Abstract
Inflammation plays a key role in cardiovascular disease by contributing to atherothrombosis. The PLATelet inhibition and patient Outcomes (PLATO) study (NCT00391872) compared ticagrelor to clopidogrel in patients with acute coronary syndromes and demonstrated fewer cardiovascular events with ticagrelor but lower white blood cell counts (WBC) with clopidogrel. In this further analysis of the PLATO biomarker substudy, we assessed associations between WBC and clinical characteristics, biomarker levels, and CYP2C19 polymorphisms.
On-treatment mean (SD) WBC in the clopidogrel group was mildly reduced at each stage of follow-up compared with either the ticagrelor group (1 month: 7.27 (2.1) and 7.67 (2.23) x109/L for clopidogrel and ticagrelor, respectively; p < .001) or following cessation of clopidogrel (7.23 (1.97) x109/L, at 6 months vs 7.56 (2.28) x109/L after treatment cessation; P < .001). This occurred independently of baseline biomarkers and CYP2C19 genotype (where known). Adjusting for clinical characteristics and other biomarkers, no significant interaction was detected between clinical risk factors and the observed effect of clopidogrel on WBC.
Clopidogrel weakly suppresses WBC, independent of clinical characteristics, baseline inflammatory biomarker levels, and CYP2C19 genotype. Further work is required to determine the mechanism for this effect and whether it contributes to clopidogrel’s efficacy as well as therapeutic interaction with anti-inflammatory drugs.
Introduction
In the PLATelet inhibition and patient Outcomes (PLATO) study of patients with acute coronary syndrome (ACS), ticagrelor reduced both cardiovascular and all-cause mortality compared with clopidogrel[Citation1]. Subsequent post-hoc analyses of adverse events in the PLATO study demonstrated lower rates of mortality following pulmonary infection and sepsis in the ticagrelor group though this was not replicated in a comparison of ticagrelor and clopidogrel in a separate cohort of patients with peripheral arterial disease [Citation2,Citation3]. In PLATO, slightly lower levels of inflammatory markers were seen in the clopidogrel group, consistent with differential effects of clopidogrel and ticagrelor on inflammatory status[Citation2]. White blood cell (WBC) counts and high-sensitivity C-reactive protein (CRP) levels have both been shown to predict cardiovascular morbidity and mortality [Citation4–6]. Thus, differential effects of clopidogrel and ticagrelor on inflammatory status may have clinical implications.
In order to further characterize the differential effects of clopidogrel and ticagrelor on inflammatory status, we analyzed whether clinical variables, CYP2C19 genotype, and baseline levels of inflammatory markers influenced these effects in the PLATO study.
Methods
PLATO was an international, multicentre, double-blind, randomized controlled trial of ticagrelor compared with clopidogrel in 18,624 moderate-to-high-risk ACS patients. The study design and results have been published previously [Citation1,Citation7]. Some participants consented to participation in the pre-specified biomarker substudy (n = 17,095) and/or the PLATO genetic substudy (n = 10,379) [Citation8,Citation9]. Collection and analysis of DNA samples was subject to separate informed consent and approval by local ethics committees.
CYP2C19 allele status
The alleles genotyped in the PLATO genetics population were CYP2C19 loss-of-function (LOF) alleles *2, *3, *4, *5, *6, *7, and *8 and the ‘gain-of-function’ mutation CYP2C19*17. Previous publications have considered the *17 polymorphism to cause a rapid or ultra-rapid metabolism of clopidogrel to its active metabolite. However, recent analyses have demonstrated that *2 and *17 alleles are in linkage disequilibrium, completely attenuating any gain-of-function effect previously attributed to the *17 allele itself[Citation10]. We therefore classified LOF alleles as any of *2 to *8 and reclassified the *17 polymorphism as equivalent to *1 (wild-type).
Statistical methods
Patients with biomarker results at both baseline and follow-up visits were included, and all analyses used all available data. Thus, the number of patients differed between analyses for different biomarkers, at different timepoints and between unadjusted analyses and analyses incorporating several background variables. Biomarkers were analyzed as continuous variables as far as possible. Biomarkers under the lower limit of quantification were imputed as half this limit. Biomarkers above the upper limit of quantification were imputed as equal to this limit. No other imputation was performed. Levels were log-transformed before analysis. Multivariable regression models were fitted with the continuous inflammatory biomarker at follow-up visit as the dependent variable, to estimate mean biomarker levels and test interactions between treatment and risk factors. Geometric means were calculated using the antilogs of the model-adjusted means, and the relative increase between subgroups was calculated using ratios. Non-linear associations were modeled and plotted using restricted cubic splines with four knots. All models included the baseline value of the corresponding inflammatory biomarker variable to adjust for any pre-treatment differences.
Results are presented as point estimates with corresponding 95% confidence intervals (CI). P-values were not adjusted for multiple testing. All statistical analyses were performed at Uppsala Clinical Research Center using SAS version 9.4 or R version 4.0.0 statistics software.
Results
Biomarkers
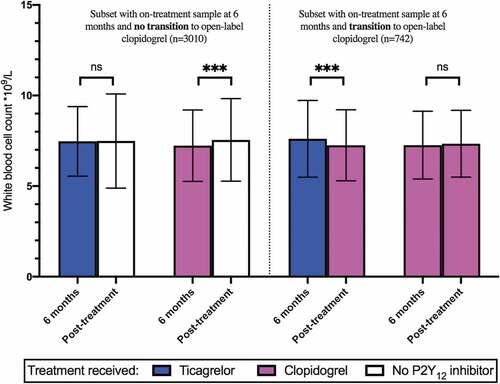
The demographics of the PLATO biomarkers substudy have been reported previously[Citation8]. Mean (SD) WBC counts were lower in clopidogrel-treated individuals, compared with those treated with ticagrelor, at 1 month (7.27 (2.1) vs 7.67 (2.23) x109/L, p < .001), 3 months (7.28 (1.99) vs 7.59 (2.19) x109/L, p < .001) and 6 months (7.21 (2.02) vs 7.48 (1.97) x109/L; p < .001), despite similar distributions of WBC in the two groups (). In the subset of patients with an on-treatment sample at 6 months and no transition to open-label clopidogrel after treatment cessation (n = 3,010), a significant increase in mean (SD) post-treatment WBC count was observed in the clopidogrel group (7.23 (1.97) x109/L, at 6 months vs 7.56 (2.28) x109/L after treatment discontinuation; p < .001) but not the ticagrelor group (7.47 (1.92) at 6 months vs 7.49 (2.60) x109/L after treatment discontinuation) ().
Figure 1. Violin plots showing distribution of white blood cell counts at different stages of follow-up in the entire biomarkers cohort, by treatment assignment. Medians, quartiles, Tukey-style whiskers (Q1-1.5*IQR and Q3 + 1.5*IQR) and outliers are overlaid. Data exclude one patient in the ticagrelor group with chronic lymphatic leukemia. ***, P < .001. ns, not significant.
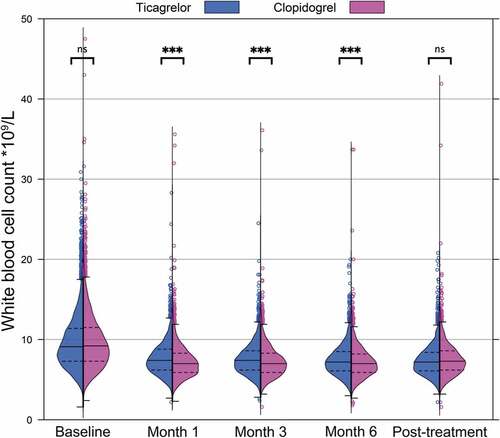
Figure 2. White blood cell counts before and after cessation of study medication. Blue columns indicate ticagrelor treatment assignment. Purple columns indicate patients assigned to clopidogrel during the study medication period and patients receiving open-label clopidogrel 1 month after cessation of study medication; white columns indicate patients who did not transition to open-label clopidogrel after cessation of study treatment. ***, P < .001. ns, not significant; WBC, White blood cell count.
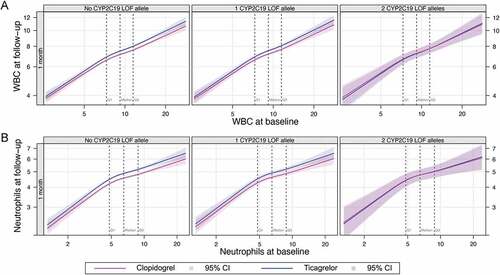
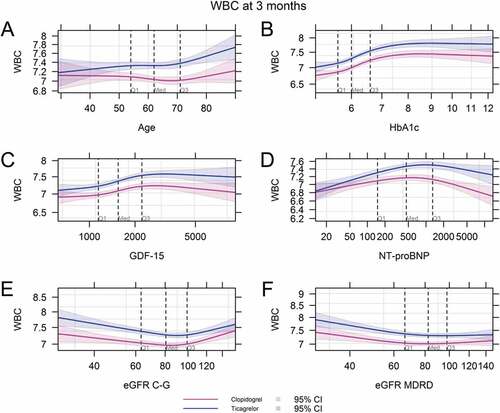
No significant interaction was observed between treatment assignment and risk factors with respect to WBC count or neutrophil count in either our unadjusted model ( and Online Supplementary Table S1) or a model adjusting for baseline clinical risk factors (Online Supplementary Tables S2 and S3, respectively). The relationship between treatment assignment and WBC was consistent across the observed range of baseline WBC counts, neutrophil counts, and CRP (, Online Supplementary Figure S1). The correlation did not vary with age or baseline levels of HbA1c, GDF-15, NT-proBNP or GFR for either total WBC count or neutrophils ( and Online Supplementary Figure S2). No significant differences were observed for levels of CRP or IL-6 at any baseline level of those markers, at any timepoint (Online Supplementary Figure S3).
Table I. Multivariable effect of randomized treatment, risk factor and interaction between treatment and risk factors on WBC at follow-up visit (unadjusted analysis)
Figure 3. White blood cell (a) and neutrophil (b) count by treatment arm at each stage of follow up, with varying baseline levels of continuous risk factor. WBC, White blood cell count.
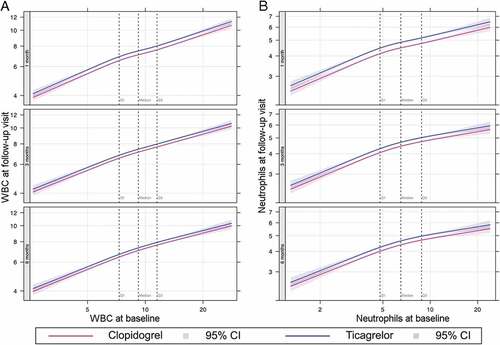
Figure 4. White blood cell counts at 3 months by treatment arm according to varying baseline levels of continuous risk factors: age (A), HbA1c (B), GDF-15 (C), NT-proBNP (D), eGFR C-G (E), eGFR MDRD (F). WBC, White blood cell count; HbA1c, hemoglobin A1c; GDF-15; growth differentiation factor 15; NT-proBNP, N-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate; C-G, Cockroft-Gault method; MDRD, modification of diet in renal diseases method.
Rates of leukopenia (defined as WBC < 3 × 109/L) and neutropenia (neutrophil count < 1.5 × 109/L) were low in both treatment groups and did not explain the observed differences in WBC (Online Supplementary Tables S4 and S5). Six cases of leukopenia were observed in clopidogrel-treated patients at the 6-month timepoint, and none in those randomized to ticagrelor (p = .039), but no significant differences were observed at other timepoints.
CYP2C19 allele status
For those with CYP2C19 polymorphism data available (n = 9,843), 7,071 had no LOF alleles, 2526 had one LOF allele and 246 had two LOF alleles (Online Supplementary Table S6). No significant differences were observed between CYP2C19 allele status, treatment assignment and WBC, neutrophil count, CRP or IL-6 at follow-up visits ( and Online Supplementary Figures S4-7).
Discussion
ACS patients randomized to clopidogrel have lower WBC counts while on treatment than those treated with ticagrelor. After discontinuation of the study medication, in those who did not transition to open-label clopidogrel, a significant increase in WBC count was observed after cessation of clopidogrel, but not ticagrelor. Conversely, in those who stopped ticagrelor and did transition to open-label clopidogrel, a small reduction in WBC count was observed. These findings suggest WBC is suppressed by clopidogrel, rather than ticagrelor mediating an increase in WBC. We show that this effect is independent of baseline biomarkers and clinical risk factors and is consistent across the 1-, 3- and 6-month timepoints.
Thienopyridines, such as clopidogrel and ticlopidine, have been associated with bone marrow suppression. Indeed, the clinical utility of the latter has been limited by this adverse effect[Citation11]. Clinically overt marrow suppression with clopidogrel is much less common and severe leukopenia is rare but nevertheless cases of associated thrombocytopenia, leukopenia or pancytopenia have been reported and attributed to non-P2Y12-mediated myelotoxic effects of clopidogrel metabolites in susceptible individuals [Citation12–15]. Here, the WBC reduction seen with clopidogrel was not driven by higher rates of severe leukopenia.
Clopidogrel is metabolized via hepatic cytochrome P450 (CYP) enzymes to its active form, which inhibits platelets by irreversibly binding to the P2Y12 receptor [Citation11,Citation16], and via plasma esterases to its inactive metabolite. Either the parent drug or inactive or active metabolites may be responsible for off-target effects but while the parent form and active metabolite appear to cause myelotoxicity in vitro, myelotoxic effects seen clinically are thought to be secondary to carboxylate metabolites[Citation12]. Given the lack of structural homology between ticagrelor and either clopidogrel or its metabolites, off-target (i.e., non-P2Y12-mediated) effects of clopidogrel are unlikely to be shared with ticagrelor and are the most likely cause of the observations in the present study.
In previous analyses of platelet counts in the PLATO study, the median platelet count increased in the first month after ACS in both groups and remained similarly elevated over the subsequent 5 months of follow-up but there was significantly less increase at 1 month in the clopidogrel group compared to the ticagrelor group (1.0 × 109/L versus 10.0 × 109/L; P < .0001)[Citation17].
Elevated inflammatory markers increase the risk of cardiovascular events while modulation of inflammation is increasingly recognized as a therapeutic target in contemporary medicine [Citation8,Citation18–20]. Anti-inflammatory agents such as colchicine and canakinumab have been shown to reduce cardiovascular events compared with placebo, although methotrexate was not shown to be effective at reducing events in CIRT, suggesting differential effects depending on which inflammatory pathways are inhibited [Citation21–24]. Although elevated WBC is associated with cardiovascular disease, it is unclear whether leukocytosis itself is a causative factor or an epiphenomenon, perhaps reflecting the extent of atherosclerosis. Nevertheless, a modest off-target anti-inflammatory effect of clopidogrel may conceivably modulate inflammation-mediated atherothrombotic risk. This phenomenon, if present, could more markedly affect outcomes in stable patients who have a lower immediate risk of acute thrombosis compared with the longer-term risk of progression of inflammation-mediated atherosclerosis. Such effects could explain the unexpected findings of the EUCLID trial, where ticagrelor was not superior to clopidogrel in a population at high risk of atherothrombotic events despite superior antiplatelet efficacy[Citation3].
Limitations: PLATO compared ticagrelor and clopidogrel, thus we compared relative effect of these two medications for the duration of the trial, including non-randomized transition to open-label clopidogrel or no P2Y12 inhibitor following completion of study medication; there is no true control arm for these analyses. These post-hoc analyses are subject to the biases of observational study so the results, including p-values, should be considered hypothesis-generating.
Conclusions
Clopidogrel therapy modestly suppresses WBC independently of baseline characteristics, pre-treatment inflammatory status and CYP2C19 genotype. The mechanism and clinical significance of this phenomenon are yet to be determined.
Authorship roles
T. A. Nelson prepared the manuscript, including tables and figures, in consultation with R.F. Storey and assisted by W. A. E. Parker. T. Ghukasyan Lakic and Johan Westerbergh performed the statistical analyses and created the draft figures. S. K. James, A. Siegbahn, Richard C. Becker, Anders Himmelmann, L. Wallentin, and R.F. Storey were members of the committees overseeing the PLATO study and biomarker substudy, and all critically revised the manuscript.
Disclosure of potential conflicts of interest
SK James reports research grants, lecture and consulting fees to institution from AstraZeneca, Jansen, Bayer, The MedCo, Chiesi, Novartis and Amgen. A Siegbahn reports institutional research grants from AstraZeneca, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, Roche Diagnostics, and Boehringer-Ingelheim; and consultancy fees from Olink Proteomics. L Wallentin reports grants from AstraZeneca, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, Merck & Co, Roche Diagnostics, Boehringer Ingelheim; and patents EP2047275B1 and US8951742B2 licensed to Roche Diagnostics; and personal fees from Abbott. RF Storey reports institutional research grants/support from AstraZeneca, Cytosorbents, GlyCardial Diagnostics and Thromboserin; consultancy fees from AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Cytosorbents, GlyCardial Diagnostics, HengRui, Idorsia, PhaseBio, Portola, Sanofi Aventis and Thromboserin; and honoraria from AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Intas Pharmaceuticals and Medscape. Other authors have no relevant disclosures.
Additional information
Funding
References
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H. et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2009;361(11):1045–1057. 10.1056/NEJMoa0904327.
- Storey RF, James SK, Siegbahn A, Varenhorst C, Held C, Ycas J, Husted SE, Cannon CP, Becker RC, Steg PG. et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets. 2014;25(7):517–525. 10.3109/09537104.2013.842965.
- Hiatt WR, Fowkes FGR, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS. et al. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N Engl J Med. 2017;376(1):32–40. 10.1056/NEJMoa1611688.
- Shankar A, Mitchell P, Rochtchina E, Wang JJ. The association between circulating white blood cell count, triglyceride level and cardiovascular and all-cause mortality: population-based cohort study. Atherosclerosis. 2007;192(1):177–183. 10.1016/j.atherosclerosis.2006.04.029.
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. 10.1016/S0140-6736(09)61717-7.
- Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White Blood Cell Count and Incidence of Coronary Heart Disease and Ischemic Stroke and Mortality from Cardiovascular Disease in African-American and White Men and Women: atherosclerosis Risk in Communities Study. Am J Epidemiol. 2001;154(8):758–764. 10.1093/aje/154.8.758.
- James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R. et al. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157(4):599–605. 10.1016/j.ahj.2009.01.003.
- Lindholm D, James SK, Gabrysch K, Storey RF, Himmelmann A, Cannon CP, Mahaffey KW, Steg PG, Held C, Siegbahn A. et al. Association of Multiple Biomarkers With Risk of All-Cause and Cause-Specific Mortality After Acute Coronary Syndromes: a Secondary Analysis of the PLATO Biomarker Study. JAMA Cardiol. 2018;3(12):1160. 10.1001/jamacardio.2018.3811.
- Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH. et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376(9749):1320–1328. 10.1016/S0140-6736(10)61274-3.
- Lewis JP, Backman JD, Reny J-L, Bergmeijer TO, Mitchell BD, Ritchie MD, J-p D, Pakyz RE, Gong L, Ryan K. et al. Pharmacogenomic polygenic response score predicts ischaemic events and cardiovascular mortality in clopidogrel-treated patients. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):203–210. 10.1093/ehjcvp/pvz045.
- Quinn MJ, Fitzgerald DJ. Ticlopidine and Clopidogrel. Circulation. 1999100:1667–1672.
- Maseneni S, Donzelli M, Taegtmeyer AB, Brecht K, Krähenbühl S. Toxicity of clopidogrel and ticlopidine on human myeloid progenitor cells: importance of metabolites. Toxicology. 2012;299(2–3):139–145. 10.1016/j.tox.2012.05.017.
- Montalto M, Porto I, Gallo A, Camaioni C, Della Bona R, Grieco A, Crea F, Landolfi R. Clopidogrel-induced neutropenia after coronary stenting: is cilostazol a good alternative? Int J Vasc Med. 2011;2011:867964. 10.1155/2011/867964.
- Zacharia G, Randhawa A, Marino N, Spaccavento C. Severe Bone Marrow Suppression Associated with Use of Clopidogrel. J Case Reports Stud. 2016;4(4):1–4. 10.15744/2348-9820.4.401.
- Ibáñez L, Vidal X, Ballarín E, Laporte J-R. Population-Based Drug-Induced Agranulocytosis. Arch Intern Med. 2005;165(8):869–874. 10.1001/archinte.165.8.869.
- Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics. 2010;20(7):463–465. 10.1097/FPC.0b013e3283385420.
- Lowenstern A, Storey RF, Neely M, Sun J-L, Angiolillo DJ, Cannon CP, Himmelmann A, Huber K, James SK, Katus HA. et al. Platelet-related biomarkers and their response to inhibition with aspirin and P2Y12-receptor antagonists in patients with acute coronary syndrome. J Thromb Thrombolysis. 2017;44(2):145–153. 10.1007/s11239-017-1516-y.
- Libby P. Inflammation in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. 10.1161/ATVBAHA.108.179705.
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ. et al. Inflammatory Markers and the Risk of Coronary Heart Disease in Men and Women. N Engl J Med. 2004;351(25):2599–2610. 10.1056/NEJMoa040967.
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-Reactive Protein and Low-Density Lipoprotein Cholesterol Levels in the Prediction of First Cardiovascular Events. N Engl J Med. 2002;347(20):1557–1565. 10.1056/NEJMoa021993.
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD. et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. 10.1056/NEJMoa1707914.
- Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS. et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381(26):2497–2505. 10.1056/NEJMoa1912388.
- Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61(4):404–410. 10.1016/j.jacc.2012.10.027.
- Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E. et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380(8):752–762. 10.1056/NEJMoa1809798.