Abstract
In this post hoc study, arachidonic acid (AA)-induced platelet aggregation during pregnancy with and without acetylsalicylic acid (ASA) treatment was studied in 323 women with unexplained recurrent first-trimester miscarriage and in 59 healthy women with normal pregnancies. All women had normal AA-induced platelet aggregation in the non-pregnant state. Women with recurrent miscarriage were treated with 75 mg ASA or placebo daily. AA-induced platelet aggregation was measured with multiple electrode impedance aggregometry and presented in units (U), where 1 U = 10 aggregation units x minutes. There were no significant differences in platelet aggregation between placebo-treated women with recurrent miscarriage and healthy women. The mean differences were
-0.7 (95%CI; −7.0; 5.6) U in the non-pregnant state, 3.8 (95%CI; −4.6; 12.2) U during the late first trimester and 1.7 (95%CI; −6.7; 10.3) U and 4.1 (95%CI; −3.9; 12.0) U during the early and late third trimester, respectively. ASA reduced platelet aggregation by median −84.0% (Q1; Q3; −89.8; −76.3), −79.9% (−84.7; −69.2) and −75.7% (−83.5; −49.5), respectively, during pregnancy. The degree of inhibition by ASA decreased during the third trimester (p < .0001). There were two (1.9%) complete non-responders to ASA and 32.1% with a partial response. The rate of subsequent miscarriage was not affected by ASA, which did not seem to influence the rate of early miscarriage if treatment was initiated when a viable pregnancy was detectable by ultrasound.
Introduction
It is well known that acetylsalicylic acid (ASA) inhibits platelet aggregation [Citation1–4]. More pronounced platelet aggregation has been suggested to play an important role in placenta-mediated obstetric complications such as recurrent miscarriage, preeclampsia, intrauterine growth retardation, impaired placental circulation and pregnancy-induced hypertension [Citation5–10]. However, the importance of platelet aggregation in unexplained recurrent miscarriage, affecting 1–2% of fertile couples [Citation11], has not been clarified [Citation5,Citation6,Citation8]. The results of clinical studies of ASA treatment are conflicting [Citation12–17]. Some studies show that ASA reduces placenta-mediated obstetric complications [Citation14,Citation16,Citation18], while others do not [Citation11,Citation19,Citation20]. Moreover, neither the optimal time for starting treatment nor the optimal ASA dose have as yet been identified [Citation1,Citation14].
The primary aim of this study was to compare arachidonic acid (AA)–induced platelet aggregation during pregnancy in women with and without unexplained recurrent miscarriage. The secondary aim was to study the effect of ASA on AA-induced platelet aggregation during pregnancy as well as on recurrence of miscarriage.
Methods
Study populations
This is a post hoc study based on a placebo-controlled RCT, conducted between 2008 and 2016, that investigated whether low-dose plain, not enteric-coated ASA (75 mg daily), prevented recurrent first-trimester pregnancy loss [Citation20]. In this study, we evaluated 160 women given ASA and 163 women given placebo. The women had had between three and seven first-trimester miscarriages. All women fulfilled the inclusion criteria for the RCT, including normal thrombophilia tests, no bleeding disorders and no medication affecting hemostasis. Only women with normal non-pregnant, AA-induced platelet aggregation were included. Mild thyroid disease, anxiety disorders and allergy, mostly to pollen, were allowed. Smoking was not an exclusion criterion for this study and was registered before pregnancy and at gestational week (gw) 13.
The control group consisted of 59 women with normal non-pregnant, AA-induced platelet aggregation from a previous longitudinal study of platelet aggregation during pregnancy and postpartum [Citation21]. They were randomly selected among women in very early pregnancy attending an ordinary antenatal clinic. The controls were healthy nonsmokers with none or one previous pregnancy loss, no previous complicated pregnancy, no family history of thromboembolic or bleeding disorders, no current medication affecting hemostasis (including platelet function) and normal non-pregnant, AA-induced platelet aggregation.
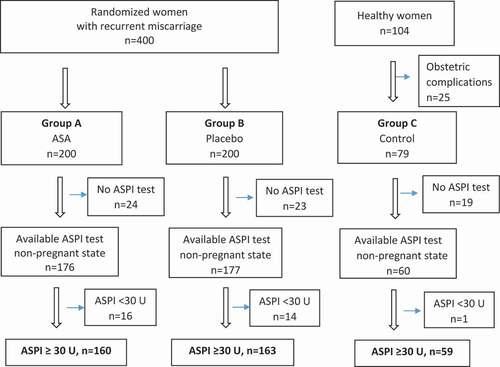
The flow of patients into the final study groups is depicted in , in which “ASPI test” denotes AA induction of platelet aggregation (see below).
Figure 1. Flow chart of potentially eligible and ultimately included women. Normal AA-induced platelet aggregation: ASPI test ≥ 30 U.
All participants received oral and written information before inclusion in the studies and written consent was given. The participants did not influence on study planning.
Blood sampling and laboratory analysis
Blood samples for platelet aggregation analysis were collected on three occasions during pregnancy: late first trimester and early and late third trimester. Samples were drawn from the cohort of healthy women approximately three months (mean 13.7 (SD 2.0) weeks) postpartum to represent the non-pregnant state, as it would have been very difficult to include healthy women before pregnancy. Brachial vein blood samples were drawn between 7 am and 3 pm at the Department of Clinical Chemistry at Södra Älvsborg Hospital, Borås, Sweden. Fresh whole blood, plasma and serum were used for the analyses.
Platelet aggregation
Fresh whole blood samples were collected in two 3-ml tubes containing 25 µg/ml hirudin (Hirudin Blood Tube, Roche, Switzerland). Platelet aggregation analyses were performed by multiple electrode impedance aggregometry (Multiplate Platelet Function Analyzer, Roche Diagnostics International Ltd, Rotkreutz, Switzerland), utilizing the ASPI test, in which AA is the initiator. Analyses were performed, following manufacturers’ instructions, by specially trained staff at the Department of Clinical Chemistry at Södra Älvsborg Hospital.
Platelet aggregation was measured as area under the aggregation curve (AUC), by multiplying aggregation units (AU), on the y axis, with time in minutes, on the x axis. Platelet aggregation was presented as units (U), where 1 U = 10 AU x minutes. An ASPI value <30 U during ASA treatment was used as the cutoff for normal ASA response [Citation22–24]. Non-response to ASA was defined as ≥30 U on every testing occasion during ASA treatment. Full response was defined as <30 U on every testing occasion during ASA treatment. Partial response was defined as at least one sample ≥30 U on any testing occasion during ASA treatment. Changes in AA-induced aggregation, respectively, corresponding to a 30%, 40% and 50% reduction, compared to the non-pregnant state, were also determined.
To study the effect of smoking on AA-induced platelet aggregation, smokers were compared with nonsmokers before and during pregnancy, when applicable.
Routine laboratory analyses
Blood samples were collected before pregnancy and at 20 gws from women treated with ASA or placebo, for analysis of hemoglobin (Hb), platelet count (PC), activated partial thromboplastin time (APTT) and prothrombin complex (INR). PC was analyzed in healthy controls at gws 8–15, 20–23, 30–33 and 37–40, and three months postpartum.
The blood samples for analysis of Hb, PC, INR, and APTT were collected in Vacurette K2EDTAs (Greiner Bio-One, Bad Haller str. 32, 4550 Kremsmünster, Austria) and in BD Vacutainers in 2.7 ml buffered 0.109 M sodium citrate (Becton, Dickinson and Company, Franklin Lakes, NJ 07417, USA), as appropriate. They were sorted, centrifuged at 2000 g and at 20°C for 10 minutes, and analyzed within 30 minutes.
Statistical methods
The sample sizes were determined by the availability of non-pregnant ASPI test results in the eligible subjects in the two original studies [Citation20,Citation21]. Continuous variables are presented as mean and standard deviation (SD), median, and quartiles 1 and 3 (Q1; Q3). The non-parametric Wilcoxon Signed Rank test and the Mann-Whitney U test were used for comparison within groups and for pairwise comparison of continuous variables between groups, respectively. Mean differences were calculated and presented with 95% confidence intervals (CI) for all main results, based on bootstrapping with 10,000 repetitions. Categorical variables are presented as numbers and percentages and Fisher´s exact test was applied for comparison between groups. All significance tests were two-sided and conducted at the 5% significance level. Analyses were performed using the SAS System version 9, SAS Institute, Cary, NC, USA.
Results
General
Demographic variables, previous medical and obstetric history, medication, platelet aggregation and PC in the non-pregnant state for the three groups are presented in . The women from the RCT had a mean age of 32.1 years and the controls’ mean age was 29.3 years. Mean BMIs were 24.0 kg/m2 and 23.1 kg/m 2, respectively.
Table I. Demographics, non-pregnant platelet aggregation after activation by arachidonic acid and platelet count at baseline (non-pregnant state)
Before pregnancy, there were 17 (10.6%) smokers in the ASA group and nine (5.5%) smokers in the placebo group. When pre-pregnancy smokers and nonsmokers were compared among women with recurrent miscarriage, there was no significant difference in AA-induced platelet aggregation (data not shown). At gw 13, there were missing data concerning smoking in two women, one in each group, while two women were still smokers in the ASA group and none in the placebo group. There were no smokers among the healthy controls, according to the inclusion criteria. In women treated with ASA or placebo, Hb, PC, APTT and INR were within the normal ranges before pregnancy, except one woman, in whom PC was 76 and 66 x109/L, respectively, on two sampling occasions. As anticipated, all PC levels were significantly lower at gw 20 than in the non-pregnant state. PC was above 100x10⁹/L during pregnancy and postpartum in all healthy controls [Citation21].
Platelet aggregation in the placebo group and in healthy controls
No significant differences were observed in AA-induced platelet aggregation at any time-point when placebo-treated women with recurrent miscarriage were compared with healthy women with normal pregnancy. The mean differences were −0.7 (95%CI; −7.0; 5.6) U in the non-pregnant state, 3.8 (95%CI; −4.6; 12.2) U during the late first trimester and 1.7 (95%CI; −6.7; 10.3) U and 4.1 (95%CI; −3.9; 12.0) U, respectively, during the early and late third trimester. No adjusted analysis excluding smokers was necessary, since all women (except one with unknown smoking habits at gw 13) stopped smoking once they became pregnant. However, significantly less AA-induced platelet aggregation was found in the non-pregnant state, compared with gws 8–15 and 37–40 (), in healthy women. There were no changes over time within the placebo group.
Table II. Absolute changes in arachidonic acid-induced platelet aggregation (ASPI test) in women with unexplained recurrent miscarriage and healthy women with normal pregnancy. Comparisons of the non-pregnant state and during pregnancy, within and between the study groups, treated with ASA or placebo or no treatment
Platelet aggregation in the ASA group
Women administered 75 mg ASA daily had significantly decreased platelet aggregation at gws 13, 30 and 36, compared with their non-pregnant state (). This group had significantly lower AA-induced platelet aggregation at every time-point during pregnancy, compared with placebo-treated women and healthy controls ().
Figure 2. AA-induced platelet aggregation in women with unexplained recurrent miscarriage and in healthy women with normal obstetric history and normal pregnancies and deliveries at term. Participants were given ASA or placebo. No treatment was given to healthy controls. All women had normal AA-induced platelet aggregation in the non-pregnant state and had available results from all testing occasions. Boxplots show mean, median, 25th and 75th percentiles, min and max. O denotes outliers.
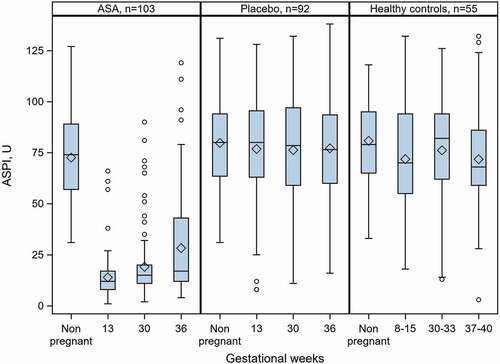
Relative changes in AA-induced platelet aggregation within the group, from the non-pregnant state to gws 13, 30 and 36, were highly significant. The relative reductions (presented as median (Q1;Q3), p-value within group and number of women) were observed at gw 13 (−84.0%;-89.8; −76.3; p < .0001; n = 123), gw 30 (−79.9%; −84.7; −69.2; p < .0001; n = 116) and gw 36 (−75.7%; −83.5; −49.5; p < .0001; n = 113). The degree of AA-induced inhibition at gw 36 was lower than that at any other gw ().
Sixty-eight (66.0%) of 103 women with blood samples taken at all three time-points during pregnancy exhibited full response to ASA at all three time-points. Two (1.9%) women were non-responders to ASA (>30 U at all measurements). The remaining 33 (32.1%) women exhibited varying degrees of platelet aggregation during the different periods of pregnancy and were identified as partial responders.
An analysis based on smoking status during pregnancy revealed no differences in AA-induced aggregation, as all women (except two, or possibly three, of 17) stopped smoking once they became pregnant.
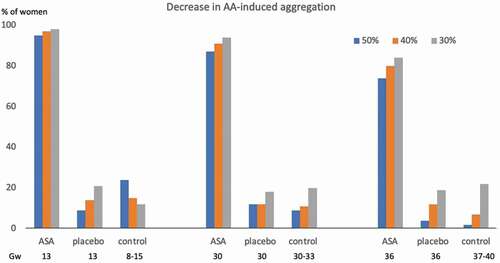
summarizes changes in AA-induced aggregation, respectively, corresponding to a 30%, 40% and 50% reduction, compared to the non-pregnant state, in all three groups.
Figure 3. Proportion with different degrees of decrease in AA-induced platelet aggregation, according to gw, in women with unexplained recurrent miscarriage, treated with ASA or placebo, and in healthy women with normal pregnancy AA: arachidonic acid, ASA: acetylsalicylic acid, gw: gestational week.
Obstetric outcome
First-trimester miscarriage occurred in 13.2% and 13.5% of the ASA and placebo groups, respectively. Second-trimester miscarriage occurred in 2.5% and 1.2%, respectively, in the two groups. The live birth rate after 22 gestational weeks was 83.8% and 84.7%, respectively, in the ASA- and placebo-treated women, of whom 75.6% and 73.6%, respectively, delivered at term.
Discussion
The main findings in this study were that there were no significant differences when AA-induced platelet aggregation in women with at least three unexplained consecutive first-trimester miscarriages was compared with that in healthy women, that low-dose ASA treatment resulted in markedly reduced platelet aggregation during pregnancy and that ASA treatment did not affect the early miscarriage rate if initiated when a viable pregnancy was detectable by ultrasound. Platelet aggregation was reduced by more than 50% among the majority of ASA-treated women throughout their entire pregnancies. There were very few non-responders to ASA. Less inhibition of platelet aggregation by ASA was observed as pregnancy progressed, although most of the women had platelet aggregation levels below the suggested cutoff for ASA effect during the entire pregnancy.
A strength of this study is the large size of the randomized cohort. Very few women failed to complete their follow-up and an overwhelming majority adhered completely to the entire study protocol. The fact that the women were followed longitudinally strengthens the results and the healthy control group enabled the evaluation of platelet aggregation in relation to normal pregnancy.
Smoking has been reported to be associated with impaired AA-induced platelet aggregation response to ASA [Citation25]. Any skewed distribution of smokers between groups might thus have had an impact on the results, if smoking cessation had not been almost universal, beginning from early pregnancy. The placebo group lacked smokers, as did the healthy control group, enabling a direct comparison. Moreover, the ASA group had very few smokers during pregnancy (only two). These low smoking rates generally strengthen the validity of our results.
One limitation is the large inter-individual variation when multiple electrode impedance aggregometry is used to determinate platelet aggregation. This has also been found in other populations [Citation26,Citation27] and limits the possibility to evaluate minor changes within individuals related to different treatments. Intra-individual variation does not differ significantly between impedance platelet aggregometry, measured by the Multiplate system, and turbidimetric platelet aggregation [Citation28]. The median coefficient of variation for multiple electrode impedance aggregometry has been reported to be 11.4% for AA induction [Citation28].
Another limitation is that demographic variables such as educational level and other socio-economic parameters were not available for the healthy control group. However, we believe that difference in the variable preexisting illness reflects the healthy control group being even more healthy than the ASA and placebo groups. The loss to follow-up of women in the control group three months postpartum is also a limitation. As anticipated, the willingness to participate three months after delivery declined.
The absence of any significant differences in platelet aggregation between healthy women without recurrent pregnancy loss and the women with a history of recurrent miscarriage, including after controlling for smoking, suggests that platelet aggregation is not a major cause of recurrent miscarriage after viable pregnancy has been verified. Our previous results, based on intention to treat analysis and comparing live birth rates in women treated with ASA and placebo, strengthen this finding [Citation20].
We did find low platelet aggregation during early and late pregnancy in the non-treated healthy women, but not in the placebo-treated women with previous recurrent first-trimester pregnancy loss. This diminished platelet aggregation during normal pregnancy has been reported in other studies investigating agonists other than AA [Citation5,Citation29]. These findings may indicate that less AA-induced platelet aggregation is beneficial for development of normal pregnancy. However, contrary to our findings, increased platelet reactivity has been reported in the second and third trimesters [Citation29]. Increased platelet aggregation as a cause of very early miscarriage, and prevention by ASA, cannot be ruled out, as no analysis of platelet aggregation was performed at miscarriage or before gw 13. Furthermore, ASA treatment was not initiated until a viable pregnancy had been verified by ultrasound. Thus, it cannot be ruled out that initiating treatment at ovulation or during very early pregnancy might affect the rate of miscarriage.
In this study, we measured the response to 75 mg ASA daily. Markedly lower AA-induced platelet aggregation during treatment was observed in the majority of treated participants. This has also been reported in other studies [Citation2,Citation3]. We found a more than 50% reduction in AA-induced platelet aggregation in most ASA-treated women during pregnancy. This has not been reported previously. The decreased inhibition of platelet aggregation as pregnancy progressed could be a result of a shorter platelet lifespan [Citation30,Citation31], increased levels of fibrinogen and von Willebrand factor [Citation32,Citation33] or changes in BMI [Citation34]. The finding may suggest that ASA treatment for any condition may require higher dosage in late pregnancy.
We found only two women (1.9%) with unaffected platelet aggregation in response to ASA treatment. The prevalence of ASA non-responsiveness has previously been reported to vary between 29% and 39% in an obstetric population [Citation35–37], corresponding to our findings if we include partial responders among the non-responders. In other studies, a significant number of pregnant women exhibited no response to 81 mg ASA [Citation1,Citation38]. Plausible explanations to non-response are noncompliance, body weight above 100 kg, and enteric coating of ASA [Citation39], none of which were applicable in the present study.
Changes in Hb, INR, APTT, and PC, from the non-pregnant state to gw 20, were as anticipated for pregnant women. The PC was above 100x10⁹/L in all but one woman and was therefore assessed as not having influenced the analyses of AA-induced aggregation.
The inhibitory influence of ASA on platelet aggregation can be measured by different methods, e.g. light transmission aggregometry [Citation5,Citation6], multiple electrode impedance aggregometry [Citation2,Citation36], the Platelet Function Analyzer (PFA)-100 [Citation38] and measurement of thromboxane, a sensitive method for detection of inhibition of AA-induced aggregation by ASA [Citation40,Citation41].
There are different platelet activators and AA is used in all methods for the detection of ASA effect [Citation2,Citation4,Citation33]. Multiple electrode impedance aggregometry (Multiplate analyzer) was chosen because the method has many advantages: easy to handle, uses whole blood, no centrifugation time, results available within six minutes and requires only a very small amount of blood. The method is often recommended and used for determination of platelet aggregation inhibition by ASA [Citation42,Citation43].
In conclusion, there was no significant difference in AA-induced platelet aggregation when women with unexplained recurrent miscarriage were compared with healthy women with normal pregnancies. However, the profile of AA-induced platelet aggregation in the latter group exhibited less platelet aggregation during early and late pregnancy. These findings may indicate that less AA-induced platelet aggregation is beneficial for development of normal pregnancy.
Previous presentation
This study has been presented as oral presentation at the Congress of the International Society for the Study of Hypertension in Pregnancy (ISSHP) and the International Society of Obstetric Medicine (ISOM) in Amsterdam, the Netherlands, October 6-9, 2018.
Contribution to authorship
Lennart Blomqvist, Margareta Hellgren and Annika Strandell designed the study. LB was responsible for the conductance of the clinical trials. Anders Jeppsson took part in the analysis and interpretation of data, together with LB, MH and AS. All authors also analyzed data in collaboration with the statistician. LB wrote the first draft under the supervision of MH. All authors have revised the manuscript and have approved the final version.
Acknowledgements
We thank biostatistician Nils-Gunnar Pehrsson and his staff at Statistiska Konsultgruppen, Gothenburg, for assistance with the statistical analyses. We also thank Joy Ellis for her professional revision of the English language.
Disclosure statement
The authors declare no conflicts of interest with respect to the authorship and publication of this article.
Additional information
Funding
References
- Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P, Tsatsaris V. Aspirin for prevention of preeclampsia. Drugs 2017;77(17):1819–1831. https://doi.org/10.1007/s40265-017-0823-0
- Jambor C, Weber C, Gerhardt K, Dietrich W, Spannagl M, Heindl B, Zwissler B. Whole blood multiple electrode aggregometry is a reliable point-of-care test of aspirin-induced platelet dysfunction. Anesth Analg 2009;109(1):25–31. https://doi.org/10.1213/ane.0b013e3181a27d10
- Norris L, Sheppard B, Bonnar J. Increased whole blood platelet aggregation in normal pregnancy can be prevented in vitro by aspirin and dazmegrel. BJOG 1992;99(3):253–257. https://doi.org/10.1111/j.1471-0528.1992.tb14508.x
- Oscarsson A, Öster S, Fredriksson M, Lindahl T, Entrei C. Platelet function assessed by whole blood aggregometry in patients undergoing non-cardiac surgery. Eur J Anaesthesiol 2011;28(5):363–369. https://doi.org/10.1097/EJA.0b013e32834448ed
- Dempsey MA, Flood K, Burke N, Murray A, Cotter B, Mullers S, Dicker P, Fletcher P, Geary M, Malone F, et al. Platelet function in patients with a history of unexplained recurrent miscarriage who subsequently miscarry again. Eur J Obstet Gynecol Reprod Biol 2015;188:61–65. https://doi.org/10.1016/j.ejogrb.2015.02.003
- Flood K, Peace A, Kent E, Tedesco T, Dicker P, Geary M, Malone F, Kenny D. Platelet reactivity and pregnancy loss. Am J Obstet Gynecol 2010;203(3):281.e1-5. https://doi.org/10.1016/j.ajog.2010.06.023
- Dogan Gun B, Numanoglu G, Oguz Oxdamar S. The comparison of vessels in elective and spontaneous abortion decidua in first trimester pregnancies: importance of vascular changes in early pregnancy losses. Acta Obstet Gynecol 2006;85(4):402–406. https://doi.org/10.1080/00016340500501731
- Whigham KA, Howie PW, Drummond AH, Prentice CR. Abnormal platelet function in preeclampsia. BJOG 1978;85(1):28–32. https://doi.org/10.1111/j.1471-0528.1978.tb15820.x
- Sultana R, Karim F, Atia F, Ferdousi S, Ahmed S. Platelet count in preeclampsia. J Dhaka National Med Coll Hos 2012;18(2):24–26. https://doi.org/10.3329/jdnmch.v18i2.16018
- Morrison R, Crawford J, MacPherson M, Heptinstall S. Platelet behaviour in normal pregnancy, pregnancy complicated by essential hypertension and pregnancy-induced hypertension. Thromb Haemost 1985;54(3):607–611. https://doi.org/10.1055/s-0038-1660080
- Brigham S, Conlon C, Farquharson R. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod 1999;14(11):2868–2871. https://doi.org/10.1093/humrep/14.11.2868
- Bujold E, Morency AM, Roberge S, Lacasse Y, Forest J-C GY. Acetylsalicylic acid for the prevention of preeclampsia and intrauterine growth restriction in women with abnormal uterine artery doppler: a systematic review and meta-analysis. JOGC 2009; 31:818-826.
- Coomarasamy A, Honest H, Papaioannou S, Gee H, Saeed Khan K. Aspirin for prevention of preeclampsia in women with historical risk factors: a systematic review. ACOG 2003;101:1319–1325.
- Meher S, Duley L, Hunter K, Askie L. Antiplatelet therapy before or after 16 weeks gestation for preventing preeclampsia: an individual participant data meta-analysis. Am J Obstet Gynecol 2016;216:121-128.
- Dekker GA, Baha M, Sibai M. Low-dose aspirin in the prevention of preeclampsia and fetal growth retardation: rationale, mechanisms and clinical trials. Am J Obstet Gynecol 1993;168(1):214–227. https://doi.org/10.1016/S0002-9378(12)90917-5
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 2017;216(2):110–120.e6. https://doi.org/10.1016/j.ajog.2016.09.076
- Cui Y, Zhu B, Zheng F. Low-dose aspirin at ≤16 weeks of gestation for preventing preeclampsia and its maternal and neonatal adverse outcomes: a systematic review and meta-analysis. Exp Ther Med 2018;15(5):4361–4369. https://doi.org/10.3892/etm.2018.5972
- Rolnik DL, Wright D, Poon LC, O´Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Nicolaides K, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;337(7):613–622. https://doi.org/10.1056/NEJMoa1704559
- Kaandorp S, Di Nisio M, Goddjin M, Middeldorp S. Aspirin or anticoagulans for treating recurrent miscarriage in women without antiphospholipid syndrome. The Cochrane Library 2009;1:CD004734.
- Blomqvist L, Hellgren M, Strandell S. Acetylsalicylic acid does not prevent first trimester unexplained recurrent pregnancy loss: a randomized controlled trial. Acta Obstet Gynecol Scand 2018;97(11):1365–1372. https://doi.org/10.1111/aogs.13420
- Blomqvist L, Strandell A, Baghaei F, Hellgren M. Platelet aggregation in healthy women during normal pregnancy – a longitudinal study. Platelets 2019;30(4):438–444. https://doi.org/10.1080/09537104.2018.1492106
- Calatzis A, Wittwer M, Kreuger B. A new approach to platelet function analysis in whole blood – the Multiplate analyzer. Platelets 2004;15:485–486.
- Lenk E, Spannagl M. Platelet function testing-guided antiplatelet therapy. International Federation of Clinical Chemistry and Laboratory Medicine 2014;24:90-96.
- Toth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost 2006;96(12):781–788. https://doi.org/10.1160/TH06-05-0242
- Patel D, Moonis M. Clinical implications of aspirin resistance. Expert Rev Cardiovasc Ther 2007;5(5):969–975. https://doi.org/10.1586/14779072.5.5.969
- Rubak P, Villadsen K, Hvas A-M. Reference intervals for platelet aggregation assessed by multiple electrode platelet aggregometry. Thromb Res 2012;130(3):420–423. https://doi.org/10.1016/j.thromres.2012.06.017
- Peerschke E, Castellone D, Stroobants A, Francis J. Reference range determination for whole-blood platelet aggregation using the Multiplate analyzer. Am J Clin Pathol 2014;142(5):647–656. https://doi.org/10.1309/AJCPP43SEYCBJLHJ
- Seyfert U, Haubelt H, Vogt A, Hellstern P. Variables influencing Multiplate whole blood impedance aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets 2007;18(3):199–206. https://doi.org/10.1080/09537100600944277
- Burke N, Flood K, Murray A, Cotter B, Dempsey M, Fay L, Dicker P, Geary M, Kenny D, Malone F. Platelet reactivity changes significantly throughout all trimesters of pregnancy compared with the non-pregnant state: a prospective study. BJOG 2013. doi:https://doi.org/10.1111/1471-0528.12394
- Wallenburg H, van Kessel P. Platelet life span in pregnancies resulting in small-for-gestational age infants. Am J Obstet Gynecol 1979;134(7):739. https://doi.org/10.1016/0002-9378(79)90939-6
- Wallenburg H, van Kessel P. Platelet lifespan in normal pregnancy as determined by a nonradioisotopic technique. Br J Obstet Gynaecol 1978;85(1):33–36. https://doi.org/10.1111/j.1471-0528.1978.tb15821.x
- Kjellberg U, Andersson NE, Rosén S, Tengborn L, Hellgren M. APC resistance and other haemostatic variables during pregnancy and puerperium. Thromb Haemost 1999;81(4):527–531. https://doi.org/10.1055/s-0037-1614518
- Wand S, Adam E, Wetz A, Meybohm P, Kunze-Szikszay N, Zacharowski K, Popov A, Moritz A, Moldenhauer L, Weber C, et al. The prevalence and clinical relevance of ASA nonresponse after cardiac surgery: a prospective bicentric study.Thromb Hemost 2018;24:179–185.
- McCall M, Peace A, Tedesco A, Foley D, Conroy R, Cox D. Weight as an assay-independent predictor of poor response to enteric aspirin in cardiovascular patients. Platelets 2020;18(4):530–535. https://doi.org/10.1080/09537104.2019.1667495
- de Weg J, Abheiden C, Fuijkschot W, Harmsze A, de Boer M, Thijs A, de Vries J. Resistance of aspirin during and after pregnancy: a longitudinal cohort study. Pregnancy Hypertens 2020;19:25–30. https://doi.org/10.1016/j.preghy.2019.11.008
- Navaratnam K, Alfirevic A, Jorgensen A, Alfirevic Z. Aspirin non-responsiveness in pregnant women at high-risk of preeclampsia. Eur J Obstet Gynecol Reprod Biol 2018;221:144–150. https://doi.org/10.1016/j.ejogrb.2017.12.052
- Navaratman K, Alfirevic A, Alfirevic Z. Low dose aspirin and pregnancy: how important is aspirin resistance? BJOG 2016;123(9):1481–1487. DOI:https://doi.org/10.1111/1471-0528.13914
- Caron N, Rivard G-E, Michon N, Morin F, Pilon D, Moutquin J-M RE, Rey É. Low-dose ASA response using PFA-100 in women with high-risk pregnancy. J Obstet Gynecol Can 2009;31(11):1022–1027. https://doi.org/10.1016/S1701-2163(16)34346-8
- Peace A, McCall M, Tedesco T, Kenny D, Conroy RM, Cox D, COX D. The role of weight and enteric coating on aspirin response in cardiovascular patients. J Thromb Haemost 2010;8(10):2323–2325. https://doi.org/10.1111/j.1538-7836.2010.03997.x
- Rozalski M, Watala C, Golanski J. Various laboratory protocols for measuring thromboxane A2 generation to detect the effectiveness of acetylsalicylic acid therapy: a comparative study. Blood Coagul Fibrinolysis 2014;25(1):46–51. https://doi.org/10.1097/MBC.0b013e32836551b5
- Armstrong P, Truss N, Ali F, Dhanji A, Vojnovic I, Zain Z, Bishop-Bailey D, Paul-Clark M, Tucker A, Warnet T, et al. Aspirin and the in vitro linear relationship between thromboxane A2-mediated platelet aggregation and platelet production of thromboxane A2. J Thromb Haemost 2008;6(11):1933–1943. DOI:https://doi.org/10.1111/j.1538-7836.2008.03133.x
- Zimmermann N, Hohlfeld T. Clinical implications of aspirin resistance. Thromb Haemost 2008;100(9):379–390. https://doi.org/10.1160/TH08-01-0056
- Mansour K, Taher A, Musallam K, Alam S. Aspirin resistance. Adv Hematol 2009;2009:1–10. DOI:https://doi.org/10.1155/2009/937352
