Abstract
The relationship between platelet count and risk of major bleeding in patients with venous thromboembolism (VTE) during anticoagulation remains unclear. We therefore investigated the association between platelet count, measured at VTE diagnosis and before the thrombotic event, and risk of major bleeding. Participants comprised 744 patients with incident VTE derived from the Tromsø Study. Major bleedings were recorded during the first year after VTE. Cox-regression was used to calculate hazard ratios (HRs) for major bleeding across platelet count quartiles.
There were 55 major bleedings (incidence rate 9.1/100 person-years, 95% confidence interval [CI] 7.0–11.8). The major bleeding risk increased across quartiles of platelet count measured at VTE diagnosis (P for trend<0.02). In the age- and sex-adjusted model, subjects with platelet count in the highest quartile (≥300x109/L) had a 4.3-fold (95% CI 1.7–10.9) higher risk of major bleeding compared to those with platelet count in the lowest quartile (≤192x109/L), and exclusion of patients with cancer yielded similar results. When platelet count was measured on average 7 years before a VTE, the corresponding HR was 2.5 (95% CI 0.9–6.7). Our results suggest that increasing platelet count, assessed several years before and at VTE diagnosis, is associated with a higher risk of major bleeding, and could be a stable individual marker of major bleeding risk in VTE-patients.
Introduction
Major bleeding events are feared and severe complications of anticoagulant therapy associated with high costs, morbidity and mortality in the treatment of venous thromboembolism (VTE) [Citation1–4]. Depending on type, intensity and duration of anticoagulation, major bleeding has been reported to occur annually in 3 to 9 per 100 person-years in non-interventional studies of VTE patients [Citation5–7]. The assessment of major bleeding risk is essential to guide decisions regarding treatment duration in unprovoked VTE [Citation3]. Furthermore, an accurate risk stratification of major bleeding may identify patients at high risk of bleeding, who would benefit from targeted preventive measures during the initial period of anticoagulant treatment. Known risk factors for major bleeding are predominantly of clinical nature, such as advanced age, active cancer and co-morbidities, which display only modest discriminatory ability when combined in risk assessment models for major bleeding risk [Citation8–10]. The addition of biomarkers to clinical risk factors and age improved prediction of major bleeding in patients with atrial fibrillation in the ABC-model [Citation11]. Therefore, biomarkers could be promising candidates to improve discrimination between those at high and low risk of major bleeding during anticoagulant treatment in VTE.
Platelets are potential attractive biomarkers for bleeding given their crucial role in hemostasis [Citation12], and the fact that measurement of platelet count is inexpensive and easily obtainable. However, the relationship between platelet count and risk of major bleeding in VTE patients remains unclear. For instance, previous data have shown that both low and high platelet counts were associated with increased risk of major bleeding in VTE patients [Citation13,Citation14]. Elucidating the role of platelet count in the risk of major bleeding may be challenging, as several conditions associated with VTE can affect platelet count but also increase the bleeding risk during anticoagulation, such as cancer, liver disease, major surgery, and trauma [Citation3,Citation15–18]. It is noteworthy that even though environmental factors influence platelet count, genetics largely contribute to variation in platelet-related phenotypes [Citation19]. Indeed, family and twin studies indicate a high heritability of platelet count and indices related to platelet size, including mean platelet volume (MPV) [Citation19–21]. Moreover, the intra-individual variation in platelet count has been shown to be substantially less than the inter-individual variation in healthy subjects [Citation22]. In light of available data, platelet count appears to be a stable phenotype within an individual over time.
As a potentially stable phenotype, an individual’s platelet count could influence the predisposition to bleeding during exposure to anticoagulant therapy. In order to clarify the association of platelet count with risk of major bleeding in VTE patients under anticoagulant treatment, we hypothesized that platelet count measured at the time of VTE diagnosis and several years before the thrombotic event were both associated with major bleeding. To address our study hypothesis, we investigated the association between platelet count, measured at VTE diagnosis, and risk of major bleeding during the first year after an incident VTE. Then, using the same study population, we explored whether platelet count assessed several years prior to the incident VTE was associated with major bleeding.
Methods
Study Population
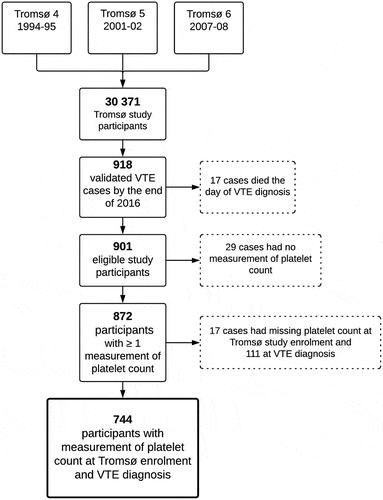
Study participants originated from the fourth (1994–95), fifth (2001–02) and sixth (2007–08) surveys of the Tromsø study, a single-center, population-based cohort in Tromsø, Norway [Citation23]. Members of the population living in the municipality of Tromsø were invited to participate in the surveys, and altogether 30 371 unique individuals aged 25–97 years participated. Identification and subsequent adjudication of potential VTE cases from the source population have been previously described in detail [Citation24]. In short, potential VTE cases were identified by searching the hospital discharge diagnosis registry, the autopsy registry, and the radiology procedure registry at the University hospital of North Norway (UNN). Identified cases were adjudicated by trained personnel and only included when signs and symptoms of deep vein thrombosis (DVT) or pulmonary embolism (PE) were combined with objective confirmation by radiological procedures that resulted in a VTE diagnosis requiring treatment. The study was approved by the Regional Committee of Research and Medical Health Ethics, and all study participants provided informed written consent.
From the date of inclusion in one of the three surveys until December 31 2016, a total of 918 participants developed an incident VTE (). Seventeen participants who died on the same day of their VTE diagnosis were excluded, leaving 901 eligible study participants. Among these, 29 participants did not have any measurement of platelet count. Of the remaining 872 participants, 17 had missing platelet count values at Tromsø study enrollment and 111 at VTE diagnosis and were therefore excluded. The resulting study population consisted of 744 VTE cases with available platelet count measurement at Tromsø study enrollment and VTE diagnosis ().
Clinical Characteristics of VTE Events
Medical records were searched at time of and 12 weeks preceding the VTE diagnosis for clinical information on VTE characteristics and provoking factors. Factors that classified the VTE event as provoked were major surgery, trauma or acute medical conditions (acute myocardial infarction, ischemic stroke, or major infectious disease) within 12 weeks prior to VTE event, marked immobilization (confined to bed >3 days, wheel-chair, or long-distance travel exceeding 4 hours within the last 14 days prior to VTE event), or any other factor(s) specifically described in the medical records to have provoked the VTE (e.g. intravascular catheter or plaster cast). Presence of known active cancer at the time of VTE diagnosis was regarded as a provoked VTE. In the case of a concurrent DVT and PE diagnosis, the VTE event was classified as a PE.
The presence of comorbidities, such as hypertension, renal dysfunction, and anemia, was assessed in medical records of VTE patients. A systolic blood pressure above 160 mmHg defined hypertension. The estimated glomerular filtration rate (eGFR) was calculated with the chronic kidney disease epidemiology collaboration equation based on creatinine levels, age, gender and race [Citation25]. Anemia was defined as a hemoglobin level below 11.5 g/dL for women and below 13.0 g/dL for men at VTE diagnosis. A history of bleeding was recorded if a previous bleeding event was specifically noted in the medical records of VTE cases.
To account for type and duration of VTE treatment, we considered the planned treatment (i.e. heparin, vitamin k antagonist [VKA] or direct oral anticoagulant) and duration of anticoagulation that were stated by the attending physicians in the medical records at the time of VTE diagnosis. Duration of anticoagulant therapy was categorized into 3, 6, 12, and more than 12 months, as previously described [Citation26].
Platelet Count Measurements
Measurement of platelet-related phenotypes at Tromsø study enrollment, i.e. platelet count and MPV, has previously been described elsewhere [Citation24]. Briefly, non-fasting blood samples were collected from an antecubital vein into 5-mL vacutainers containing EDTA as an anticoagulant (K3- EDTA 40 µL, 0.37 mol/L per tube), and analyzed within 12 hours in an automated blood cell counter (Coulter Counter®, Coulter Electronics, Luton, UK). For the platelet count measurement at VTE diagnosis, we considered the first blood sample drawn for the diagnostic work-up of VTE, as described in the medical records of each VTE patient at the UNN. According to the protocol of the Department of Clinical Chemistry at the UNN, blood samples were collected in vacutainers containing EDTA.
Major Bleeding Events
The medical records for all study participants were searched for bleeding events during the 365 days following the VTE at the UNN. All second-line care and advanced emergency medicine, such as transfusion of blood products, is exclusively provided by the UNN. The UNN is situated in the middle of Tromsø municipality, with a vicinity of approximately 250 km to the nearest hospital providing comparable health-care functions. Two reviewers (trained medical personnel from the UNN) adjudicated the bleeding events independently in accordance with the criteria proposed by the International Society on Thrombosis and Hemostasis (ISTH) [Citation27]. In short, a bleeding event that was fatal, and/or symptomatic in a critical area or organ, and/or requiring blood transfusion of ≥2 units of whole blood or red blood cells or causing a fall in hemoglobin level of ≥20 g/L, was considered major. In case of disagreement, the event was discussed in an endpoint committee (HSJ and JBH) to reach consensus.
Statistical Analyses
Subjects were followed from the date of their first VTE to the date of an incident major bleeding, death, migration, or end of follow-up (i.e. 365 days after the first VTE), whichever came first. Subjects who died or migrated out of the municipality of Tromsø were censored at the time of the respective event. Statistical analyses were performed with STATA version 15.0 MP (Stata Corp. College Station, Texas, United States).
Platelet count was divided into quartiles based on platelet count distribution measured at VTE diagnosis, and the first quartile was set as the reference. Crude incidence rates (IRs) with 95% confidence intervals (CIs) of major bleeding were calculated across quartiles of platelet count and expressed as number of events per 100 person-years at risk. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) with 95% CIs for major bleeding. HRs were adjusted for age and sex in a first model, with the addition of body mass index (BMI) and planned duration of anticoagulation therapy to a second model. Risk estimates were adjusted for treatment duration because knowledge of platelet count at VTE diagnosis might have influenced the decision on preplanned treatment length. In a final fully adjusted model, we included surgery, acute medical conditions, eGFR, hypertension, history of bleeding and anemia. The proportional hazards assumption was assessed by evaluating the parallelism of the log-log survivor function across quartiles of platelet count, and tested using Schoenfeld residuals. In order to assess potential non-linearity between platelet count and major bleeding risk, the association was visualized by a generalized additive regression plot using R version 3.6.1. Platelet count was modeled with a smoothing spline fit in a Cox model adjusted for age, sex, BMI and planned duration of treatment.
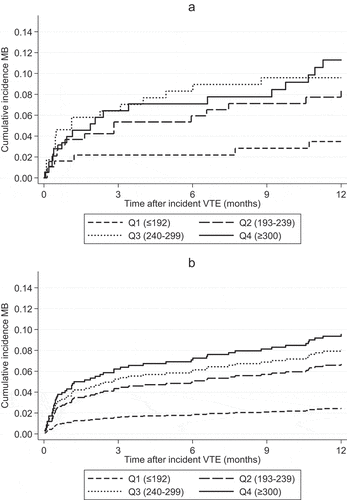
The risk of death has been reported to be higher in elderly with high and low platelet counts compared to those with a normal platelet count [Citation28]. We therefore additionally performed competing risk by death analyses and calculated the sub-distribution hazard ratios (SHRs) to limit overestimation of the relative risk differences of major bleeding between platelet count categories [Citation29]. The 1-year cumulative incidences of major bleeding across platelet count quartiles were visualized in traditional one minus Kaplan-Meier (1-KM) plots and in cumulative incidence function plots corrected for competing risk by death.
In addition to possible chemotherapy-induced thrombocytopenia, cancer has the potential to induce thrombocytosis [Citation17]. Cancer is also a well-known risk factor for bleeding during anticoagulation in VTE [Citation3], and we therefore conducted a sensitivity analysis excluding all patients with active cancer at VTE diagnosis. Using a similar rationale, we stratified analyses according to provoking status, as cases with provoked VTE are more likely to be exposed to factors that may affect platelet count and risk of major bleeding, such as major surgery and trauma [Citation3,Citation18].
Platelet count measured at Tromsø study enrollment was categorized using the same cutoff values as for platelet count at VTE diagnosis. HRs for major bleeding were adjusted for age and sex in a first model, and BMI was added to a second model. It is well established, and previously shown in the Tromsø study [Citation24], that platelet count is negatively correlated with MPV, an indice of platelet size. Platelet size is regarded as a marker of platelet function, with studies showing that large platelets are more reactive and adhere and aggregate faster ex vivo than small platelets [Citation30,Citation31]. Therefore, differences in platelets size could potentially explain the association between platelet count and major bleeding. In order to investigate the potential of platelet size, measured as MPV, to mediate the association between platelet count and major bleeding, we further adjusted HRs for MPV measured at Tromsø study enrollment.
Results
Baseline characteristics across quartiles of platelet count measured at VTE diagnosis are presented in . The mean age and proportion of male subjects decreased across increasing quartiles of platelet count. Subjects in the highest quartile were more likely to have anemia, active cancer, provoked VTE, recent surgery and acute medical conditions compared to those in the lower quartiles. The planned treatment type and duration of anticoagulant therapy did not appear to vary in any consistent manner across quartiles of platelet count.
Table I. Baseline characteristics according to quartiles of platelet count measured at venous thromboembolism diagnosis
Among the 744 patients with incident VTE, there were 55 major bleeding events within 1 year of VTE diagnosis during 605 person-years (IR 9.1 per 100 person-years, 95% CI 7.0–11.8), with a median time from VTE events to major bleeding of 35 days (interquartile range [IQR] 11–183 days). Major bleeding characteristics and classification according to ISTH criteria are presented in Supplemental Table I. Three bleeding events were fatal (within 1 week), and 40% of the major bleedings were symptomatic in critical areas or organs.
The crude IRs and relative risks for major bleeding according to quartiles of platelet count measured at VTE diagnosis are presented in . IRs for major bleeding increased across quartiles of platelet count, from 3.8 per 100 person-years (95% CI 1.7–8.6) in the lowest category (≤192 x109/L) to 12.8 per 100 person-years (95% CI 8.1–20.3) in the highest category (≥300 x109/L). Likewise, in the age- and sex-adjusted model, HRs for major bleeding increased with increasing platelet count in a dose-response fashion. Compared to the first quartile, HRs for major bleeding were 2.7 (95% CI 1.0–6.9), 3.1 (95% CI 1.2–8.0) and 4.3 (95% CI 1.7–10.9) for quartiles 2 to 4, respectively. Further adjustment for BMI and planned duration of anticoagulant treatment yielded essentially similar results (). With additional adjustments for surgery, acute medical conditions, eGFR, hypertension, history of bleeding and anemia, the risk estimates were somewhat attenuated, with an HR of 3.4 (95% CI 1.3–8.8) for the highest vs. the lowest quartile of platelet count, with virtually no changes in risk estimates after taking the presence of death as competing event (HR 3.2, 95% CI 1.2–8.6). In all models of the overall population, there was a trend (P for trend ≤0.02) for increased risk of major bleeding by increasing quartiles of platelet count. In the sensitivity analysis, the risk estimates were slightly attenuated after excluding patients with cancer, and the HRs were 1.9 (95% CI 0.6–5.7), 1.8 (95% CI 0.5–6.1) and 2.9 (95% CI 0.9–9.5) for quartiles 2 to 4, respectively, compared to the first quartile in the fully adjusted model (). Supplemental describes the stepwise adjustment for the aforementioned covariates for the overall population and those without cancer. In analyses stratified according to planned type of anticoagulant, the results did not appear to differ substantially between subjects treated with heparins+VKAs compared to subjects treated with heparins only, even though there were relatively few individuals in the heparin group (Supplemental Table III).
Table II. The 1-year risk of major bleeding according to quartiles of platelet count measured at venous thromboembolism diagnosis
shows the risk of major bleeding as a continuous function of platelet count. As indicated in the density plot, the major bleeding risk increased linearly within the 25–75th percentile range of platelet count (192–299 x 109/L). The 1-year cumulative incidences of major bleeding across quartiles of platelet count were estimated by 1-KM (), and by the cumulative incidence function in the presence of competing risk by death (). The cumulative incidences of major bleeding increased with increasing quartiles of platelet count (), and the results remained essentially similar after taking competing risk by death into account (). The majority of the major bleeding events occurred in the first 3 months after the VTE, and the 3-month cumulative incidences of major bleeding were 1.6%, 4.3%, 5.2% and 6.2% for quartiles 1–4, respectively (). Of note, in the six major bleeding events occurring among patients in the lowest quartile, platelet counts ranged from 123 to 181 x 109/L.
Figure 2. The risk of major bleeding as a continuous function of platelet count measured at venous thromboembolism diagnosis (a, adjusted for age, sex, body mass index and planned duration of anticoagulation) and at Tromsø study enrollment (b, adjusted for age, sex and body mass index) in a generalized additive regression model. The solid line shows hazards ratios, enclosed by shaded areas indicating 95% confidence intervals. The distribution of platelet count is shown in a density plot at the bottom, and vertical lines indicate quartile cutoffs
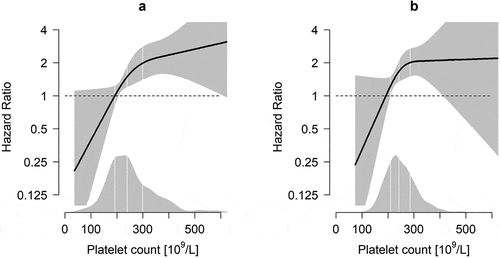
Figure 3. One-year cumulative incidence of major bleeding by quartiles of platelet count measured at venous thromboembolism diagnosis estimated by 1-Kaplan-Meier (a) and in the presence of death as competing event (b)
In subgroups (), the HRs for major bleeding in patients with provoked VTE were similar in quartiles 2–4 compared to the reference, ranging from 3.5 (95% CI 1.0–12.7) to 4.5 (95% CI 1.3–16.0) in the age- and sex-adjusted models. For unprovoked VTE, subjects with a platelet count in the two highest quartiles had a 2- to 3-fold higher risk of major bleeding compared to those with a platelet count in the first quartile, but CIs were wide and included unity.
Table III. The 1-year risk of major bleeding according to quartiles of platelet count measured at venous thromboembolism diagnosis in provoked and unprovoked cases
In our cohort, the median time from blood sampling in Tromsø 4–6 to VTE diagnosis was 6.8 years (IQR 3.1–9.2). There was a positive correlation between platelet count assessed at Tromsø study enrollment and VTE diagnosis (Spearman’s rho = 0.52, P< .001). Moreover, as expected, platelet count at Tromsø study enrollment was negatively associated with MPV (Spearman’s rho = −0.42, P< .001). As described in , the risk of major bleeding increased with increasing platelet count measured several years before the incident VTE. In the age- and sex adjusted model, subjects in the highest category of platelet count (i.e. ≥300 x 109/L) had a 2.5-fold (HR 2.5, 95% 0.9–6.7) higher risk of major bleeding after developing a VTE compared to subjects with a platelet count in the lowest category (i.e. ≤192 x 109/L). Risk estimates did not materially change after adjusting for BMI, but were somehow attenuated when MPV was added to the regression models, mainly for the highest category of platelet count (HR 2.0, 95% CI 0.7–5.6). When the risk of major bleeding was visualized as a function of platelet count measured at Tromsø study enrollment, the association displayed a pattern similar to the results for platelet count assessed at VTE diagnosis ().
Table IV. The 1-year risk of major bleeding in patients with venous thromboembolism according to platelet count measured at Tromsø study enrollment
Discussion
In this study, we found that an increasing platelet count measured at VTE diagnosis was associated with a higher risk of major bleeding in a dose-response fashion during the first year after the VTE. Exclusion of patients with active cancer and subgroup analyses stratified by provoked and unprovoked VTE yielded similar results. When platelet count was measured in the same study participants several years prior to the development of the incident VTE, an increasing platelet count was also associated with a higher risk of major bleeding. Our results suggest that platelet count is a stable phenotype within an individual over time that may influence the susceptibility to bleeding during anticoagulant treatment after a VTE.
In our study of patients derived from the general population, the overall major bleeding rate of 9.1 per 100 person-years was considerably higher than the rate of about 1.0 per 100 person-years found in randomized controlled trials (RCTs) involving VTE patients [Citation32]. However, unselected patients derived from the general population have more often serious comorbidities and are managed under less intensive surveillance as compared to patients selected into RCTs. In addition, RCTs evaluating the efficacy and safety of anticoagulants are more likely to exclude patients with a bleeding predisposition. Notably, 24% of our patients had active cancer at the time of VTE diagnosis, which is an established risk factor for bleeding during anticoagulation [Citation3]. Our rate of major bleeding is in agreement with a prospective cohort comprising 842 VTE patients treated with anticoagulant therapy in the community [Citation33]. In this study, the rate of major bleeding was 10.6 per 100 person-years, and it was particularly high in analysis restricted to VTE patients with cancer (15.7 per 100 person-year). Another clinically relevant finding in our study was the fact that the median time from incident VTE to major bleeding was 35 days, with the majority of the major bleeding events occurring within the first 3 months after VTE. These results are consistent with previous data [Citation1] and underscore the concept that patients with an underlying predisposition to bleeding are more likely to develop a major bleeding shortly after initiation of anticoagulant therapy.
To the best of our knowledge, we are the first to investigate the association of platelet count, measured within the same subjects prior to and at VTE diagnosis, with risk of major bleeding. A few previous studies have explored the relationship between platelet count and major bleeding in VTE [Citation13,Citation14,Citation34]. In a study comprising 3012 VTE patients, Yamashita et al. assessed the influence of platelet count at VTE diagnosis on the risk of major bleeding [Citation34], and reported a higher risk of major bleeding in patients with a moderate to severe thrombocytopenia (<100 x 109/L) compared to those with no thrombocytopenia (>150 x 109/L). Apparently, these findings are in contrast with our results. However, the study by Yamashita et al. is not necessarily comparable to the present study. For example, the platelet count cutoffs were determined according to clinical preferences in their study (i.e. 100 x 109/L and 150 x 109/L) and these cutoffs are included within the lowest quartile in our analyses. Furthermore, the authors did not treat death as a competing risk in their analyses, which could have led to an overestimation of the association between thrombocytopenia and major bleeding. The relationship between platelet count measured at VTE diagnosis and major bleeding was also investigated in the RIETE registry [Citation13,Citation14]. Di Micco et al. assessed the three-month risk of major bleeding in 43078 VTE patients according to categories of platelet counts ranging from <80 x 109/L to >450 x 109/L [Citation13]. In patients with and without cancer, both the highest (>450 x 109/L) and lowest (<80 x 109/L) categories were associated with increased risk of major bleeding when compared to a platelet count ranging from 150 to 300 x 109/L [Citation13]. Similar to our findings, Di Micco et al. found that a high platelet count was associated with increased risk of major bleeding.
Here we found a dose-response relationship between an increasing platelet count, assessed at VTE diagnosis, and risk of major bleeding. Although the statistical power in sensitivity and subgroup analyses were limited due to the lower number of participants, cancer or other comorbidities with high mortality rates did not appear to substantially contribute to this relationship, as risk estimates were only slightly attenuated after excluding patients with active cancer and when competing risk of death was taken into account [Citation29]. Other comorbidities or conditions, including surgery, acute medical conditions (acute myocardial infarction, ischemic stroke and major infectious disease), hypertension, renal function (i.e. eGFR), history of bleeding and anemia appeared to partially explain the relationship, as adjustment for these conditions had a marginal impact on the risk estimates, with an increasing platelet count still being associated with a higher risk of major bleeding. Moreover, a positive association between platelet count and major bleeding risk was found not only in patients with provoked VTE but also in those with unprovoked events, albeit less pronounced in the latter group with the 95% CIs of risk estimates including unity. Thus, transient factors related to provoked VTEs that can induce an increase in platelet count, like major surgery or trauma [Citation18], did not seem to contribute to a great extent to the relationship between platelet count and major bleeding.
In the present study, a platelet count measured on average 7 years prior to the incident VTE still showed a dose-response relationship with major bleeding in analyses adjusted for age, sex, and BMI, although the confidence intervals of risk estimates included unity. When compared to the lowest quartile of platelet count, the highest quartile was associated with a 2.5-fold (95% CI 0.9–6.8) higher risk of major bleeding. Furthermore, the platelet count measured at VTE diagnosis displayed a significant positive correlation with the platelet count measured several years before the thrombotic event. Such findings reinforce the notion that platelet count is a stable phenotype within an individual over time, as previously demonstrated by others [Citation20,Citation22]. This notion is consistent with the high degree of heritability reported for some platelet-related phenotypes, including platelet count and indices of platelet size, such as MPV [Citation19–21]. In our study, platelet count and MPV, measured at Tromsø study enrollment, showed a negative moderate correlation, and the impact of platelet count on major bleeding risk was somehow attenuated after adjusting for MPV. This finding suggests that differences in platelet size could partly explain the association between platelet count and major bleeding. Large and small platelets have been shown to substantially differ in their functional roles in the hemostatic system. Compared to small platelets, large platelets are associated with increased reactivity, shortened bleeding time, faster adhesion to collagen and aggregation ex vivo, and increased expression of glycoproteins on their membranes [Citation30,Citation31]. Reticulated platelets, which are large and hyperreactive platelets, display a prothrombotic profile, as recently revealed in transcriptome analysis [Citation35]. Moreover, results from epidemiological studies, including the Tromsø Study, show that an increased MPV is associated with a higher risk of arterial cardiovascular disease [Citation36] and VTE [Citation24], thereby supporting the higher prothrombotic potential of large platelets [Citation24]. It is of interest that in the presence of substantial thrombocytopenia (<20 x 109/L), a low MPV has been shown to be a stronger predictor of bleeding than platelet count [Citation37]. In light of these data, we can speculate that an increasing platelet count, even within a normal range, would be associated with a lower platelet reactivity, as reflected by a decrease in MPV, which could predispose to major bleeding during anticoagulant treatment after a VTE.
Platelet count, as a phenotype that is stable over time within an individual, seems a promising biomarker to improve stratification of major bleeding risk during anticoagulant treatment. However, the use of platelet count in risk assessment models in patients with VTE and atrial fibrillation has yielded controversial results, with studies using different cutoff values of platelet count [Citation38–40]. Whether an elevated platelet count at the appropriate cutoff value can improve discrimination of VTE patients with high and low risk of major bleeding would require further investigation.
The inclusion of subjects derived from the general population, the complete and validated registry of VTE events, and the strict criteria used to define major bleeding based on the ISTH recommendations are among the main strengths of the present study. Additionally, the exclusivity of UNN as the sole healthcare provider enhances the probability to capture all relevant major bleeding events. The study also has some limitations. There were 157 subjects (17% of eligible participants) with missing values on platelet count who were excluded from our analyses (). However, there were no substantial differences in the baseline characteristics among subjects with and without missing values of platelet count (data not shown), indicating that missing values was presumably at random. The number of major bleeding events was low in some subgroups, which could have resulted in limited statistical power. Our results should therefore be interpreted with caution, especially in subgroup analysis. Only a few patients (15 out of 744 subjects included in the analyses) had a platelet count <100 x 109/L at the time of VTE diagnosis. Therefore, we were not able to evaluate the role of moderate to severe thrombocytopenia on the risk of major bleeding. It is noteworthy that the association between platelet count and major bleeding was less pronounced when platelet count was measured at Tromsø study enrollment as compared to the assessment at VTE diagnosis. Even though platelet count is subject to less intra-individual compared to inter-individual variability [Citation22], individual changes due to advancing age or environmental factors (such as onset of diseases) may occur over time [Citation18,Citation41]. In our cohort study, with a long period of follow-up, values of platelet count might have changed over time in participants with or without a major bleeding event. This could have led to an underestimation of the association between platelet count measured at Tromsø study enrollment and major bleeding, due to regression dilution bias, a phenomenon that occurs in the long-term follow-up of cohort studies [Citation42]. Unfortunately, we did not have information on concomitant use of drugs that might have affected the bleeding risk, such as antiplatelet agents. However, we conducted a sensitivity analysis excluding VTE cases with a known medical history of myocardial infarction or stroke (n = 131), as these would be the most likely users of antiplatelet medication (Supplemental Table IV). The association between an increasing platelet count and major bleeding remained, albeit somewhat attenuated compared to the main analyses, suggesting that the association was not primarily driven by antiplatelet medications. Finally, MPV is not commonly part of the diagnostic work-up for subjects with suspected acute VTE, and information on MPV at VTE diagnosis was therefore not available.
In conclusion, our results suggest that an increasing platelet count, measured several years before and at VTE diagnosis, is associated with a higher risk of major bleeding during the first year after an incident VTE. Our findings imply that a platelet count measured at VTE diagnosis is a stable phenotype within an individual over time that has the potential to improve risk stratification of major bleeding after a VTE.
Disclosure Of Conflict Of Interest
The authors report that they have no conflicts of interest.
Supplemental Material
Download MS Word (33.1 KB)Acknowledgements
K. G. Jebsen TREC is supported by an independent grant from Stiftelsen K.G. Jebsen.
H. S. Johnsen receives a grant from the North Norwegian regional health authorities (Helse-Nord).
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003;139(11):893–900.
- Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med 2010;152(9):578–589. doi: 10.7326/0003-4819-152-9-201005040-00008.
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149(2):315–352.doi: 10.1016/j.chest.2015.11.026.
- Gustafsson N, Poulsen PB, Stallknecht SE, Dybro L, Johnsen SP. Societal costs of venous thromboembolism and subsequent major bleeding events: a national register-based study. Eur Heart J Qual Care Clin Outcomes 2020;6(2):130-137.
- Lutsey PL, Zakai NA, MacLehose RF, Norby FL, Walker RF, Roetker NS, Adam TJ, Alonso A. Risk of hospitalised bleeding in comparisons of oral anticoagulant options for the primary treatment of venous thromboembolism. Br J Haematol 2019;185(5):903–911.doi: 10.1111/bjh.15857.
- Veeger NJ, Piersma-Wichers M, Tijssen JG, Hillege HL, van der Meer J. Individual time within target range in patients treated with vitamin K antagonists: main determinant of quality of anticoagulation and predictor of clinical outcome. A retrospective study of 2300 consecutive patients with venous thromboembolism. Br J Haematol 2005;128(4):513–519. doi: 10.1111/j.1365-2141.2004.05348.x.
- Khan F, Datta YH. Risk of bleeding during long-term anticoagulation with warfarin: a tertiary care center experience. Blood Coagul Fibrinolysis 2015;26(1):110–112. doi: 10.1097/MBC.0000000000000186.
- Piovella C, Dalla Valle F, Trujillo-Santos J, Pesavento R, López L, Font L, Valle R, Nauffal D, Monreal M, Prandoni P, et al. Comparison of four scores to predict major bleeding in patients receiving anticoagulation for venous thromboembolism: findings from the RIETE registry. Intern Emerg Med 2014;9(8):847–852.doi: 10.1007/s11739-014-1073-8.
- Palareti G, Antonucci E, Mastroiacovo D, Ageno W, Pengo V, Poli D, Testa S, Tosetto A, Prandoni P. The American college of chest physician score to assess the risk of bleeding during anticoagulation in patients with venous thromboembolism. J Thrombosis Haemostasis 2018;16(10):1994–2002.doi: 10.1111/jth.14253.
- van Es N, Wells PS, Carrier M. Bleeding risk in patients with unprovoked venous thromboembolism: A critical appraisal of clinical prediction scores. Thromb Res 2017;152:52–60. doi: 10.1016/j.thromres.2017.02.016.
- Hijazi Z, Oldgren J, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet 2016;387(10035):2302–2311.doi: 10.1016/S0140-6736(16)00741-8.
- Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev 2015;29(3):153–162. doi: 10.1016/j.blre.2014.10.003.
- Di Micco P, Ruiz-Gimenez N, Nieto JA, Aujesky D, Molino FD, Valle R, Barrón M, Maestre A, Monreal M. Platelet count and outcome in patients with acute venous thromboembolism. Thromb Haemost 2013;110(5):1025–1034.doi: 10.1160/TH13-04-0352.
- Giorgi-Pierfranceschi M, Di Micco P, Cattabiani C, Guida A, Pagán B, Morales MDV, Salgado E, Suriñach JM, Tolosa C, Monreal M, et al. Platelet count and major bleeding in patients receiving vitamin K antagonists for acute venous thromboembolism, findings from real world clinical practice. Medicine (Baltimore) 2015;94(47):e1915.doi: 10.1097/MD.0000000000001915.
- Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program 2005;1–12.doi:10.1182/asheducation-2005.1.1
- Lambert MP. Platelets in liver and renal disease. Hematol Am Soc Hematol Educ Program 2016;2016(1):251–255. doi: 10.1182/asheducation-2016.1.251.
- Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer 2012;130(12):2747–2760. doi: 10.1002/ijc.27441.
- Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical significance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med 1999;245(3):295–300. doi: 10.1046/j.1365-2796.1999.00452.x.
- Johnson AD. The genetics of common variation affecting platelet development, function and pharmaceutical targeting. J Thromb Haemost 2011;9(Suppl 1):246–257. doi: 10.1111/j.1538-7836.2011.04359.x.
- Whitfield JB, Martin NG. Genetic and environmental influences on the size and number of cells in the blood. Genet Epidemiol 1985;2(2):133–144. doi: 10.1002/gepi.1370020204.
- Pujol-Moix N, Vazquez-Santiago M, Morera A, Ziyatdinov A, Remacha A, Nomdedeu JF, Fontcuberta J, Soria JM, Souto JC. Genetic determinants of platelet large-cell ratio, immature platelet fraction, and other platelet-related phenotypes. Thromb Res 2015;136(2):361–366.doi: 10.1016/j.thromres.2015.06.016.
- Ross DW, Ayscue LH, Watson J, Bentley SA. Stability of hematologic parameters in healthy subjects. Intraindividual versus interindividual variation. Am J Clin Pathol 1988;90(3):262–267. doi: 10.1093/ajcp/90.3.262.
- Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I. Cohort profile: the Tromso Study. Int J Epidemiol 2012;41(4):961–967. doi: 10.1093/ije/dyr049.
- Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Mean platelet volume is a risk factor for venous thromboembolism: the Tromso Study, Tromso, Norway. J Thrombosis Haemostasis 2010;8(1):157–162. doi: 10.1111/j.1538-7836.2009.03498.x.
- Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–612.doi: 10.7326/0003-4819-150-9-200905050-00006.
- Johnsen HS, Hindberg K, Bjori E, Brodin E, Brækkan S, Morelli V, Hansen J-B. D-Dimer measured at diagnosis of venous thromboembolism is associated with risk of major bleeding. TH Open 2019;3(1):e77–e84.doi: 10.1055/s-0039-1683395.
- Schulman S, Kearon C. Subcommittee on control of anticoagulation of the S, standardization committee of the international society on T, haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x.
- Tsai MT, Chen YT, Lin CH, Huang TP, Tarng DC. U-shaped mortality curve associated with platelet count among older people: a community-based cohort study. Blood 2015;126(13):1633–1635. doi: 10.1182/blood-2015-06-654764.
- Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41(3):861–870. doi: 10.1093/ije/dyr213.
- Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res 1983;32(5):443–460. doi: 10.1016/0049-3848(83)90255-4.
- Handtke S, Steil L, Palankar R, Conrad J, Cauhan S, Kraus L, Ferrara M, Dhople V, Wesche J, Völker U, et al. Role of platelet size revisited-function and protein composition of large and small platelets. Thromb Haemost 2019;119(3):407–420.doi: 10.1055/s-0039-1677875.
- Ost D, Tepper J, Mihara H, Lander O, Heinzer R, Fein A. Duration of anticoagulation following venous thromboembolism: a meta-analysis. JAMA 2005;294(6):706–715. doi: 10.1001/jama.294.6.706.
- Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100(10):3484–3488.doi: 10.1182/blood-2002-01-0108.
- Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, Oi M, Akao M, Kobayashi Y, Toyofuku M, et al. Influence of baseline platelet count on outcomes in patients with venous thromboembolism (from the COMMAND VTE registry). Am J Cardiol 2018;122(12):2131–2141.doi: 10.1016/j.amjcard.2018.08.053.
- Bongiovanni D, Santamaria G, Klug M, Santovito D, Felicetta A, Hristov M, von Scheidt M, Aslani M, Cibella J, Weber C, et al. Transcriptome analysis of reticulated platelets reveals a prothrombotic profile. Thromb Haemost 2019;119(11):1795–1806.doi: 10.1055/s-0039-1695009.
- Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thrombosis Haemostasis 2010;8(1):148–156.doi: 10.1111/j.1538-7836.2009.03584.x.
- Eldor A, Avitzour M, Or R, Hanna R, Penchas S. Prediction of haemorrhagic diathesis in thrombocytopenia by mean platelet volume. Br Med J (Clin Res Ed) 1982;285(6339):397–400. doi: 10.1136/bmj.285.6339.397.
- Nieuwenhuis HK, Albada J, Banga JD, Sixma JJ. Identification of risk factors for bleeding during treatment of acute venous thromboembolism with heparin or low molecular weight heparin. Blood 1991;78(9):2337–2343. doi: 10.1182/blood.V78.9.2337.2337.
- Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151(3):713–719.doi: 10.1016/j.ahj.2005.04.017.
- Nieto JA, Solano R, Ruiz-Ribo MD, Ruiz-Gimenez N, Prandoni P, Kearon C, Monreal M. Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registry. J Thromb Haemost 2010;8(6):1216–1222.doi: 10.1111/j.1538-7836.2010.03852.x.
- Biino G, Santimone I, Minelli C, Sorice R, Frongia B, Traglia M, Ulivi S, Di Castelnuovo A, Gögele M, Nutile T, et al. Age- and sex-related variations in platelet count in Italy: a proposal of reference ranges based on 40987 subjects‘ data. PLoS One 2013;8(1):e54289.doi: 10.1371/journal.pone.0054289.
- Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ 2010;340(jun23 2):c2289. doi: 10.1136/bmj.c2289.