Abstract
Platelet function assays and global haemostasis assays are essential in diagnosing bleeding tendencies, with light transmission aggregometry (LTA) as golden standard. The Multiple Electrode Aggregation (Multiplate), platelet function assay (PFA) and rotational thromboelastometry (ROTEM) are mostly used as whole-blood screening tests. Currently, patients have to travel to specialized laboratories to undergo these tests, since specific expertise is required. Pre-analytical variables, like storage time and temperature during transport, are still considered to be the most vulnerable part of the process and may lead to discrepancies in the test results. We aim to give a first impression on the stability of blood samples from healthy volunteers during storage and investigate the effect of storage time (1, 3, 6 and 24 hours) and temperature (4°C, room temperature and 37°C) on the Multiplate, PFA, ROTEM and LTA test results. Our data indicated that, for the PFA, whole blood can be stored for 3 hours at room temperature. Whole blood used for the Multiplate and ROTEM can be stored for 6 hours of storage. For LTA, PRP and whole blood were stable up to 3 hours at 4°C or room temperature and 6 hours at room temperature, respectively.
Introduction
Sustaining normal blood flow, without the risk of bleeding or developing thrombosis, is a critically and tightly regulated process between pro-coagulant and anti-coagulant factors, also known as haemostasis. Haemostasis consists of four phases; 1) during the primary haemostasis, a platelet plug is formed, 2) while in the secondary haemostasis, the formation of the fibrin network occurs, 3) the clot is becoming stable during prolongation phase and 4) during fibrinolysis, the blood clot is demolished. Platelets originate from megakaryocytes in the bone marrow and play an important role in regulating haemostasis. Pathological conditions affecting platelet count or function can result in both bleeding or thrombotic disorder [Citation1–3]. To screen for or identify these haemostatic disorders, platelet function assays are essential, thereby helping clinicians with diagnosing the patient and making clinical decisions.
Therefore, laboratory diagnostics must be performed under high-quality standards to minimalize analytical errors within (haemostasis) laboratory testing[Citation4]. Overall, laboratory testing can be subdivided into three critical phases, which are essential for obtaining reliable test results; the pre-analytical, analytical and post-analytical phase [Citation4–6]. Unfortunately, the pre-analytical phase is still considered to be the most vulnerable part in the whole process of laboratory diagnostics. Inconsistency in performing pre-analytical procedures such as patient preparation, sample collection, specimen acquisition, handling and storage, are contributing to the high incidence of pre-analytical errors [Citation7–12].
Where most pre-analytical variables are easier to standardize, the optimization and consensus in the optimal storage time and temperature of haemostasis tests are difficult to control [Citation8–10]. Generally, specialized haemostatic tests, e.g. platelet function assays, are performed in specialized laboratories. Currently, patients have to travel to the hospitals with the correct expertise to undergo a platelet function assay or global haemostasis assay. Ideally, if optimal pre-analytical conditions of blood could be determined, resulting in increased storage times, specimens could be sent to these specialized hospitals to perform the platelet function assays. Overall, there is need for a general consensus regarding the stability duration of the samples, storage times and conditions of primary tubes or whole blood for these specialized haemostatic tests.
Therefore, we investigated the optimal duration, in which whole blood and platelet-rich plasma are stable during different time intervals and temperatures, for platelet function assays and global haemostasis assays. Here, we determined the blood and plasma stability in the following platelet function assays and global haemostasis assays; Multiplate (Multiple Electrode Aggregation), platelet function analyzer (PFA), rotational thromboelastometry (ROTEM) and light transmission aggregometry (LTA).
Materials and methods
Study design
To elucidate the optimal time duration and temperature, in which whole blood is stable and can be used for platelet function assays and global haemostasis assays, whole blood was collected from healthy volunteers. Approval was given by the local ethics board of the MUMC+ and healthy volunteers were asked to sign an informed consent form. The volunteers were asked to fill in a questionnaire regarding their age, sex, medication and bleeding history. Furthermore, a complete blood count (CBC) was performed for each included blood sample (). For each platelet function assay and global haemostasis assay, whole blood was collected from five healthy volunteers using a venepuncture performed by an experienced phlebotomist. Exclusion criteria for this study were the use of platelet inhibitors or drugs containing acetylsalicylic acid 7 days before blood collection or non-steroidal anti-inflammatory drugs 3 days before blood collection. Volunteers with a history of bleeding were also excluded. During the course of the study, the use of oral contraceptives was allowed.
Table I. Baseline characteristics of healthy volunteers
Blood collection for specialized haemostatic tests
For platelet function assays and global haemostasis assays, whole blood from five healthy volunteers was collected in the morning (between 8.30 and 9.00 AM) by an experienced phlebotomist. Volunteers were allowed to drink water and tea, eat a light, nonfat meal. Blood was collected into different tubes depending on the type of test; an EDTA tube (BD Vacutainer 7.2 mg K2EDTA; 4.0 mL) was collected to measure hematological parameters. For the Multiplate analysis, whole blood was collected in hirudin tubes (Roche Hirudin Blood tube, 3.0 mL) containing a thrombin inhibitor. For the PFA, ROTEM and LTA, whole blood was collected in citrate tubes (BD Vacutainer 9NC) containing 0.109 M (3.2%) sodium citrate in a ratio of 1:10. Whole blood or platelet-rich plasma (PRP) was stored statically in a fridge at around 4°C, room temperature (18°C-22°C) or in a water bath which was set at 37°C (all samples were capped), to investigate the stability of stored samples over time and determine the optimal storage condition (). Before the start of the test, all blood samples were mixed gently. Each time point was compared to a reference condition, which was chosen based on the manufacturer’s instructions ().
Table II. Investigated storage time, temperature and reference condition per test, including the optimal storage condition according to our study
Multiplate
300 μL hirudin anticoagulated blood was diluted with 300 μL NaCl 0.9% and incubated for 3 min at 37°C. Fibrinogen adhered to the coated sensors, after which 20 μL of platelet activator (Adenosine diphosphate (ADP) 6.4 μM (Roche) or collagen 3.2 μg/mL (Chronolog)) was added. Platelets then adhered to the sensors and increased the electrical resistance between the sensors. This change in electrical resistance was detected by the Multiplate. The aggregation response was followed for 6 min at 37°C under constant stirring, resulting in an aggregation curve. The area under the curve was determined and used as a measure of maximal aggregation. The used reference ranges were based on the manufacturer’s instructions.
Platelet function analyzer
Platelet function, in whole blood, was measured using the platelet function analyzer (PFA-200; Siemens Innovance). The process of platelet adhesion and aggregation was stimulated using a collagen/epinephrine (CEPI; Siemens) or a collagen/ADP (CADP; Siemens) cartridge. 800 μL of whole citrated blood was added to the cartridge. The blood flowed through the cartridge under high shear stress. The activated platelets will aggregate to the coated membrane of the cartridges and the time needed to fully close the aperture is measured (closure time (CT)). The used reference ranges were based on the manufacturer’s instructions.
Rotational thromboelastometry
Rotational thromboelastometry (ROTEM; Sigma) is a viscoelastic test that measures general coagulation in whole blood, based on a cup and a permanent oscillating vertical axis. When coagulation occurs, the blood clot increases the resistance on the axis, resulting in a reduced movement. In this research, we performed the EXTEM (star-tem and extem reagent) and FIBTEM (extem and fibtem reagent) test. 300 μL of whole citrated blood was added to the disposable cup together with reagents (Tem Innovations). The 0.2 M CaCl2 (star-tem reagent) recalcified the blood, the extem reagent activated the coagulation by containing recombinant Tissue Factor, and the fibtem reagent inhibited platelets by containing platelet inhibitor cytochalasin D. The parameters measured were clotting time (CT), and A10. By comparing EXTEM and FIBTEM, the contribution of platelets can be determined. The used reference ranges were based on the manufacturer’s instructions.
Light transmission aggregometry
Platelet aggregation was measured using light transmission aggregometry (LTA) on the Chronolog 700 (Aggro/Link version 8). PRP was made by centrifuging the blood for 10 min at 170 g (without using a break). PRP was transferred to another tube and a thrombocyte count was performed by Sysmex XN-9000 (Sysmex). Platelet poor plasma (PPP) was made by centrifuging the remaining plasma for 5 min at 2500 g. This plasma then was transferred to another tube and centrifuged in the ultracentrifuge for 10 min at 10.000 g. Autologous PPP was used to standardize PRP to 250 × 109 platelets/L in order to compare volunteers. To activate the aggregation, two agonists, ADP (diluted in 0.9% NaCl to 5 μmol/L) and collagen (diluted in isotonic glucose solution to 2 μg/mL) were added. The used reference ranges were based on a paper published by our laboratory[Citation13].
Statistics
Statistical analysis of platelet function assays was performed using Graphpad Prism (GraphPad). A Kruskall–Wallis test was used to investigate the influence of pre-analytical variables on the different platelet assays. All median values, including the range of all values, were plotted in the graphs. A p-value of <0.05 was considered a significant difference.
Results
Multiplate
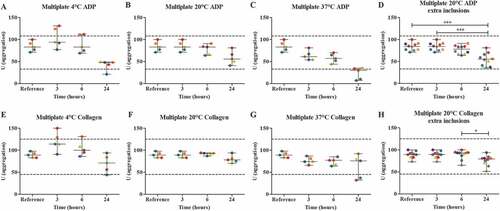
Multiplate analysis was performed in blood from five healthy volunteers using two agonists; ADP and collagen. Blood was stored in a fridge at around 4°C, room temperature (18°C-22°C) or in a water bath which was set at 37°C for 3, 6 and 24 hours. The platelet count in samples stored for 3 h, 6 h, and 24 h was 270 (39), 268 (41), and 273 (48) respectively (). Storage at room temperature, only showed a decrease of the median area under the curve (AUC) after 24 hours for both ADP and collagen ( A-C + E-G). However, storage of blood for 24 hours at 37°C, did show three results (ADP) and two results (collagen) that exceeded the lower reference range ( C + G) and showed errors in haematological parameters, such as the presence of hypochromia and immature granulocytes (data not shown). Also, a downward trend is observed over time, when samples were stored at 4°C and 37°C. Since blood samples, stored at room temperature, showed less variation between samples compared to storage at 4°C, we used this condition for further testing. Five additional volunteers were added to increase the statistical power of the results, leading to a significant difference between the median AUC of the reference value and 24 hours of storage (p < .001) for ADP ( D + H).
Figure 1. The effect of storage time and temperature on the Multiplate analysis (n = 5), using two platelet agonists, 6.4 μM ADP (A-D) and 3.2 μg/mL collagen (E-H). Whole blood was stored for 3, 6 or 24 hours at 4°C (A + E), room temperature (B + F) or 37°C (C + G). For the room temperature condition, 5 additional volunteers were included (D + H). Storage for 3 hours at room temperature was used as the reference value. Values are expressed as median ± range. * p < .05, *** p < .001. Each color represents one volunteer. The reference ranges (ADP: 33–108 U; collagen: 45–125 U) are depicted as dotted lines.
Platelet function analyzer
The PFA was performed in blood from five healthy volunteers using collagen/ADP and collagen/EPI cartridges. Blood was stored in a fridge at around 4°C, room temperature (18°C-22°C) or in a water bath which was set at 37°C for 1, 3, 6 and 24 hours. The platelet count in samples stored for 1 h, 3 h, 6 h, and 24 h was 251 (55), 254 (57), 249 (59), and 257 (53) respectively (). For both collagen/ADP and collagen/EPI, no apparent differences were observed between the median closure time (CT) of the reference value compared to 1, 3, 6 and 24 hours of storage at all storage temperatures ( A-C + E-G). However, storage at 4°C and 37°C do appear to show an upward trend for the collagen/ADP cartridge ( A + C). For the collagen/ADP cartridge, after 3, 6 and 24 hours of storage at 4°C, one, three and one sample exceeded the upper reference range respectively. For the collagen/EPI cartridge, after 1, 3 and 6 hours of storage, one, one and three samples exceeded the upper reference range. Both cartridges showed one sample exceeding the lower reference range after 24 hours of storage ( A + E). After 24 hours of storage at room temperature, only one patient sample provided results below the lower reference range for collagen/ADP and after 6 and 24 hours of storage, two and three samples had measurements above the upper reference range for collagen/EPI, respectively ( B + F). Storage at 37°C showed similar results for collagen/ADP, but for collagen/EPI, one, four and three patients exceeded the upper reference range after 3, 6 and 24 hours of storage respectively ( C + G). Also, the XN-9000 showed errors in the measurements of the haematological parameters in samples stored at 37°C.
Figure 2. The effect of storage time and temperature on the Platelet Function Analyzer analysis (PFA) (n = 5) with cartridges collagen/ADP (A-D) and collagen/EPI (E-H). Whole blood was stored for 1, 3, 6 or 24 hours at 4°C (A + E), room temperature (B + F) or 37°C (C + G). For the room temperature condition, 5 additional volunteers were included (D + H). Storage for 1 hour at room temperature was used as the reference value. Values are expressed as median ± range. * p < .05. Each color represents one volunteer. The reference ranges (CADP: 68–121 seconds; CEPI: 84–160 seconds) are depicted as dotted lines.
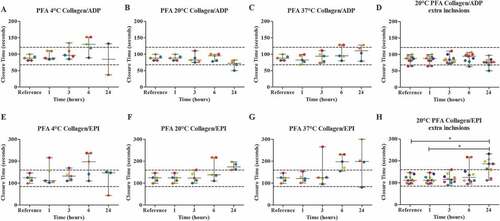
Storage of blood at room temperature showed the most promising results, so we included five extra volunteers for this condition. However, for collagen/ADP, these extra volunteers did not result in a significant difference between the median CT of the reference value in comparison to all other time points. For collagen/EPI, inclusion of extra volunteers did result in a significant difference between the CT of the reference value compared to 24 hours of storage ( D + H).
Rotational thromboelastometry
Blood of five healthy volunteers was used to perform a ROTEM, measuring the EXTEM and FIBTEM with two parameters, clotting time (CT) and the firmness of the clot after 10 minutes (A10 in mm). Blood was stored in a fridge at around 4°C, room temperature (18°C-22°C) or in a water bath which was set at 37°C for 1, 3, 6 and 24 hours. The platelet count in samples stored for 1 h, 3 h, 6 h, and 24 h was 261 (54), 258 (61), 259 (57), and 259 (54) respectively (). Storage of blood at 4°C and room temperature showed no apparent difference between the median CT of the reference value compared to all time points for both the EXTEM and FIBTEM results ( A + B + E + F). Storage at 37°C showed similar results, however, after 24 hours, one patient sample exceeded the upper reference range of the EXTEM CT, possibly interpretable as “abnormal” value ( C + G). For both the EXTEM and FIBTEM CT, the median of the measurements appears to be increased after 24 hours of storage, compared to the reference condition, although this is not significant. Again, haematological parameters showed errors at 37°C. For the EXTEM A10, storage at 4°C and 37°C resulted in samples that exceeded the lower reference range ( A + C + E + G). For all storage temperatures, the FIBTEM A10 did not show an apparent difference between the reference value and all time points ( A-C + E-G).
Figure 3. The effect of storage time and temperature on the clotting time in seconds (CT), using a ROTEM (n = 5) with EXTEM (A-D) and FIBTEM (E-H) as parameters. Whole blood was stored for 1, 3, 6 or 24 hours at 4°C (A + E), room temperature (B + F) or 37°C (C + G). For the room temperature condition, 5 additional volunteers were included (D + H). Storage for 1 hour at room temperature was used as the reference value. Values are expressed as median ± range. Each color represents one volunteer. The reference ranges (EXTEM: 38–79 seconds; FIBTEM: 38–79 seconds) are depicted as dotted lines.
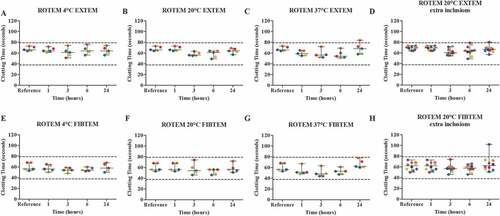
Figure 4. The effect of storage time and temperature on the clot firmness after 10 minutes (A10 in mm), using a ROTEM (n = 5) with EXTEM (A-D) and FIBTEM (E-H) as parameters. Whole blood was stored for 1, 3, 6 or 24 hours at 4°C (A + E), room temperature (B + F) or 37°C (C + G). For the room temperature condition, 5 additional volunteers were included (D + H). Storage for 1 hour at room temperature was used as the reference value. Values are expressed as median ± range. Each color represents one volunteer. The reference ranges (EXTEM: 43–65 mm; FIBTEM: 7–23 mm) are depicted as dotted lines.
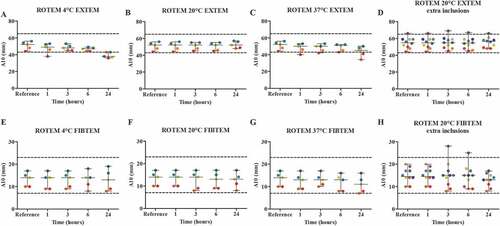
For room temperature, five additional volunteers were included, since this condition showed the best results for both clotting time and firmness of the clot after 10 minutes. The EXTEM analysis did not show a significant difference between the median CT of the reference value and all of the storage times, though, 24 hours of storage showed one value that exceeded the upper reference range ( D). For the EXTEM A10, no significant difference was observed between the median A10 of the reference value and all time points ( D). For the FIBTEM CT, no significant differences were found between the median CT of the reference value and all time points, however, one result exceeded the upper reference range after 24 hours of storage ( D). For the FIBTEM A10, no significant difference was observed between the median A10 of the reference value and 24 hours of storage. The same healthy volunteer exceeded the upper reference range after 3 and 6 hours of storage, but not after 24 hours ( H).
Light transmission aggregometry
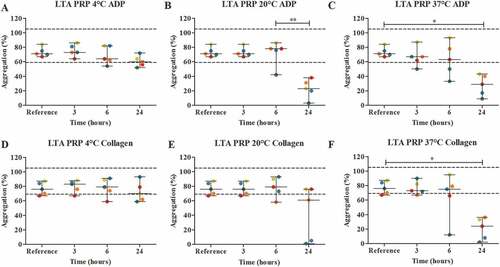
After blood collection, either platelet-rich plasma (PRP) was made and stored in a fridge at around 4°C, room temperature (18°C-22°C) or in a water bath which was set at 37°C for 3, 6 and 24 hours, or whole blood was stored and PRP was made just before analysis. Both methods were performed with blood of five healthy volunteers; ADP and collagen were chosen as agonists for platelet aggregation. For both ADP and collagen, storage of PRP over time showed no significant differences between the median percentage of aggregation of the reference value in comparison to the median percentage of aggregation for all temperatures and all time points ( A-F). At 4°C, we observed one and two patient samples that exceeded the lower reference value after 6 and 24 hours of storage respectively (ADP) and one patient sample that exceeded the lower reference value after 6 and 24 hours of storage (collagen). For storage at room temperature, five (ADP) and two (collagen) patient samples exceeded the lower reference value after 24 hours of storage. At 37°C, all five results exceeded the lower reference range for both ADP and collagen after 24 hours of storage. At 37°C, a lot of variation was observed in the results of both ADP and collagen, and again, errors in haematological parameters occurred ( C + F).
Figure 5. The effect of storage time and temperature on the maximal platelet aggregation (%) using light transmission aggregometry (LTA) (n = 5) with agonists 5 μmol/L ADP (A-C) and 2 μg/mL collagen (D-F). PRP was stored for 3, 6 or 24 hours at 4°C (A + D), room temperature (B + E) or 37°C (C + F). Storage for 3 hours at room temperature was used as the reference value. Values are expressed as median ± range. * p < .05, ** p < .01. Each color represents one volunteer. The reference ranges (60–100%) are depicted as dotted lines.
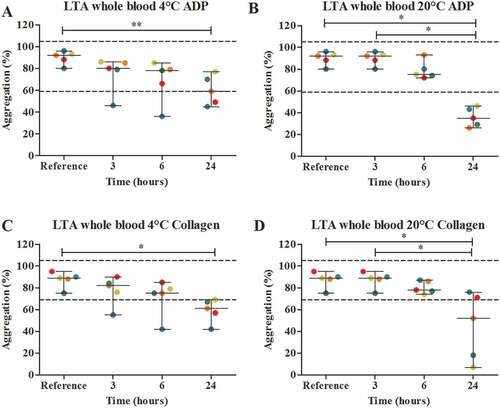
Storage of whole blood over time, did show a significant differences between the median percentage of aggregation of the reference value compared to the median percentage of aggregation of 24 hours of storage for both 4°C and room temperature and for both ADP and collagen ( A-D). Storage of whole blood at 4°C, did result in one sample exceeding the lower reference value after 3 and 6 hours of storage and two samples exceeding the lower reference value after 24 hours of storage for both ADP and collagen. Storage at 20°C, only showed samples exceeding the lower reference value after 24 hours of storage ( A + C).
Figure 6. The effect of storage time and temperature on the maximal platelet aggregation (%) using light transmission aggregometry (LTA) (n = 5) with agonists 5 μmol/L ADP (A + B) and 2 μg/mL collagen (C-D). Whole blood was stored for 3, 6 or 24 hours at 4°C (A + C), room temperature (B + D). Storage for 3 hours at room temperature was used as the reference value. Values are expressed as median ± range. * p < .05, ** p < .01. Each color represents one volunteer. The reference ranges (60–100%) are depicted as dotted lines.
Discussion
For platelet function assays and global haemostasis assays, we investigated the optimal duration in which whole blood is stable during different time intervals and temperatures. A first impression of the optimal storage condition and time for the Multiplate, PFA, ROTEM and LTA tests was given.
Multiplate
Multiplate analysis was performed in blood from five healthy volunteers using two agonists; ADP and collagen. Our results suggest that blood, used for the Multiplate (Multiple Electrode Aggregometry (MEA)), can be stored for 6 hours, but not 24 hours after blood collection at room temperature. These results were not in line with literature. Two papers have already observed significant differences, when comparing 1 hour of storage to 3 hours of storage [Citation14,Citation15]. They concluded that a Multiplate analysis must be performed within 1 hour after receiving the blood sample. Dézsi et al[Citation15]. even preferred performing the Multiplate within 30 minutes, while Hughes et al. suggested that whole blood can be stored up to 72 hours at 19°C without a decrease in platelet aggregation investigated in 10 participants. Though, storage at 25°C did show a decrease in platelet aggregation[Citation16]. Our samples were stored at room temperature, which can fluctuate between 19°C and 25°C. The exact storage temperature could differ between days, which might explain why our results showed a decrease in platelet aggregation for both adenosine diphosphate (ADP) and collagen. Platelets that are stored at 37°C become less viable compared to platelets stored at lower temperatures, because at 37°C, platelets have a rapid adenosine triphosphate (ATP) turnover and therefore a higher metabolic rate[Citation17]. It is important to take into account that normally more agonists are measured with a Multiplate analysis; however, this would have required too much blood from our volunteers. Therefore, we decided to investigate only two agonists.
Platelet function analyzer
The PFA was performed in blood from five healthy volunteers using collagen/ADP and collagen/EPI cartridges. Based on our study, the optimal condition for storing blood for PFA tests is room temperature. Some samples, stored at 4°C and 37°C, exceeded the reference range. Literature suggested that storage at 4°C will change the discoid shape of the platelets to spherical, resulting in decreased function[Citation18]. Bertino et al[Citation19]. showed a loss in viability of the platelets and an increased risk of contamination with bacteria, when blood was stored at 37°C. Jilma-Stohlawetz et al[Citation14]. did not find any significant differences in PFA results in both healthy volunteers and patients on clopidogrel or prasugrel, when comparing 1 and 3 hours of storage; however, they used a specific P2Y12 cartridge for their measurements, which might have a slightly different maximal storage time and optimal storage temperature compared to the cartridges we used. Hellstern et al[Citation20]. concluded that collagen/ADP can even be measured up to 24 hours of storage, while the collagen/EPI increased rapidly after 8 hours of storage. Our results were concordant with Hellstern et al[Citation20]., however, for collagen/EPI, the CT results were already above the reference range at 6 hours of storage. Since the cartridges are always requested at the same time, blood can be stored at room temperature for 3 hours, but not 6 hours after blood collection.
Rotational thromboelastometry
Blood of five healthy volunteers was used to perform a ROTEM, measuring the EXTEM and FIBTEM with two parameters, clotting time (CT) and the firmness of the clot after 10 minutes (A10 in mm). Our results showed, that the EXTEM CT and FIBTEM CT can be tested up to 6 hours after the blood was collected, while the EXTEM A10 can still be measured after the blood was stored for 24 hours. It is important to take into account that normally more parameters are tested in the ROTEM, not just the EXTEM and FIBTEM. Literature suggested that the platelet function will be stable when stored at 4°C, this is concordant to our results, which also showed a stable median CT for both EXTEM and FIBTEM over time[Citation21]. For the EXTEM A10, some samples exceeded the lower reference range when stored at 4°C and 37°C, this could probably be explained by the loss of platelet viability [Citation18,Citation19]. Therefore, the most optimal storing condition appeared to be room temperature. After 3 and 6 hours of storage, one of the healthy volunteers exceeded the upper reference range of the FIBTEM A10. This could potentially be an artifact, since this abnormality was not observed in the EXTEM A10. Since the EXTEM and FIBTEM are always requested at the same time, blood collected can be stored for 6 hours, but not 24 hours after blood collection.
Light transmission aggregometry
Light transmission aggregometry (LTA) was performed with whole blood or freshly made PRP of five healthy volunteers; ADP and collagen were chosen as agonists for platelet aggregation. Based on our light transmission aggregometry measurements, PRP appears to be most stable when stored at 4°C or room temperature. PRP can be stored for 3 hours after blood collection, but not 6 hours. Our found results are not in line with literature. Ho et al[Citation22]. suggested that PRP can be stored up to 6 hours at both 4°C and room temperature without a change in platelet aggregation.
For whole blood, LTA measurements appear to be stable for 6 hours at room temperature. When whole blood was stored for 24 hours, several samples, stored at 4°C and room temperature, exceeded the lower reference range for both ADP and collagen, however, all time points between 6 hours and 24 hours of storage were not tested. This was in line with a paper of Choi et al[Citation23]., who showed that, when whole blood was stored at 4°C and PRP was made just before the analysis, the responsiveness of ADP was impaired after 24 hours. Choi et al[Citation23]. also reported that storage of whole blood for 24 hours at room temperature resulted in an impaired responsiveness to ADP (20–60% aggregation). This is concordant with our results for ADP. However, they also reported that the responsiveness to collagen was not affected after 24 hours, though almost all of our volunteers showed aggregation under the lower reference range[Citation23].
Finally, for all assays, the platelet counts () and blood volume over time were similar. This observation suggests that the platelet counts probably did not affect our results.
Limitations
In this pilot study, the number of donors included in our study was limited, which made it difficult to find significant differences. Also, in general, only a limited number of studies on the stability of blood for platelet function assays have been performed. Therefore, future research should focus on investigating the pre-analytical effects in larger cohorts with both healthy volunteers and patient material. Furthermore, these experiments should now be performed with all agonists, additional parameters and tests. Also, more additional time points between 6 and 24 hours should be included.
Conclusion
In our study, we have given a first impression of the optimal storage condition and time for whole blood or PRP using several platelet function assays. The optimal storage conditions per test according to our study are shown in . Additional studies are needed to create a standardized protocol that can be used in every hospital, since it is important that in all hospital laboratories, the pre-analytical variables are taken into account and potentially facilitate transport of blood samples for platelet function assays to academic hospitals.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
References
- Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in haemostasis. Physiol Rev. 2013;93(1):327–358. 10.1152/physrev.00016.2011.
- Bonar RA, Lippi G, Favaloro EJ. Overview of haemostasis and thrombosis and contribution of laboratory testing to diagnosis and management of hemostasis and thrombosis disorders. Methods Mol Biol (Clifton, NJ). 2017;1646:3–27.
- George JN. Platelets. Lancet (London, England). 2000;355(9214):1531–1539. 10.1016/S0140-6736(00)02175-9.
- Hawkins R. Managing the pre- and post-analytical phases of the total testing process. Ann Lab Med. 2012;32(1):5–16. 10.3343/alm.2012.32.1.5.
- Lundberg GD. Acting on significant laboratory results. Jama. 1981;245(17):1762–1763. 10.1001/jama.1981.03310420052033.
- Lundberg GD. How clinicians should use the diagnostic laboratory in a changing medical world. Clin Chim Acta. 1999;280(1–2):3–11. 10.1016/S0009-8981(98)00193-4.
- Hammerling JAA. Review of Medical Errors in Laboratory Diagnostics and Where We Are Today. Lab Med. 2012;43(2):41–44. 10.1309/LM6ER9WJR1IHQAUY.
- Favaloro EJ, Lippi G, Adcock DM. Preanalytical and postanalytical variables: the leading causes of diagnostic error in haemostasis? Semin Thromb Hemost. 2008;34(7):612–634. 10.1055/s-0028-1104540.
- Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med. 2007;45(6):720–727. 10.1515/CCLM.2007.167.
- Lippi G, Simundic AM. The EFLM strategy for harmonization of the preanalytical phase. Clin Chem Lab Med. 2018;56(10):1660–1666. 10.1515/cclm-2017-0277.
- Adcock DM, Favaloro EJ, Lippi G. Critical pre-examination variables in the haemostasis laboratory and their quality indicators. Clin Biochem. 2016;49(18):1315–1320. 10.1016/j.clinbiochem.2016.08.022.
- Favaloro EJ, Funk DM, Pre-analytical LG. Variables in coagulation testing associated with diagnostic errors in haemostasis. Lab Med. 2012;43(2):1–10. 10.1309/LM749BQETKYPYPVM.
- Munnix ICA, Van Oerle R, Verhezen P, Kuijper P, Hackeng CM, Hopman-Kerkhoff HIJ, Hudig F, Van De Kerkhof D, Leyte A, De Maat MPM, et al. Harmonizing light transmission aggregometry in the Netherlands by implementation of the SSC-ISTH guideline. Platelets. 2020;Jun. 1–8. 10.1080/09537104.2020.1771549.
- Jilma-Stohlawetz P, Ratzinger F, Schorgenhofer C, Jilma B, Quehenberger P. Effect of preanalytical time-delay on platelet function as measured by multiplate, PFA-100 and VerifyNow. Scand J Clin Lab Invest. 2016;76(3):249–255. 10.3109/00365513.2016.1143115.
- Dezsi DA, Merkely B, Skopal J, Barabas E, Varnai K, Falukozy J, Veress G, Alotti N, Aradi D. Impact of test conditions on ADP-induced platelet function results with the multiplate assay: is further standardization required? J Cardiovasc Pharmacol Ther. 2018;23(2):149–154. 10.1177/1074248417728287.
- Hughes JD, Macdonald VW, Hess JR. Warm storage of whole blood for 72 hours. Transfusion. 2007;47(11):2050–2056. 10.1111/j.1537-2995.2007.01429.x.
- Michelson AD. Platelets: Academic Press; 2013, 1353p.
- Wandall HH, Hoffmeister KM, Sorensen AL, Rumjantseva V, Clausen H, Hartwig JH, Slichter SJ. Galactosylation does not prevent the rapid clearance of long-term, 4 degrees C-stored platelets. Blood. 2008;111(6):3249–3256. 10.1182/blood-2007-06-097295.
- Bertino AM, Qi XQ, Li J, Xia Y, Kuter DJ. Apoptotic markers are increased in platelets stored at 37 degrees C. Transfusion. 2003;43 (7):857–866. doi: 10.1046/j.1537-2995.2003.t01-4-00431.x.
- Hellstern P, Sturzebecher U, Wuchold B, Haubelt H, Seyfert UT, Bauer M, Vogt A, Sturzebecher J. Preservation of in vitro function of platelets stored in the presence of a synthetic dual inhibitor of factor Xa and thrombin. J Thrombosis Haemostasis: JTH. 2007;5(10):2119–2126. 10.1111/j.1538-7836.2007.02716.x.
- Pidcoke HF, McFaul SJ, Ramasubramanian AK, Parida BK, Mora AG, Fedyk CG, Valdez-Delgado KK, Montgomery RK, Reddoch KM, Rodriguez AC. et al. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion. 2013;53(Suppl 1):137s–49s. 10.1111/trf.12048.
- Ho CH, Chan IH. The influence of time of storage, temperature of storage, platelet number in platelet-rich plasma, packed cell, mean platelet volume, hemoglobin concentration, age, and sex on platelet aggregation test. Ann Hematol. 1995;71(3):129–133. 10.1007/BF01702648.
- Choi JW, Pai SH. Influence of storage temperature on the responsiveness of human platelets to agonists. Ann Clin Lab Sci. 2003;33(1):79–85.
