 ± 3.14 ng/mL, p = 0.23) (, ). There was no evidence of a carryover effect between the dosing regimens: no significant differences in pre- or post-dose TXB2 measurements were observed between the participants who received 20 mg BD in the first period vs. the second (pre-dose p = 0.768, 2 h post-dose p = 0.774, data not shown) and between those who received 75 mg OD in the first vs. the second (pre-dose p = 0.907, post-dose p = 0.914, data not shown).
Table III. Results of analyses of pharmacodynamic endpoints during each aspirin dosing regimen at steady state. Values shown are mean ± SD. p values were generated from paired t-tests.
Figure 2. Serum TXB2 (a) and bleeding time (b) in ACS patients receiving two regimens of aspirin and ticagrelor in the WILLOW ACS study. Bars represent mean + SD. Dots and lines represent paired values for the individual participants. p values shown were generated by paired t-tests between the groups (see text and tables for further details). BD, twice-daily; OD, once-daily s, seconds; sTXB2, serum thromboxane B2. the groups (see text and tables for further details). Scale on the y-axis in Figure 2(a) is logarithmic. BD, twice-daily; OD, once-daily s, seconds; TXB2, thromboxane B2.
Bleeding Time
Bleeding time measured 2 h post-dose in each treatment period was significantly shorter when receiving aspirin 20 mg BD compared to 75 mg OD (679.5 ± 305.5 vs. 833 ± 385.7 s, p = 0.04), a mean reduction of 154.5 s (18.5%) (, ). For comparison, mean ± SD bleeding time in five healthy drug-free volunteers was 342 ± 72 s, with a mean intrasubject variation of 24 s (7%).
Light Transmittance Aggregometry
There were no significant differences in arachidonic acid- or adenosine diphosphate-induced maximum aggregation responses between the regimens (, ).
Figure 3. Maximum platelet aggregation responses to arachidonic acid, adenosine diphosphate and collagen assessed by light transmittance aggregometry pre- and post-aspirin dose at the end of each treatment period in the WILLOW ACS study. Bars indicate mean + SD. p values were generated using prespecified paired t-tests. AA, arachidonic acid; ADP, adenosine diphosphate; BD, twice-daily; OD, once-daily.
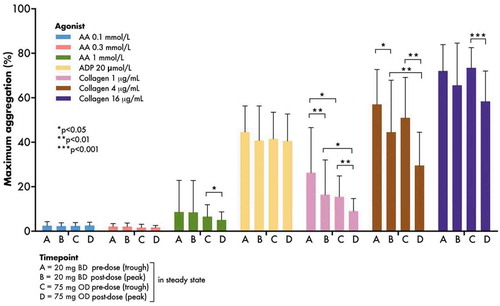
Two-hours post-dose maximum aggregation responses to collagen 1 and 4 µg/mL (but not 16 µg/mL) were greater when receiving aspirin 20 mg BD compared to 75 mg OD (mean ± SD: 20 mg BD 16.5 ± 15.6% vs. 75 mg OD 9.0 ± 5.7%, p = 0.03; and 20 mg BD 44.6 ± 23.3 vs. 75 mg OD 29.6 ± 14.9%, p = 0.007 for collagen 1 and 4 µg/mL, respectively). Pre-dose maximum aggregation to collagen 1 µg/mL (26.3 ± 20.3 vs. 15.5 ± 9.4%, p = 0.03), but not 4 or 16 µg/mL, was greater when receiving aspirin 20 mg BD (, ). Given the universally low, though expected, responses to arachidonic acid, the efficacy of the agonist was tested in drug-free platelet-rich plasma from healthy volunteers (n = 8): mean (± SD) maximum aggregation in response to stimulation with 1 mmol/L arachidonic acid was 79.1 ± 7.4%.
Urinary Prostanoids
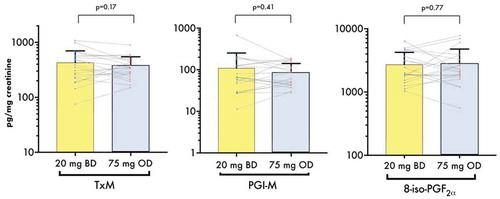
Urinary TX metabolite, which represents COX-1 activity over a broader time range than serum TXB2, showed no significant difference comparing BD vs. OD regimens (430.0 ± 269.7 vs. 371.5 ± 176.6 pg/mg creatinine, p = 0.17 for paired comparison). Urine PGI-M and 8-iso-PGF2α levels did not significantly differ between the two groups (, ).
Figure 4. Urinary levels of TX metabolite, PGI2 metabolite 8-iso-PGF2α measured at the end of each treatment period. Bars represent mean + SD. Dots and lines represent paired values for the individual participants. p values shown were generated by paired t-tests between the groups. Scale on the y-axis is logarithmic. 8-iso-PGF2α, 8-iso prostaglandin F2α; BD, twice- daily; PGI-M, prostacyclin metabolite; OD, once-daily; TxM, thromboxane metabolite.
Fibrin Clot Parameters
Given the importance of fibrin network lysis in cardiovascular outcome following acute coronary syndrome [Citation47], and the effects of aspirin on the fibrin clot [Citation41], we studied potential differences in a number of fibrin clot properties comparing the two different dosing regimens of aspirin. We found no significant differences in lag time (a measure of clotting tendency), clot maximum absorbance (a measure of clot density and fiber thickness) or lysis time (a measure of resistance of clot to breakdown) between the regimens pre- or post-aspirin dosing in the two groups studied (supplementary material).
Safety
There were no PLATO-defined major or minor bleeding events and no SAEs occurred during the study, including any thrombotic events. Two participants suffered trivial bleeding events during the aspirin 75 mg OD period, compared to none whilst receiving aspirin 20 mg BD (Supplementary Table I). No participant stopped study medication prematurely.
Effect of Weight and Body Mass Index
Study participants had a median enrolment weight of 86 kg (range 55–106) and body mass index (BMI) of 28.6 kg/m2 (22.6–37.1). There were no significant correlations between body weight or BMI and TX metabolite or PGI2 metabolite when receiving either the novel or standard regimen (Supplementary Figure 1), suggesting the efficacy of the novel regimen was maintained across the range of weight and BMI.
Discussion
DAPT with aspirin and ticagrelor represents a standard maintenance antithrombotic therapy that is recommended as first-line treatment following ACS [Citation3,Citation4,Citation49]. Typically given for at least 1 year, continuation for longer remains an effective strategy for preventing MACE in high-risk patients but comes at the price of increased bleeding [Citation6]. Even though the overall balance of mortality risks appears to favor use of longer-term ticagrelor-based DAPT in high-risk patients [Citation50–Citation52], clinicians and patients alike may be reluctant to extend DAPT therapy due to the bleeding risk. It has been proposed that aspirin can safely be stopped in ticagrelor-treated patients with a history of percutaneous coronary intervention [Citation53], an approach tested in the recently reported GLOBAL LEADERS study, which showed that 1 month of DAPT followed by 23 months of ticagrelor monotherapy was not superior to 12 months of DAPT followed by 12 months of aspirin monotherapy, although this was limited by heterogeneity of P2Y12 receptor antagonists in the control group [Citation54]. Even if ticagrelor monotherapy is effective in preventing stent thrombosis, there are likely to remain a significant group of patients at high risk of ongoing native plaque rupture events in whom DAPT is needed to optimize protection against future MACE.
Strategies maintaining the combined antithrombotic effect of DAPT, whilst reducing bleeding tendency, therefore have the potential to improve overall clinical outcomes. We have shown that a novel regimen of very-low-dose BD aspirin given to ticagrelor-treated ACS patients broadly maintains the inhibitory effects of aspirin on TXA2 synthesis and arachidonic acid-induced platelet activation, but reduces peak inhibition and is associated with a significant reduction in bleeding time (). It remains to be determined whether this will translate into a reduction in clinical bleeding events, but similar doses have previously been shown to have clinical antithrombotic efficacy. The European Stroke Prevention Study 2 investigated a similar very-low-dose, BD aspirin regimen (25 mg BD) alone or in combination with another antiplatelet drug (dipyridamole) in 6602 stroke patients, showing a significant benefit of BD aspirin, alone or in combination, vs. placebo in preventing recurrent cerebrovascular events [Citation55]. Giving aspirin BD may also improve symmetry of DAPT effect when given with ticagrelor, which is also given BD, and may simplify drug intake, including the possibility of developing a combination tablet. This might help to address the under-recognized issue of treatment compliance that can limit the efficacy of treatment strategies in coronary artery disease patients [Citation56], and can be improved by reducing the number of tablets they receive [Citation57].
Figure 5. Conceptual figure summarizing the anticipated profiles of COX-1 inhibition and hemostasis provided by maintenance aspirin doses of 75 mg OD or 20 mg BD in combination with ticagrelor 90 mg BD, during steady-state. Figure for illustrative purposes only, scale on the y-axis is arbitrary. Dashed line represents effects on COX-1 and hemostasis at steady-state trough levels. BD, twice-daily; COX-1, cyclo-oxygenase 1; mg, milligrams; OD, once-daily.
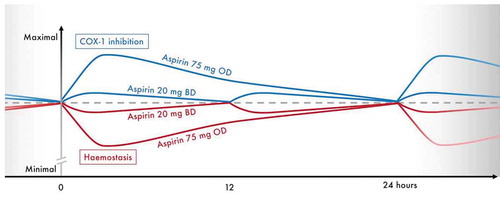
The use of very-low-dose twice-daily aspirin in this setting is only considered feasible when given in combination with ticagrelor which, in contrast to older drugs, provides potent and reliable P2Y12 inhibition [Citation58]. P2Y12 inhibitors reduce AA-induced platelet aggregation independently of aspirin [Citation59], meaning there may be a rationale for reducing the intensity of aspirin therapy. However, the results of our study also illustrate the fact that aspirin continues to provide additional antiplatelet effects even in the presence of potent P2Y12 inhibition, given that we saw differences in TX-related biomarkers and platelet function between the two dosing regimens at 2 h post-dose. This is consistent with previous studies of the relationship between aspirin and P2Y12 receptor inhibition or deficiency [Citation60]. Hence, there is justification for an approach that continues to include aspirin but seeks to reduce its intensity to improve hemostasis whilst maintaining adequate levels of platelet inhibition.
In pre-dose samples, we saw similar levels of overall platelet inhibition with the two regimens, but it is possible there may be differences at the platelet level in the pattern of inhibition between the two regimens at this timepoint given that some newly formed platelets will be more rapidly exposed to aspirin with the BD regimen. Even a small number of uninhibited platelets can form the basis for thrombosis [Citation61]. However, the clinical efficacy and safety of a BD aspirin regimen remains to be explored.
The study was limited by a small sample size and therefore was unable to determine whether the very-low-dose aspirin regimen significantly improves in vivo prostacyclin biosynthesis so this will require assessment in a larger study. The goal of the current study was to provide reassurance from a pharmacodynamic study that such a larger study is appropriate. Similarly, we only compared one novel regimen with standard therapy, rather than including multiple permutations; however, our results suggest that aspirin 20 mg BD and ticagrelor 90 mg BD achieve our goal of reducing peak-trough variation in effect and improving hemostasis.
We saw no significant correlation between weight or BMI and COX-1 inhibition in the present study (supplementary material) but lacked sufficient numbers of obese patients, with or without diabetes, to determine levels of COX-1 inhibition in these individuals who tend to have diminished response to low-dose aspirin [Citation62]. Consequently, further pharmacodynamic studies focussing on obese or diabetes patients are required to understand how aspirin regimens may potentially be modified in these individuals when combined with ticagrelor.
In conclusion, aspirin dose modification represents a novel and feasible strategy to be investigated for optimizing the balance of antithrombotic benefits and bleeding-related risks in ticagrelor-treated ACS patients, and demands further study to determine whether this translates into improvements in net clinical outcomes.
Statement of contribution
W.A.E. Parker, R.F. Storey, H. M. Judge and J. Hanson designed the protocol; R.F. Storey was principal investigator for the study; W.A.E. Parker and R.F. Storey drafted the manuscript; W.A.E. Parker and R.F. Storey supervised patient recruitment and study procedures; W.A.E. Parker, R.C. Orme, J. Hanson, H.M. Stokes, P.A. Shaw and H.M. Judge conducted study procedures; W.A.E. Parker and R.F. Storey designed the statistical analyses and analyzed the data; K. Thorneycroft and W.A.E. Parker obtained healthy volunteer data; B. Rocca, G. Petrucci and B. Porro undertook urinary prostanoid measurements; W. Sumaya and R. A. Ajjan oversaw tests of fibrin clot dynamics; all authors carried out study procedures, critically revised the manuscript for important intellectual content and approved the final manuscript.
Declarations of interest
William A. E. Parker: nominated by University of Sheffield as inventor on patent application related to the study results. Wael Sumaya: speakers fees from Bayer. Bianca Rocca: institutional research grants from the Italian Medicines Agency (AIFA); consultancy fees from Bayer AG, speaker fees from Amgen, Celgene, Daiichi Sankyo Italia, Novartis Farma, and Sanofi. Robert F. Storey: institutional research grants from AstraZeneca and PlaqueTec; consultancy fees from AstraZeneca, Avacta, Bayer, Bristol Myers Squibb, Haemonetics, Idorsia, Novartis, Thromboserin and PlaqueTec; speaker fees from AstraZeneca and Bayer; nominated by University of Sheffield as inventor on patent application related to the study results. The other authors have no relevant disclosures.
Supplemental Material
Download MS Word (1.8 MB)Acknowledgments
The authors thank Dr. Nana Theodorou and Dr. Erica Wallis (Clinical Research Office), Kim Ryalls and Tim Hopkins (Pharmacy), and Charlotte Culver (Cardiology and Cardiothoracic Surgery Directorate) from Sheffield Teaching Hospitals NHS Foundation Trust; and Kathleen Baster from the Statistical Services Unit, University of Sheffield. This work was funded by University of Sheffield via the Higher Education Innovation Funding scheme. The study was supported by the National Institute for Health Research (NIHR) award to Sheffield Clinical Research Facility; the views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol 2013;61:e78–e140. doi:10.1016/j.jacc.2012.11.019
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol 2014;64:e139–228. doi:10.1016/j.jacc.2014.09.017
- Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. doi:10.1093/eurheartj/ehs215
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2015;37:267–315.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057.
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. New Engl J Med 2015;372:1791–1800.
- Ducrocq G, Schulte PJ, Becker RC, Cannon CP, Harrington RA, Held C, Himmelmann A, Lassila R, Storey RF, Sorbets E, et al. Association of spontaneous and procedure-related bleeds with short- and long-term mortality after acute coronary syndromes: an analysis from the PLATO trial. EuroIntervention 2015;11:737–745.
- Bonaca MP, Bhatt DL, Oude Ophuis T, Steg PG, Storey R, Cohen M, Kuder J, Im K, Magnani G, Budaj A, et al. Long-term tolerability of ticagrelor for the secondary prevention of major adverse cardiovascular events: a secondary analysis of the PEGASUS-TIMI 54 Trial. JAMA Cardiol 2016;1:425–432.
- Mahaffey KW, Wojdyla DM, Carroll K, Becker RC, Storey RF, Angiolillo DJ, Held C, Cannon CP, James S, Pieper KS, et al. Ticagrelor compared with clopidogrel by geographic region in the platelet inhibition and patient outcomes (PLATO) trial. Circulation 2011;124:544–554.
- FDA. Highlights of prescribing information - Brillinta (Ticagrelor). DE, USA: AstraZeneca; 2016.
- Teng R, Maya J, Butler K. Evaluation of the pharmacokinetics and pharmacodynamics of ticagrelor co-administered with aspirin in healthy volunteers. Platelets 2013;24:615–624.
- Davi G, Custro N, Novo S, Mattina A, Strano A. The effect of two low doses of aspirin on whole blood thromboxane and prostacyclin generation in healthy subjects. Thromb Haemost 1983;50:669–670.
- Weksler BB, Pett SB, Alonso D, Richter RC, Stelzer P, Subramanian V, Tack-Goldman K, Gay WA Jr. Differential inhibition by aspirin of vascular and platelet prostaglandin synthesis in atherosclerotic patients. N Engl J Med 1983;308:800–805.
- Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest 1982;69:1366–1372.
- FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ, Lawson JA, Brash AR. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest 1983;71:676–688.
- Bally M, Dendukuri N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, Brophy JM. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ 2017;357:j1909. doi:10.1136/bmj.j2024
- Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet 2004;364:2021–2029.
- Kirkby NS, Lundberg MH, Wright WR, Warner TD, Paul-Clark MJ, Mitchell JA. COX-2 protects against atherosclerosis independently of local vascular prostacyclin: identification of COX-2 associated pathways implicate Rgl1 and lymphocyte networks. PLoS One 2014;9:e98165. doi:10.1371/journal.pone.0098165
- Nanhwan MK, Ling S, Kodakandla M, Nylander S, Ye Y, Birnbaum Y. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase-2- dependent effect. Arterioscler Thromb Vasc Biol 2014;34:2078–2085.
- Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol 2015;35:1805–1814.
- Kirkby NS, Lundberg MH, Chan MV, Vojnovic I, Solomon AB, Emerson M, Mitchell JA, Warner TD. Blockade of the purinergic P2Y12 receptor greatly increases the platelet inhibitory actions of nitric oxide. Proc Natl Acad Sci U S A 2013;110:15782–15787.
- Arazi HC, Doiny DG, Torcivia RS, Grancelli H, Waldman SV, Nojek C, Fornari MC, Badimon JJ. Impaired anti-platelet effect of aspirin, inflammation and platelet turnover in cardiac surgery. Interact Cardiovasc Thorac Surg 2010;10:863–867.
- Henry P, Vermillet A, Boval B, Guyetand C, Petroni T, Dillinger JG, Sideris G, Sollier CB, Drouet L. 24-hour time-dependent aspirin efficacy in patients with stable coronary artery disease. Thromb Haemost 2011;105:336–344.
- Cavalca V, Rocca B, Veglia F, Petrucci G, Porro B, Myasoedova V, De Cristofaro R, Turnu L, Bonomi A, Songia P, et al. On-pump cardiac surgery enhances platelet renewal and impairs aspirin pharmacodynamics: effects of improved dosing regimens. Clin Pharmacol Ther 2017;102:849–858.
- Paikin JS, Hirsh J, Ginsberg JS, Weitz JI, Chan NC, Whitlock RP, Pare G, Johnston M, Eikelboom JW. Multiple daily doses of acetyl-salicylic acid (ASA) overcome reduced platelet response to once-daily ASA after coronary artery bypass graft surgery: a pilot randomised controlled trial. J Thromb Haemost 2015;13:448–456.
- Pascale S, Petrucci G, Dragani A, Habib A, Zaccardi F, Pagliaccia F, Pocaterra D, Ragazzoni E, Rolandi G, Rocca B, et al. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood 2012;119:3595–3603. doi:10.1182/blood-2011-06-359224
- Rocca B, Santilli F, Pitocco D, Mucci L, Petrucci G, Vitacolonna E, Lattanzio S, Mattoscio D, Zaccardi F, Liani R, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost 2012;10:1220–1230.
- Cavalca V, Rocca B, Squellerio I, Dragani A, Veglia F, Pagliaccia F, Porro B, Barbieri SS, Tremoli E, Patrono C. In vivo prostacyclin biosynthesis and effects of different aspirin regimens in patients with essential thrombocythaemia. Thromb Haemost 2014;112:118–127.
- Larsen ML, Pedersen OH, Hvas AM, Niekerk P, Bonlokke S, Kristensen SD, Grove EL. Once- versus twice-daily aspirin treatment in patients with essential thrombocytosis. Platelets 2018;1–7. (epub ahead of print). doi:10.1080/09537104.2018.1430356
- De Stefano V, Rocca B, Tosetto A, Soldati D, Petrucci G, Beggiato E, Bertozzi I, Betti S, Carli G, Carpenedo M, et al. The Aspirin Regimens in Essential Thrombocythemia (ARES) phase II randomised trial design: implementation of the serum thromboxane B2 assay as an evaluation tool of different aspirin dosing regimens in the clinical setting. Blood Cancer J 2018;8:49.
- Dillinger JG, Drissa A, Sideris G. Bal dit Sollier C, Voicu S, Manzo Silberman S, Logeart D, Drouet L, Henry P. Biological efficacy of twice daily aspirin in type 2 diabetic patients with coronary artery disease. Am Heart J 2012;164:600–606.
- Spectre G, Arnetz L, Ostenson CG, Brismar K, Li N, Hjemdahl P. Twice daily dosing of aspirin improves platelet inhibition in whole blood in patients with type 2 diabetes mellitus and micro- or macrovascular complications. Thromb Haemost 2011;106:491–499.
- Addad F, Chakroun T, Elalamy I, Abderazek F, Chouchene S, Dridi Z, Gerotziafas GT, Hatmi M, Hassine M, Gamra H. Antiplatelet effect of once- or twice-daily aspirin dosage in stable coronary artery disease patients with diabetes. Int J Hematol 2010;92:296–301.
- Gurfinkel EP, Altman R, Scazziota A, Heguilen R, Mautner B. Fast platelet suppression by lysine acetylsalicylate in chronic stable coronary patients. Potential clinical impact over regular aspirin for coronary syndromes. Clin Cardiol 2000;23:697–700.
- Raschka C, Koch HJ. [Pharmacokinetics after oral and intravenous administration of d,l- monolysine acetylsalicylate and an oral dose of acetylsalicylic acid in healthy volunteers]. Therapie 2001;56:669–674.
- Storey RF, Angiolillo DJ, Bonaca MP, Thomas MR, Judge HM, Rollini F, Franchi F, Ahsan AJ, Bhatt DL, Kuder JF, et al. Platelet Inhibition with Ticagrelor 60 mg Compared with 90 mg Twice-daily in the PEGASUS-TIMI 54 study. J Am Coll Cardiol 2016;67:1145–1154.
- Patrono C, Ciabattoni G, Pinca E, Pugliese F, Castrucci G, De Salvo A, Satta MA, Peskar BA. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res 1980;17:317–327.
- Brash AR, Jackson EK, Saggese CA, Lawson JA, Oates JA, FitzGerald GA. Metabolic disposition of prostacyclin in humans. J Pharmacol Exp Ther 1983;226:78–87.
- Patrono C, FitzGerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol 1997;17:2309–2315.
- Ciabattoni G, Pugliese F, Davi G, Pierucci A, Simonetti BM, Patrono C. Fractional conversion of thromboxane B2 to urinary 11-dehydrothromboxane B2 in man. Biochim Biophys Acta 1989;992:66–70.
- Ajjan RA, Standeven KF, Khanbhai M, Phoenix F, Gersh KC, Weisel JW, Kearney MT, Ariens RA, Grant PJ. Effects of aspirin on clot structure and fibrinolysis using a novel in vitro cellular system. Arterioscler Thromb Vasc Biol 2009;29:712–717.
- Antovic A, Perneby C, Ekman GJ, Wallen HN, Hjemdahl P, Blomback M, He S. Marked increase of fibrin gel permeability with very low dose ASA treatment. Thromb Res 2005;116:509–517.
- Neergaard-Petersen S, Ajjan R, Hvas AM, Hess K, Larsen SB, Kristensen SD, Grove EL. Fibrin clot structure and platelet aggregation in patients with aspirin treatment failure. PLoS One 2013;8:e71150. doi:10.1371/journal.pone.0071150
- Carter A, Cymbalista C, Spector T, Grant P. Heritability of clot formation, morphology, and lysis: the EuroCLOT study. Arterioscler Thromb and Vasc Biol 2007;27:2783–2789.
- Franchi F, Rollini F, Cho JR, King R, Phoenix F, Bhatti M, DeGroat C, Tello-Montoliu A, Zenni MM, Guzman LA, et al. Effects of dabigatran on the cellular and protein phase of coagulation in patients with coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel. Results from a prospective, randomised, double-blind, placebo-controlled study. Thromb Haemost 2016;115:622–631.
- Hess K, Alzahrani SH, Price JF, Strachan MW, Oxley N, King R, Gamlen T, Schroeder V, Baxter PD, Ajjan RA. Hypofibrinolysis in type 2 diabetes: the role of the inflammatory pathway and complement C3. Diabetologia 2014;57:1737–1741.
- Sumaya W, Wallentin L, James SK, Siegbahn A, Gabrysch K, Bertilsson M, Himmelmann A, Ajjan RA, Storey RF. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: a PLATO substudy. Eur Heart J 2018;39:1078–1085. doi:10.1093/eurheartj/ehy013
- Tohgi H, Konno S, Tamura K, Kimura B, Kawano K. Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin. Stroke 1992;23:1400–1403.
- Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, et al. ESC/EACTS Guidelines on myocardial revascularization: the task force on myocardial revascularization of the european society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS) developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI). Eur Heart J 2014;35:2541–2619. doi:10.1093/eurheartj/ehu278
- Bhatt DL, Bonaca MP, Bansilal S, Angiolillo DJ, Cohen M, Storey RF, Im K, Murphy SA, Held P, Braunwald E, et al. Reduction in Ischemic Events with Ticagrelor in Diabetic Patients: from the PEGASUS-TIMI 54 Trial. J Am Coll Cardiol 2016;67:2732–2740.
- Bonaca MP, Bhatt DL, Storey RF, Steg PG, Cohen M, Kuder J, Goodrich E, Nicolau JC, Parkhomenko A, López-Sendón J, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol 2016;67:2719–2728.
- Bansilal S, Bonaca MP, Cornel JH, Storey RF, Bhatt DL, Steg PG, Im K, Murphy SA, Angiolillo DJ, Kiss RG, et al. Ticagrelor for secondary prevention of atherothrombotic events in patients with multivessel coronary disease. J Am Coll Cardiol 2018;71:489–496.
- Capodanno D, Mehran R, Valgimigli M, Baber U, Windecker S, Vranckx P, Dangas G, Rollini F, Kimura T, Collet JP, et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol 2018;15:480–496.
- Vranckx P, Valgimigli M, Juni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018;392:940–949. doi:10.1016/S0140-6736(18)31858-0
- Diener H, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996;143:1–13.
- Du L, Cheng Z, Zhang Y, Li Y, Mei D. The impact of medication adherence on clinical outcomes of coronary artery disease: a meta-analysis. Eur J Prev Cardiol 2017;24:962–970.
- Castellano JM, Sanz G, Penalvo JL, Bansilal S, Fernandez-Ortiz A, Alvarez L, Guzman L, Linares JC, Garcia F, D’Aniello F, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol 2014;64:2071–2082.
- Joshi RR, Hossain R, Morton AC, Ecob R, Judge HM, Wales C, Walker JV, Karunakaran A, Storey RF. Evolving pattern of platelet P2Y12 inhibition in patients with acute coronary syndromes. Platelets 2014;25:416–422.
- Armstrong PC, Leadbeater PD, Chan MV, Kirkby NS, Jakubowski JA, Mitchell JA, Warner TD. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost 2011;9:552–561.
- Scavone M, Femia EA, Caroppo V, Cattaneo M. Inhibition of the platelet P2Y12 receptor for adenosine diphosphate does not impair the capacity of platelet to synthesise thromboxane A2. Eur Heart J 2016;37:3347–3356. doi:10.1093/eurheartj/ehv551
- Hoefer T, Armstrong PC, Finsterbusch M, Chan MV, Kirkby NS, Warner TD. Drug-free platelets can act as seeds for aggregate formation during antiplatelet therapy. Arterioscler Thromb Vasc Biol 2015;35:2122–2133.
- Patrono C, Rocca B. Obesity, and aspirin responsiveness. J Am Coll Cardiol 2017;69:613–615.
