Abstract
Light transmission aggregometry (LTA) is considered the gold standard method for evaluation of platelet function. However, there are a lot of variation in protocols (pre-analytical procedures and agonist concentrations) and results. The aim of our study was to establish a national LTA protocol, to investigate the effect of standardization and to define national reference values for LTA. The SSC guideline was used as base for a national procedure. Almost all recommendations of the SSC were followed e.g. no adjustment of PRP, citrate concentration of 109 mM, 21 needle gauge, fasting, resting time for whole blood and PRP, centrifugation time, speed and agonists concentrations. LTA of healthy volunteers was measured in a total of 16 hospitals with 5 hospitals before and after standardization. Results of more than 120 healthy volunteers (maximum aggregation %) were collected, with participating laboratories using 4 different analyzers with different reagents. Use of low agonist concentrations showed high variation before and after standardization, with the exception of collagen. For most high agonist concentrations (ADP, collagen, ristocetin, epinephrine and arachidonic acid) variability in healthy subjects decreased after standardization. We can conclude that a standardized Dutch protocol for LTA, based on the SSC guideline, does not result in smaller variability in healthy volunteers for all agonist concentrations.
Introduction
Light transmission aggregometry (LTA) is considered the gold standard method to investigate patients with suspected abnormalities of primary hemostasis due to inherited or acquired defects of platelet function [Citation1]. In this method, the platelet aggregation pattern in response to an agonist is measured in platelet-rich plasma (PRP) by turbidometry [Citation2]. Different national and international guidelines state that LTA plays an important role in the diagnostic work-up of patients with bleeding tendency [Citation3–9]. Novel techniques based on whole-blood analysis, e.g. impedance aggregometry and platelet function analyzer (PFA), have been developed, but these lack sensitivity for detection of mild platelet function disorders [Citation10,Citation11] or are only complementary to LTA, such as flow cytometry [Citation12]. Thus, LTA continues to be one of the most helpful tools for the evaluation of suspected platelet function disorders.
However, (pre-)analytical aspects of LTA methodology have not been adequately standardized [Citation13]. There are many variables that affect the outcome of platelet aggregation, e.g. method of blood sampling, preparation of PRP or choice of agonists and their concentrations [Citation14]. Recent surveys by proficiency testing organizations have also identified variations in LTA practices and suggested the need for guidelines to standardize LTA [Citation15–17] since LTA is time-consuming, technically challenging, poorly reproducible and requires a relatively large volume of fresh blood. Standardization is also necessary to maintain this technique in small laboratories and to compare results from patients referred to other hospitals for diagnosis or treatment. During the last decade, several attempts have been made by different organizations to develop LTA guidelines, e.g. the CLSI and the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis (SSC-ISTH) guidelines [Citation18–22]. The clinical impact of using different methods for LTA is unknown, but it could lead to over and underdiagnosis of platelet disorders. In the Netherlands, the Society for Hematological Laboratories (VHL, Vereniging Hematologische Laboratoria) and the Working group Hemostasis Diagnostics of the Dutch Society for Thrombosis and Hemostasis (WHD-NVTH) collected data from 25 LTA-using laboratories in a national survey on LTA. A large variety of procedures were reported mostly on fasting, resting, smoking, caffeine use, centrifugation procedures and platelet adjustment of PRP (unpublished results). Subsequently, the VHL/WHD-NVTH developed a national LTA protocol, based on the recommendations of the Platelet Physiology Subcommittee of the SSC-ISTH [Citation18]. With the introduction of this national LTA protocol, we aimed to achieve a higher degree of standardization in the Netherlands for LTA.
This study had three aims:
To investigate whether the use of a standardized LTA protocol could reduce variation (per agonist concentration) in general by analyzing maximum aggregation in healthy volunteers.
To investigate whether the use of a standardized protocol for LTA could reduce variation in five hospitals that participated both before and after standardization by analyzing maximum aggregation in healthy volunteers.
To calculate national reference values per agonist concentration by using all healthy volunteers results after standardization.
Materials and Methods
Participating Laboratories
A total of 10 laboratories (together including 129 healthy subjects) participated in the survey in 2013 (before standardization) and 11 laboratories (134 healthy subjects) in 2016 (after standardization). Five laboratories participated before and after standardization. Details of the participants are summarized in .
Table I. The number of Dutch laboratories that participated in this survey before and after introduction of a standardized LTA protocol, including the instruments used for aggregometry
Healthy Subjects
Healthy volunteers were included anonymously (no registration of age or gender) with the only exclusion criteria of taking drugs known to interfere with platelet function. Information was recorded on fasting, diet instructions and a light meal prior to sample collection. Citrated blood was drawn from healthy volunteers according to the local venepuncture procedure, CCKL/ISO-15189 guidelines and the WMA Declaration of Helsinki.
Instruments
Most laboratories used one type of instrument for LTA, either Chrono-Log Corporation (Haverston, PA, USA), APACT, (LABiTec, Ahrensburg, Germany), PAP-8 (Biodata Corporation, Horsham, PA, USA) or Aggram (Helena BioSciences, Europe).
The instrument type per participant is shown in .
Before standardization in 2013, each participating laboratory used their local protocol for LTA which will not be specified here. Overall, 10 different procedures for LTA were used in the 10 laboratories in the Netherlands before standardization.
The diversity in reagent concentrations in 2013 (before standardization) and the fixed concentrations used after standardization in 2016 is summarized in .
Table II. Agonists concentrations used before (2013) and after (2016) standardization
In 2016 the national VHL protocol, based on the SSC-ISTH protocol according to Cattaneo et al., was approved [Citation18]. The protocol describes pre-analytical variables regarding blood collection and sample preparation, centrifuge settings, aggregometry devices and settings, which are summarized in Supplementary Tables S1 and S2.
Used reagent brands were not recorded in 2013 and the reagents used in 2016 are summarized in Supplementary Table S3.
Statistics
Data were anonymized before import into a database and analyzed using GraphPad Prism 7.0 (GraphPad Software). Differences in LTA results of healthy subjects (not normally distributed) between 2013 and 2016 were tested on significance with a non-parametric Mann–Whitney U test. Reference intervals (95% interval, normally distributed) were determined by parametric analyses using EP-evaluator (Data Innovations LLC).
Results
Overall Variation in Maximum Aggregation of Healthy Volunteers Before and After Introduction of a Standardized LTA Protocol
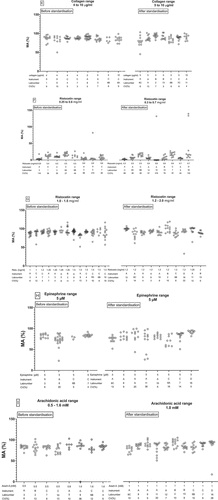
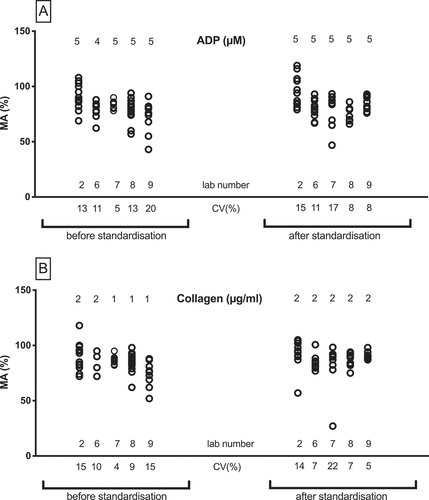
demonstrates observations of the variation in maximum platelet aggregation with various concentrations of ADP, collagen, ristocetin, epinephrine and arachidonic acid in healthy volunteers before and after standardization using different analyzers. ADP concentrations of 0.5 to 2.5 μM () show in general high coefficients of variation (CV) in maximum aggregation ranging from 5% to 107% when measured on all instruments (). The use of a standardized protocol (2 μM ADP) does not reduce the CV. Lowest variation is observed before standardization when using the slightly higher ADP concentration of 2,5 μM. shows that increasing ADP concentrations (5 or 10 µM) reduces the CV without any difference before or after standardization. Intermediate or high ADP concentrations show little variability between various analyzers in terms of maximum aggregation ranges.
Figure 1. Changes in LTA results of healthy volunteers on different analyzers before and after standardization. Graphs show the instrument type (a: Chronolog, b: PAP8, c: APACT, d: AggRAM), laboratory number and the coefficient of variation (CV, %) in maximum aggregation after stimulation of PRP from healthy subjects with: a) 0.5–2.5 µM ADP low range, b) 4–5 µM ADP intermediate range, c) 10–20 µM ADP high range, d) 0.2–2 µg/ml collagen low range, e) 4–10 µg/ml collagen high range, f) 0.25–0.6 mg/ml ristocetin low range, g) 1.0–1.5 mg/ml ristocetin high range, h) 5 µM epinephrine, i) 0.5–1.6 mM arachidonic acid. Results are expressed as % maximum aggregation
More or less the same effect is observed with collagen. Before standardization in 2013, low collagen concentrations (0.2–2 μg/mL) show more variation in maximum aggregation results of healthy volunteers in comparison to high collagen concentrations (4–10 μg/mL) on both Chronolog and APACT instruments (). After standardization in 2016, low collagen concentrations (1–2 μg/mL) show less variability in maximum aggregation on all instruments, compared to the LTA results of 2013 (). A fixed, high collagen concentration of 5 μg/mL displays roughly the same variation before and after standardization (). As expected, low concentrations of ristocetin result in very low maximal aggregation responses with a few outliers (). The standardization procedure does not affect these results. Higher ristocetin concentrations show CV ranges of 3% to 19% before and 3–22% after standardization (). For epinephrine 5 µM the CV before standardization is lower (). Finally, LTA results of arachidonic acid are relatively stable between different instruments before and after standardization ().
Observational Changes in LTA Results for five Individual Hospital Laboratories Who Participated Both Before and After Standardization
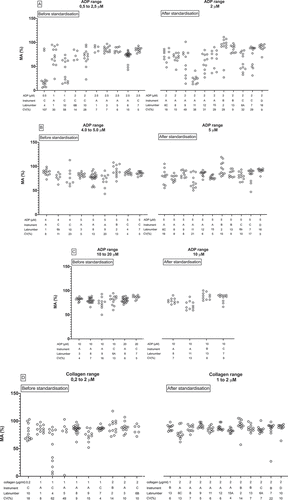
illustrates the consequence of LTA standardization with intermediate doses of ADP for five individual hospital laboratories who participated in both surveys. The variation in maximum aggregation of hospital laboratory 2 and 6 shows no obvious change between 2013 and 2016. Standardization clearly increases variation in maximum aggregation with intermediate concentrations of ADP in hospital 7. In contrast to this, variability is reduced in both hospital 8 and 9 after standardization in 2016. demonstrates the results of LTA standardization with low concentrations of collagen for the same five hospitals. Standardization evidently reduces variation in maximum aggregation with low collagen doses in 4 out of 5 hospital laboratories.
Figure 2. The consequence of standardization for five individual hospitals who participated before and after standardization. Diagrams show variation in maximum aggregation after stimulation of PRP from healthy subjects with a) 4–5 µM ADP or b) 1–2 µg/ml collagen. Results are expressed as % maximum aggregation
Determination of General Reference Ranges
Reference intervals (95% confidence intervals, normally distributed) for maximum aggregation expressed as a percentage, for each agonist, were estimated using the LTA data from all healthy controls (). For individual agonist responses, the total numbers of samples tested ranged from 42 to 107.
Table III. LTA reference intervals (95% confidence interval, normally distributed) for % maximum aggregation per agonist concentration after standardization
Maximum aggregation response to low concentrations (2 μM) of ADP in healthy volunteers shows a very broad range of results (). However, less variability was seen with intermediate (5 μM) and high (10 μM) concentrations of ADP. This also accounts for low and high doses of collagen and ristocetin, epinephrine and arachidonic acid ().
Discussion
Nowadays, LTA is still the most common method used in clinical laboratories to assess platelet function and is considered the gold standard method for detecting thrombocytopathy [Citation23–26]. However, (pre-)analytical aspects of this method have not yet been standardized worldwide [Citation15–17] despite various efforts of experts involved in the development of national or international guidelines [Citation18–22]. Novel techniques are developed, e.g. whole-blood flow cytometry [Citation12,Citation27]. Furthermore, promising results are accomplished with platelet aggregation studies under flow conditions [Citation28]. Advantages of these new methods are the use of whole blood and low sample preparation, but they also require trained technicians and specialized equipment [Citation29–34]. Likewise, standardization of the traditional LTA method is still essential here.
Despite the recently ISTH-SSC Platelet Physiology Subcommittee guideline for LTA [Citation18], a Dutch survey of LTA protocols from 25 different hospitals in the Netherlands resulted in 25 different procedures (unpublished results). Adherence to a new guideline requires many changes for the individual hospitals, which are time and money consuming and the actual improvement for patient care remains unknown. This discrepancy in LTA procedures is not new, but also evident from other surveys [Citation15–17]. Results of a worldwide survey [Citation21] showed that common practices were identified in sample collection, processing and analysis and although some were generally considered acceptable, others were not ideal. The agonist concentrations used for LTA varied, and many laboratories used ADP, collagen, epinephrine and ristocetin, at more than one concentration, in addition to arachidonic acid. The parameters commonly used to assess LTA responses were maximum amplitude or percentage aggregation, which were considered particularly important, in addition to the presence of a ‘secondary wave,’ deaggregation, shape change and a measure of the lag phase. Furthermore, many laboratories did not have appropriate determined reference intervals or used non-adjusted PRP [Citation21]. We examined the introduction of a national LTA protocol in the Netherlands and the consequences of standardization. Most and very important variables [Citation35] such as used citrate concentration (109 mM) or the window between blood drawing and analysis were completely standardized by the participants in 2016 (after standardization). Our data show that standardization was not effective for the low ADP concentration. This can be explained by the fact that ADP is a batch-dependent agonist and probably highly variable in the low concentration range. The question arises whether we should still use these low concentrations of ADP. It is unknown what the low ADP concentration adds to the analyses of bleeding tendency when no discrimination can be made between healthy volunteers and patients with a bleeding tendency. ADP requires structural and extensive quality control of lots and batches [Citation18]. Unpublished results from a small, multicentre, agonist brand study in the Netherlands on the agonists collagen and ADP showed that brand and lot differences were the most prominent for the variable aggregation patterns with low dose ADP and for deviations in the type of collagen used (non-equine collagen brands). In our study, brands were mostly coupled with instrument brands and we could not see a clear pattern in differences. Althaus et al. [Citation36] performed an interesting study which was partly comparable with our study by questioning 15 laboratories to measure maximal aggregation in 3 PRP samples of healthy volunteers using a fixed panel of agonists that were provided by shipping to the hospitals. In this study, the highest CV was observed using the lowest ADP concentration and the CV decreased with increasing ADP levels. They contributed this effect to a lower stability of ADP in shipping.
In contrast to ADP, low doses of collagen showed improvement in variation of LTA results after standardization. Presumably, this is because not every laboratory used the same collagen preparation before standardization in 2013. Differences between bovine and equine collagen preparations in LTA have been described earlier and could be a source of variance [Citation37]. Likewise, arachidonic acid displayed less variation in maximum aggregation results after standardization. This cannot be explained. In case of epinephrine no significant effect of standardization was observed.
The benefits of standardization for an individual laboratory were present but variable. Using a fixed concentration of ADP had mostly no effect and brought no reduction in variation in the majority of the hospitals. In general, there was less variation in LTA results with a fixed concentration of collagen after standardization. In conclusion, variation was not unambiguously reduced after standardization, assuming that there was protocol adherence.
A possible explanation for the fact that in general there is a low reduction in variation after standardization, might be the effect of introducing non-adjusted PRP, which was a major change for laboratories after standardization. Diluting PRP with platelet poor plasma to adjust platelet count introduces artifacts as suggested by Cattaneo et al. and should be avoided, because it artefactually inhibits platelet aggregation [Citation18,Citation38]. In addition, it may also lead to more variation, because of different platelet counts in PRP [Citation39,Citation40]. However, working with non-adjusted PRP may also result in variation between healthy volunteers. Another study shows that LTA with platelet count adjusted PRP is superior to native PRP for detecting bleeding disorders, although the benefit is small and may not be clinically significant [Citation41]. On the other hand, Althaus et al. [Citation36] used also non-adjusted PRP and published lower CV’s in healthy PRPs compared to our findings.
A limitation of our study is that healthy control subjects differ in 2013 (before standardization) and 2016 (after standardization). Furthermore, there are limited data for some agonists, e.g. less than 10 donors per hospital. This makes our research largely observational. We only investigated healthy volunteers and not patients with thrombocytopathies. Advantages of standardization could be larger in this target population; patient suspected of bleeding disorder.
The CLSI guideline requires a minimum of 120 normal subjects to establish reference intervals. For a technique such as LTA this is expensive, labor-intensive and not feasible for each individual laboratory. The proposed ranges in can give guidance for laboratories and are directorial. Each laboratory must decide and substantiate whether they can use these values in daily patient care.
Taken together, our findings illustrate that standardization of LTA procedures does not necessarily lead to less variation, especially for low agonist concentrations. For most agonist concentrations (intermediate and high ADP, low and high collagen, low and high ristocetin, epinephrine and arachidonic acid) variability decreased in healthy subjects. The VHL still encourages all LTA users to adhere to the new guideline.
Declaration Of Interest Statement
The authors report no declarations of interest.
Acknowledgements
The authors thank C. Klopper, S. Busscher, H. Nieman-Teunis, T. de Boer, J. van Empel, N. van Dijk and C. van den Nieuwenhof for performing the LTA.
References
- Nurden P, Nurden AT. Congenital disorders associated with platelet dysfunctions. Thromb Haemost 2008;99:253–263.
- Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962;194:927–929.
- Harrison P, Mackie I, Mumford A, Briggs C, Liesner R, Winter M, Machin S, British Committee for Standards in Haematology. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol 2011;155:30-44.
- Hayward CP. Diagnostic approach to platelet function disorders. Transfus Apher Sci 2008;38:65–76.
- Gresele P; Subcommittee on Platelet Physiology of the International Society on Thrombosis and Hemostasis. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost 2015;13:314–322.
- Mezzano D, Quiroga T, Pereira J. The level of laboratory testing required for diagnosis or exclusion of a platelet function disorder using platelet aggregation and secretion assays. Semin Thromb Hemost 2009;35:242–254.
- Mumford AD, Ackroyd S, Alikhan R, Bowles L, Chowdary P, Grainger J, Mainwaring J, Mathias M, O’Connell N; BCSH Committee. Guideline for the diagnosis and management of the rare coagulation disorders: a United Kingdom haemophilia centre doctors’ organization guideline on behalf of the British committee for standards in haematology. Br J Haematol 2014;167:304–326.
- Watson SP, Lowe GC, Lordkipanidzé M, Morgan NV; GAPP consortium. Genotyping and phenotyping of platelet function disorders. J Thromb Haemost 2013;11 Suppl 1:351–363.
- Dawood BB, Lowe GC, Lordkipanidzé M, Bem D, Daly ME, Makris M, Mumford A, Wilde JT, Watson SP. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood 2012;120:5041-5049.
- Moenen FCJI, Vries MJA, Nelemans PJ, van Rooy KJM, Vranken JRRA, Verhezen PWM, Wetzels RJH, Ten Cate H, Schouten HC, Beckers EAM, et al. Screening for platelet function disorders with Multiplate and platelet function analyzer. Platelets 2019;30:81–87.
- Al Ghaithi R, Drake S, Watson SP, Morgan NV, Harrison P. Comparison of multiple electrode aggregometry with lumi-aggregometry for the diagnosis of patients with mild bleeding disorders. J Thromb Haemost 2017;15:2045–2052.
- van Asten I, Schutgens REG, Baaij M, Zandstra J, Roest M, Pasterkamp G, Huisman A, Korporaal SJA, Urbanus RT. Validation of flow cytometric analysis of platelet function in patients with a suspected platelet function defect. J Thromb Haemost 2018;16:689–698.
- Breddin HK. Can platelet aggregometry be standardized? Platelets 2005;16:151–158.
- Krekels JPM, Verhezen PWM, Henskens YMC. Platelet aggregation in healthy participants is not affected by smoking, drinking coffee, consuming a high-fat meal, or performing physical exercise. Clin Appl Thromb Hemost 2018 Jan 1;1076029618782445. doi:10.1177/1076029618782445. Epub ahead of print.
- Moffat KA, Ledford-Kraemer MR, Nichols WL, Hayward CP. Variability in clinical laboratory practice in testing for disorders of platelet function: results of two surveys of the North American specialized coagulation laboratory association. Thromb Haemost 2005;93:549–553.
- Jennings I, Woods TA, Kitchen S, Walker ID. Platelet function testing: practice among UK national external quality assessment scheme for blood coagulation participants, 2006. J Clin Pathol 2008;61:950–954.
- Duncan EM, Bonar R, Rodgers SE, Favaloro EJ, Marsden K. Methodology and outcomes of platelet aggregation testing in Australia, New Zealand and the Asia-Pacific region: results of a survey from the royal college of pathologists of Australasia haematology quality assurance program. Int J Lab Hematol 2009;31:398–406.
- Cattaneo M, Cerletti C, Harrison P, Hayward CP, Kenny D, Nugent D, Nurden P, Rao AK, Schmaier AH, Watson SP et al. Recommendations for the Standardization of Light Transmission Aggregometry: A Consensus of the Working Party from the Platelet Physiology Subcommittee of SSC/ISTH. J Thromb Haemost 2013Apr 10. doi: 10.1111/jth.12231. [Epub ahead of print].
- Christie DJ, Avari T, Carrington LR, Cohen E, DeBiase B, Harrison P, Kickler TS, Kottke-Marchant K, Ledford-Kraemer M, Rand ML et al. Platelet Function Testing by Aggregometry: Approved Guideline. Wayne PA: Clinical and Laboratory Standards Institute 2008;28:1-45.
- Hayward CP, Moffat KA, Raby A, Israels S, Plumhoff E, Flynn G, Zehnder JL. Development of North American consensus guidelines for medical laboratories that perform and interpret platelet function testing using light transmission aggregometry. Am J Clin Pathol 2010;134:955-63.
- Cattaneo M, Hayward CP, Moffat KA, Pugliano MT, Liu Y, Michelson AD. Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: a report from the platelet physiology subcommittee of the SSC of the ISTH. J Thromb Haemost 2009;7:1029.
- The British Society for Haematology BCSH Haemostasis and Thrombosis Task Force. Guidelines on platelet function testing. J Clin Pathol 1988;41:1322–1330.
- Hayward CP, Pai M, Liu Y, Moffat KA, Seecharan J, Webert KE, Cook RJ, Heddle NM. Diagnostic utility of light transmission platelet aggregometry: results from a prospective study of individuals referred for bleeding disorder assessments. J Thromb Haemost 2009;7:676-684.
- Gresele P, Falcinelli E, Bury L. Laboratory diagnosis of clinically relevant platelet function disorders. Int J Lab Hematol 2018;40 Suppl 1:34–45. doi:10.1111/ijlh.12814.
- Podda G, Scavone M, Femia EA, Cattaneo M. Aggregometry in the settings of thrombocytopenia, thrombocytosis and antiplatelet therapy. Platelets 2018;29:644–649. doi:10.1080/09537104.2018.1445843. Epub 2018 Mar 14.
- Lussana F, Femia EA, Pugliano M, Podda G, Razzari C, Maugeri N, Lecchi A, Caberlon S, Gerli G, Cattaneo M. Evaluation of platelet function in essential thrombocythemia under different analytical conditions. Platelets 2019;20:1–8. doi:10.1080/09537104.2019.1584668. Epub ahead of print.
- Navred K, Martin M, Ekdahl L, Zetterberg E, Andersson NG, Strandberg K, Norstrom E. A simplified flow cytometric method for detection of inherited platelet disorders-A comparison to the gold standard light transmission aggregometry. PLoS One 2019;14:e0211130. doi:10.1371/journal.pone.0211130. eCollection 2019.
- Brouns SLN, van Geffen JP, Heemskerk JWM. High-throughput measurement of human platelet aggregation under flow: application in hemostasis and beyond. Platelets 2018;29:662–669. doi:10.1080/09537104.2018.1447660. Epub 2018 Mar 14.
- Boknäs N, Macwan AS, Södergren AL, Ramström S. Platelet function testing at low platelet counts: when can you trust your analysis? Res Pract Thromb Haemost 2019;3:285–290. doi:10.1002/rth2.12193. eCollection 2019 Apr.
- Platton S, McCormick Á, Bukht M, Gurney D, Holding I, Moore GW. A multicenter study to evaluate automated platelet aggregometry on Sysmex CS-series coagulation analyzers-preliminary findings. Res Pract Thromb Haemost 2018;2:778–789. doi:10.1002/rth2.12140. eCollection 2018 Oct.
- Bret VE, Pougault B, Guy A, Castet S, Huguenin Y, Pillois X, James C, Fiore M. Assessment of light transmission aggregometry on the routine coagulation analyzer Sysmex CS-2500 using CE-marked agonists from Hyphen Biomed. Platelets 2019;30:540–542. doi:10.1080/09537104.2018.1528346. Epub 2018 Oct 12.
- Chan MV, Leadbeater PD, Watson SP, Warner TD. Not all light transmission aggregation assays are created equal: qualitative differences between light transmission and 96-well plate aggregometry. Platelets 2018;29:686–689. doi:10.1080/09537104.2018.1466388. Epub 2018 May 1.
- Chan MV, Armstrong PC, Warner TD. 96-well plate-based aggregometry. Platelets 2018 Nov;29 7:650–655. doi:10.1080/09537104.2018.1445838. Epub 2018 Mar 15.
- Frère C, Kobayashi K, Dunois C, Amiral J, Morange PE, Alessi MC. Assessment of platelet function on the routine coagulation analyzer Sysmex CS-2000i. Platelets 2018;29:95–97. doi:10.1080/09537104.2017.1353683. Epub 2017 Sep 29.
- Germanovich K, Femia EA, Cheng CY, Dovlatova N, Cattaneo M. Effects of pH and concentration of sodium citrate anticoagulants on platelet aggregation measured by light transmission aggregometry induced by adenosine diphosphate. Platelets2018;29:21-26. doi: 10.1080/09537104.2017.1327655. Epub 2017 Jun 23.
- Althaus K, Zieger B, Bacchoul T, Jurk K. Standardisation of light transmission aggregometry for diagnosis of platelet disorders: an inter-laboratory external quality control assessment. Thromb Haemost 2019;119:1154–1161.
- Jarvis GE, Atkinson BT, Snell DC, Watson SP. Distinct roles of GPVI and integrin alpha(2)beta(1) in platelet shape change and aggregation induced by different collagens. Br J Pharmacol 2002;137:107–117.
- Cattaneo M, Lecchi A, Zighetti ML, Lussana F. Platelet aggregation studies: autologous platelet-poor plasma inhibits platelet aggregation when added to platelet-rich plasma to normalize platelet count. Haematologica 2007;92:694–697.
- Mani H, Luxembourg B, Kläffling C, Erbe M, Lindhoff-Last E. Use of native or platelet count adjusted platelet rich plasma for platelet aggregation measurements. J Clin Pathol 2005;58:747–750.
- Breet NJ, Van Werkum JW, Bouman HJ, Kelder JC, Ten Berg JM, Hackeng CM. Do not adjust the platelet count in light transmittance aggregometry when predicting thrombotic events after percutaneous coronary intervention. J Thromb Haemost 2010;8:2326-2328.
- Castilloux JF, Moffat KA, Liu Y, Seecharan J, Pai M, Hayward CPM. A prospective cohort study of light transmission platelet aggregometry for bleeding disorders: is testing native platelet-rich plasma non-inferior to testing platelet count adjusted samples? Thromb Haemost 2011;106:675-682.