Abstract
Charge interactions play a critical role in the activation of the innate immune system by damage- and pathogen-associated molecular pattern receptors. The ability of these receptors to recognize a wide spectrum of ligands through a common mechanism is critical in host defense. In this article, we argue that platelet glycoprotein receptors that signal through conserved tyrosine-based motifs function as pattern recognition receptors (PRRs) for charged endogenous and exogenous ligands, including sulfated polysaccharides, charged proteins and nanoparticles. This is exemplified by GPVI, CLEC-2 and PEAR1 which are activated by a wide spectrum of endogenous and exogenous ligands, including diesel exhaust particles, sulfated polysaccharides and charged surfaces. We propose that this mechanism has evolved to drive rapid activation of platelets at sites of injury, but that under some conditions it can drive occlusive thrombosis, for example, when blood comes into contact with infectious agents or toxins. In this Opinion Article, we discuss mechanisms behind charge-mediated platelet activation and opportunities for designing nanoparticles and related agents such as dendrimers as novel antithrombotics.
Introduction
Platelets are activated by a miscellaneous variety of charged ligands of distinct structures and sizes, ranging from polymers of sulfated polysaccharides to diesel exhaust particles (DEPs).Citation1 This includes synthetic nanoparticles which activate platelets in proportion to their surface area.Citation2 Both positively and negatively charged ligands have been shown to activate platelets, with activation mediated through glycoprotein receptors which signal through tyrosine-based signaling motifs in their cytosolic tails or associated membrane proteins. Example receptors include glycoprotein (GP)VI and C-type lectin-like receptor 2 (CLEC-2), which signal through Src family kinases (SFK) and Syk tyrosine kinases, and platelet endothelial aggregation receptor (PEAR)1, which signals through SFKs and PI 3-kinases. Crosslinking of these receptors leads to phosphorylation of tyrosine-based motifs in their cytosolic tail and activation of downstream signaling cascades (see visual abstract).Citation3
In this article, we describe the miscellaneous range of charged agonists that stimulate platelet activation through tyrosine kinase receptors, with a special focus on nanoparticles, and how this is influenced by size and surface charge. We speculate that modifications in nanoparticle design could lead to the generation of a new class of antiplatelet agent that prevents charge-mediated receptor clustering and platelet activation.
Nanoparticles in Biomedicine
Nanoparticles are small particles with dimensions under 100 nm.Citation4 They can have a range of charges and can be comprised of metallic and nonmetallic constituents. Nanoparticles can be engineered for different biomedical applications and have been exploited in several areas, including drug delivery, biosensing and medical imaging.
Metallic nanoparticles, including gold, iron and platinum, have been investigated in biomedical applications both in vitro and in vivo.Citation5–8 Gold nanoparticles of varying size under 100 nm have been used for drug delivery, especially for tumor targeting.Citation9 Gold nanoparticle conjugation to methotrexate (giving ~14 nm nanoparticles) induces cytotoxicity of tumor cells in vitro.Citation10 Surface area and charge are important factors for gold nanoparticle function and cellular uptake for tumor targeting. Gold nanoparticles (13 nm) also have biosensor utilities and can measure thrombin generation through conjugation to thrombin-binding aptamers.Citation11 Magnetite, an iron-nanoparticle derivative, entrapped with thrombin has been proposed as a novel hemostatic agent. The iron-based nanoparticles can be guided to areas of bleeding by a magnetic field and coagulation is accelerated through fibrinogen injection, shortening hemostasis time by a factor of 6.5.Citation12 Superparamagnetic iron oxide nanoparticles (SPIONs) are utilized as MRI contrast agents for visualizing liver/spleen tumors and atherosclerotic plaques.Citation13,Citation14 Platinum nanoparticles have applications in glucose biosensing,Citation15,Citation16 bioimaging of tumor cellsCitation17 and in mimicking natural enzymes in therapies for oxidative stress.Citation18
Nonmetallic nanoparticles, such as carbon nanotubes (CNTs), polystyrene, silica and polyamidoamine (PAMAM) dendrimers, have also been utilized in bioimaging and drug delivery.Citation19–22 CNTs have been developed as anticancer drugs to help combat multidrug resistanceCitation23 and antigen carriers to help in tumor antigen recognition.Citation24 Polystyrene nanoparticles (30 nm) have been functionalized to induce cell death of liver-derived tumor cells in vitroCitation21. Silica nanoparticles are being engineered as potential antigen carriers in vaccine development.Citation25,Citation26 Dendrimers have utilities in bioimaging and in drug and gene delivery.Citation22,Citation27,Citation28 They are constituted of branched repeating units, such as polymers, giving rise to a more uniformed nanostructure. Dendrimers can entrap or be conjugated to high molecular weight molecules and become hyperbranched, which are attractive characteristics for drug delivery.Citation29
There are a number of important considerations however with the emerging use of nanoparticles in biomedicine that require further attention. The in vivo plasma concentrations, half-life and clearance mechanisms of nanoparticles need to be fully characterized in addition to understanding off-target effects, such as platelet activation. The charged nanoparticles, by their very nature, will bind to plasma proteins and to proteins on cells surfaces thus effectively lowering their concentration, and potentially causing off-target effects.
Nanoparticles in Emissions
Despite their utilities in biomedicine, some nanoparticles pose a risk to health. This is illustrated by the increase in incidence of cardiovascular and other diseases in areas with high levels of air-born pollutantsCitation30–32 and with particulate matter in smoke.Citation33 DEPs (20–70 nm) are regarded as prominent sources of airborne particulate matter.Citation30 Several epidemiological studies have suggested both short-term and long-term exposure to DEPs and other particulate matter are associated with cardiovascular disease, including venous thromboembolism.Citation32 Controlled DEP exposure studies have been performed in human subjects, with typical 1–2 h exposure doses ranging for 100–300 µg particulate matter/m3, with most showing no major effects on inflammatory markers but reduced vasomotor functionCitation34 and adverse vascular endothelial effects.Citation35 Studies of direct lung translocation of particular matter and in vivo effects are mainly assessed in animal models, with several mechanisms of disease pathogenesis proposed. In animal models, nanoparticle exposure has been linked to increased inflammation and platelet activation. In mice, gold nanoparticles have been found in blood, urine and the liver of ApoE−/- mice after inhalation, with a predominance of smaller nanoparticles (<10 nm).Citation36 The inhaled gold particles accumulate in the vascular lesions of the mice and are associated with mild pulmonary inflammation.Citation36 In rats, ultrafine carbon nanoparticles can translocate into the circulation following inhalation, with significant accumulation in the liver after 18 h.Citation37 DEPs were deposited in the distal airways of mice following intratracheal installation and are associated with enhanced collagen-induced platelet aggregation.Citation38 Different DEP fractions also can induce hypercoagulable states after installation.Citation39 In rats, intratracheal instillation of DEPs (500 µg/rat) accelerated thrombosis, through reducing occlusion time in a ferric chloride thrombosis model in vivo.Citation40 However, it is important to consider how representable acute, high dose DEP installation in animals is in modeling long-term diesel particle exposure and whether DEP translocation and concentrations in blood would equate to levels observed in human settings, bearing in mind the high level of binding to plasma proteins.
Nanoparticle-mediated Platelet Activation
Several studies have reported that both metallic and nonmetallic nanoparticles induce aggregation and secretion in platelets (). However, most of these studies have been performed in washed platelets, where plasma binding is disregarded, and rarely speculate on the free blood plasma concentration. Interestingly, not only can plasma proteins negate the charge on particles, some such as albumin can form a protein corona around the nanoparticle,Citation56,Citation57 which may impede nanoparticle-mediated platelet activation but enhance other in vivo effects.Citation52 Various mechanisms have been proposed to influence platelet activation by nanoparticles including size, functional group and charge as discussed below:
Table 1. Platelet activation by metallic nanoparticles. Summary of metallic nanoparticles that have been shown to activate platelets (through aggregation, microscopy or flow cytometry studies) and the proposed mechanism behind the nanoparticle function. WP = studies performed using a form of washed platelets (no plasma proteins). PRP = studies performed using platelet-rich plasma (PRP; containing plasma proteins)
Table 2. Platelet activation by nonmetallic nanoparticles. Summary of nonmetallic nanoparticles that have been shown to activate platelets (through aggregation, microscopy or flow cytometry studies) and the proposed mechanism behind the nanoparticle function. WP = studies performed using a form of washed platelets (no plasma proteins). PRP = studies performed using platelet-rich plasma (PRP; containing plasma proteins)
Influence of Nanoparticle Size
Differentially sized nanoparticlesCitation2,Citation46,Citation52,Citation58 and different nanoparticle types, both metallic () and nonmetallic (), have been shown to stimulate aggregation of washed platelets and some in platelet-rich-plasma (PRP). There is some evidence that smaller nanoparticles have a greater potency.Citation29,Citation41,Citation52 For example, an increase in expression of platelet CD62P (P-selectin) was reported after exposure to 20 nm gold nanoparticles compared to 70 nm.Citation41 Potential explanations for this increased potency include the ability of small nanoparticles to reach charged residues close to the membrane or potentially the ease of transport of small nanoparticles into the open canicular system.Citation41 The former may be relevant for example to the activation of PEAR1 by sulfated polysaccharides from seaweed known as fucoidans. The binding of fucoidan to PEAR1 has been mapped to the heparin-binding domain in the 13th of the 15 EGF repeats in PEAR1, which lies close to the cell surface.Citation59 Small nanoparticles could potentially mimic this charge effect in fucoidans and thus activate PEAR1.
Influence of Nanoparticle Surface Area
We have recently shown that platelet activation is proportional to nanoparticle surface area and revealed that surface area is a critical factor for mediating platelet activation.Citation2 There is an inverse surface area ratio relationship, whereby smaller nanoparticles have larger surface areas. The degree of aggregation in proportion to the surface area and shows a bell-shaped relationship. The bell-shaped curve is consistent with activation being mediated by receptor clustering, with increased concentrations of nanoparticles competing with each other for receptor binding. Alternatively, the bell-shaped curve could be due to charge or steric effects preventing aggregation. It is important to note that the nanoparticles are much greater in size than the majority of platelet receptors. For example, the theoretical maximum height of CLEC-2 above the membrane is ≈12 nm, which is similar to that of the smaller nanoparticles (Supplementary ).
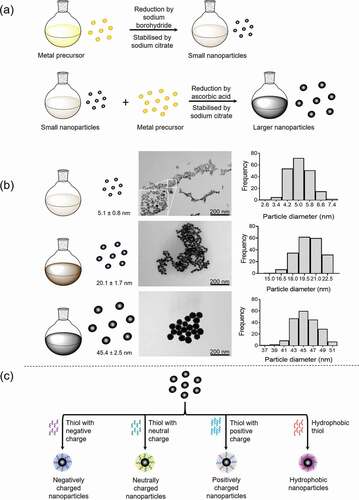
Figure 1. Nanoparticle synthesis. a) Metallic nanoparticles (gold/platinum) can be synthesized by the nucleation process, whereby small nanoparticles (seeds) are a template for larger nanoparticle (up to 100 nm) growth. Stable nanoparticles with controlled sizes are achieved by selecting the suitable reducing agents and stabilizing agents in addition to precursor (metal salts) concentrations and temperature. Small nanoparticles are initially synthesized by the reduction of the metal precursor by sodium borohydride and stabilized by sodium citrate. Larger nanoparticles are synthesized using these small nanoparticles with ascorbic acid and sodium citrate. b) Examples of different sized platinum nanoparticles synthesized by the nucleation process. Transmission electron microscopy (TEM) images are employed to obtain the average nanoparticle diameter to generate the distribution curve. c) Functionalisation of nanoparticles by thiols to produced nanoparticle with negative or positive charges (charged = also potentially hydrophilic) and hydrophobic nanoparticles
Influence of Nanoparticle Charge
Platelet activation in vitro has been shown following exposure to both negatively and positively charged metallicCitation47,Citation48 and nonmetallicCitation29,Citation51,Citation52 nanoparticles. Platelet function is not altered with exposure to nanoparticles with neutral charge.Citation29 The functional group attached to the nanoparticles could potentially be involved in nanoparticle–platelet interactions. Different carboxyl groups have been shown to affect nanoparticle potency for platelet activation.Citation52 Amine modifications of nanoparticles, giving positive charges, can increase platelet aggregation.Citation51 Large (G4-G6) cationic PAMAM dendrimers associated with increased numbers of amine groups are able to induced platelet aggregation compared to the other formulations of neutral and anionic PAMAM dendrimers.Citation29 Negatively charged platinum,Citation2 silver,Citation46 gold,Citation41 polystyreneCitation2 and ironCitation50 nanoparticles all activate platelets with tyrosine kinase dependent or rapid signaling-dependent mechanisms, leading to Ca2+ release (). Tailoring nanoparticles to have positive or negative charges will be a critical approach in determining the mechanism(s) behind charge-mediated platelet activation and in blocking these interactions.
Synthesis of Charged Nanoparticles
Methods for synthesizing nanoparticles include chemical (reduction of metal precursor), physical (laser ablation) or biological (plants, bacteria).Citation6 Chemical synthesis methods are well-suited for controlling nanoparticle size, functionalisation and monodispersity. A detailed example of chemical synthesis of metallic nanoparticles is shown in , whereby small nanoparticles (seeds) act as a template to grow larger nanoparticles.Citation6,Citation60,Citation61 Nanoparticles can be functionalized by the addition of ligands, surfactants, polymers, biomolecules or thiols during or after synthesis reactions.Citation62–65 Thiols can be physisorbed onto the nanoparticle surface forming a monolayer surrounding the nanoparticle core and can carry different terminal head groups (negatively/positively or neutral charge) and hydrophobic moieties (). Thiol addition to give a specific surface charge could be beneficial for designing nanoparticles for therapeutics.
Mechanism of Charge Mediated Platelet Activation
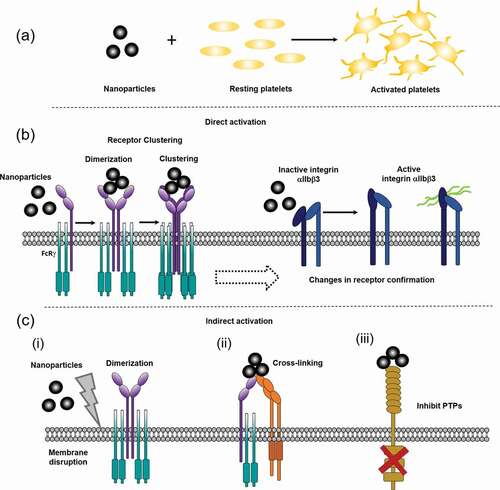
Ligand engagement of platelet receptors leads to phosphorylation of signaling proteins, increased Ca2+ flux and platelet aggregation via enhanced fibrinogen binding to integrin GPIIb/IIIa.Citation66 Charged nanoparticles can potentially induce receptor activation through homoreceptor or heteroreceptor cross-linking, either through direct receptor crosslinking on individual platelets. They can also potentially induce activation through a conformational change in the receptor or removal of inhibitory pathways as illustrated in and C. Also, they can potentially produce passive agglutination by crosslinking of proteins on adjacent platelets. This is sometimes seen with high concentrations of nanoparticles which induce GPIIb-IIIa independent aggregation. However, lower concentrations favor activation of GPIIb-IIIa in part mediated by the feedback action of secondary agonists such as ADP and thromboxane (TxA2).
Figure 2. Schematics of potential mechanisms behind nanoparticle induced platelet activation. a) Nanoparticles are small particles (<100 nm) that can activate platelets, through different proposed mechanisms. b) Direct nanoparticle driven platelet activation. Nanoparticles could potentially bind directly to platelets, such as GPVI, leading to ITAM signaling, dimerization and clustering or through causing conformational changes in receptors, like integrin αIIbβ3 (GPIIb/IIIa) changes from an inactive confirmation to an active. c) Potential in-direct mechanisms for nanoparticle driven platelet activation. (i) nanoparticles cause general membrane disruption leading to receptor dimerization, (ii) nanoparticles crosslinking different platelet receptors, (iii) nanoparticle binding/activation leads to inhibition of protein-tyrosine phosphatases (PTPs)/ ITIM receptors, promoting platelet activation. Nanoparticles are not to scale, they are represented here are small nanoparticles (<50 nm), larger nanoparticles can be larger than the receptor height above the membrane (see Supplementary )
Charge-mediated mechanisms of platelet activation are primarily restricted to glycoprotein receptors as these are activated through clustering in contrast to G protein-coupled receptors which are activated through a conformational change which require exquisite binding of the ligand. Several platelet glycoprotein receptors have been shown to be activated by charged ligands including:
GPVI
GPVI is a member of the immunoglobulin (Ig) receptor superfamily expressed on platelets and megakaryocytes and best known as a receptor for collagen.Citation67 GPVI is activated by a diverse range of endogenous and exogenous ligands (), including fibrin and fibrinogen,Citation68–70 extracellular matrix proteins (laminin, fibronectin, galectin-3Citation71 and vitronectinCitation67,Citation72), positively charged histonesCitation1 and the neuronal proteins, reelin and β-amyloid.Citation73,Citation74 Negatively charged molecules, such as chondroitin sulfate, heparins and small polyanions block fibrin-mediated aggregation and GPVI shedding, supporting a role for charge-mediated GPVI activation.Citation75 Furthermore, serglycin, a sulfated proteoglycan contained in platelet α-granules, also appears to be important in charge neutralization and regulation of charge-mediated GPVI shedding, as convulxin-mediated GPVI shedding is increased in serglycin knock-out mice.Citation76
Table 3. Multiple ligands of the tyrosine kinase receptors, GPVI, CLEC-2 and PEAR1. GPVI, CLEC-2 and PEAR1 are activated by multiple ligands. Top row = endogenous ligands recognized for GPVI, CLEC-2 and PEAR1. Bottom row = exogenous ligands for GPVI, CLEC-2 and PEAR1
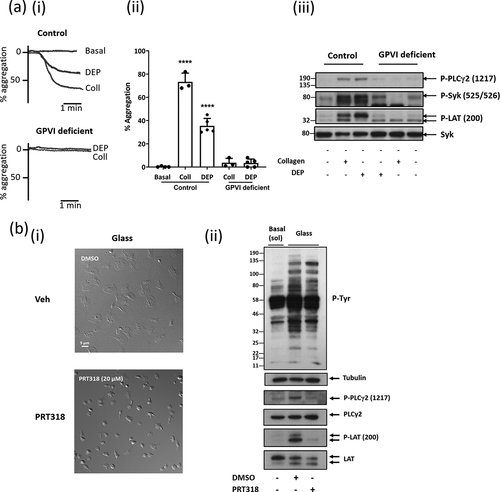
A number of proteins ligands for GPVI have regions of negative net charge, including fibronectin, fibrinogen, β-amyloid and phosphorothioate oligonucleotides.Citation77 In addition, DEP-mediated activation has previously been shown to be abolished in platelets from GPVI-deficient mice.Citation1 Here, we expanded on this to show negatively charged DEPs (20–70 nm) and negative charged surfaces also activate human platelets through GPVI, using platelets from homozygous patients (), who have a mutation in the GP6 gene preventing expressionCitation78 (see Supplementary Materials for full patient details). These patients have no GPVI expression as shown by flow cytometry and western blotting, whereas levels of other glycoprotein receptors, including CLEC-2, GPIIb/IIIa and FcγRIIA are within the normal range.Citation78,Citation79 Stimulation of tyrosine phosphorylation of proteins in the GPVI signaling pathway, including Syk, LAT and PLCγ2, by DEPs is abolished in the patients (). In line with these results, platelets undergoing spreading (i.e. filopodia and lamellipodia formation) on negatively charged glass surfaces in association with an increase in tyrosine phosphorylation including LAT and PLCγ2 (). Spreading and phosphorylation of both proteins are abolished in the presence of the Syk inhibitor PRT-060318 () consistent with the critical role for GPVI. Tyrosine phosphorylation of Syk and FcRγ-chain, and spreading, is also induced on polystyrene, with phosphorylation of both proteins blocked by PRT-060318 (Supplementary Figure 2). Tyrosine phosphorylation of several other proteins is retained in the presence of the Syk inhibitor on both surfaces demonstrating that other pathways contribute to the increase in phosphorylation but that these do not induce spreading.
Figure 3. Charged DEPs and negative charged surfaces activate human platelets through a GPVI and Syk dependent manner. a) Commercially bought DEP (50 µg/mL; see Supplementary Materials for details) induced platelet aggregation is abolished in GPVI-deficient patients. (i) Representative aggregation trace of DEP and collagen (Coll; 30 µg/mL) induced aggregation in washed platelets (4x108/mL) from controls (+/+) and GPVI deficient patients (individuals homozygous (−/−) for a mutation in GP6 resulting in truncated GPVI). For more details see Supplementary Materials. (ii) Significant reduction in DEP-aggregation and collagen-induced platelet aggregation in GPVI deficient individuals. One-way ANOVA with Tukey’s multiple comparisons test, n = 3–5, ****p < .0001 to basal. (iii) Representative western blot for tyrosine phosphorylation of PLCγ2, Syk and LAT following collagen and DEP stimulated washed platelets from GPVI-deficient individuals and control individuals. b) Platelet spreading on glass (charged surface) is abolished with Syk inhibition. (i) Washed platelets (2x107/mL) spread on glass (negatively charged surface) for 30 min. Platelets were pre-incubated with 20 μM PRT-060318 (Syk inhibitor) or DMSO (vehicle) 5 min before spreading. Scale bar = 5 μm. (ii) Western blot for tyrosine phosphorylation of signaling proteins after platelets were spread on glass. Representative of 3 experiments
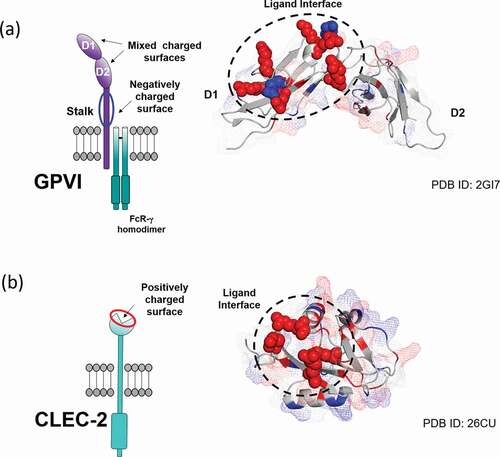
GPVI has several areas of charge in its two Ig domains as shown in , and contains a highly negatively charged stalk that is highly O-glycosylated and charged due to sialylation.Citation80,Citation81 Site directed mutagenesis studiesCitation82 along with co-crystallization of GPVI with CRP and a blocking nanobody show critical charged residues (predominantly positively charged) at positions E21, R38, E40, R46, K59, R60, R67 and R166 in the D1 domain.Citation82–84 The crystallization of GPVI with a nanoparticle or dendrimer could reveal whether these residues or other residues provide a surface on GPVI to mediate binding of charged ligands. This will also help determine whether nanoparticles and other charged ligands bind directly to specific epitopes or in ligand-binding regions on GPVI, or if GPVI activation is mainly driven by the general charge of the nanoparticles/ligands. Furthermore, developing a blocking agent to prevent GPVI-charged ligand engagement could help to disrupt thrombus propagation and reduce vessel occlusion.
Figure 4. Charge distribution and ligand-binding interface mapped onto the surface of a) GPVI (PDB: 2GI7) and b) CLEC-2 (PDB: 2C6U) extracellular domains. Charged residues of the ligand binding interface are represented as spheres with their color corresponding to charge (red: positive, blue: negative). Additional charged residues are highlighted throughout the domains with their surface coverage displayed as a mesh
CLEC-2
CLEC-2 is a receptor for the highly sialylated membrane protein podoplanin and oxidized heme (hemin).Citation85 CLEC-2 has also been shown to be activated by DEPs in human and mouse platelets, and by negatively charged poly-sulfated fucoidans in mouse platelets ().Citation1,Citation86
The extracellular domain of CLEC-2 has been co-crystallized with a podoplanin peptide (containing the conserved binding sequence) and the snake venom rhodocytin. The sequence within podoplanin, EDXXXT (single amino acid code), is known as a platelet aggregation-stimulating (PLAG) domain.Citation85,Citation87 These are conserved in mammals and mediated podoplanin-induced platelet activation. The structure revealed that threonine in the PLAG-3 domain is glycosylated and capped with sialic acid which is critical for binding to CLEC-2. The rhodocytin α-subunit possesses a unique Glu-Asp sequence which is also critical for binding to CLEC-2. The interaction with both ligands is mediated through four arginine residues (R107, R118, R152 and R157) that create a charged surface on CLEC-2 (depicted in ). Consecutive acidic residues within the PLAG-3 domain of podoplanin, and the negatively charged residues within the N-terminal loop of the α-subunit of rhodocytin bind through electrostatic contacts to the charged surface within the CLEC-2 C-type lectin-like domain, while additional polar contacts reenforce the binding interface. Ligand binding can be abolished by mutation of the essential arginine residues within CLEC-2 to uncharged residues, such as alanine,Citation87 highlighting the importance of charge during CLEC-2: ligand binding.
PEAR1
Human PEAR1 is a novel platelet and endothelial receptor that mediates powerful activation of platelet by sulfated polysaccharides, including fucoidans and dextran-sulfate.Citation59 Sulfation is critical for activation. The site of interaction has been mapped to the thirteenth EGF-like repeat in PEAR1 which contains a cluster of positively charged amino acids in a conserved heparin binding-like consensus sequence. The co-crystallization of PEAR1 with its ligands will map the critical amino acids and potentially provide information on the endogenous ligand. Interestingly, we have shown that in mouse platelets, sulfated glycopolymers mediate activation through CLEC-2 with only a partial role for PEAR1Citation59 and speculate that this may be due to the much higher expression of CLEC-2 in mouse platelets compared to humans.Citation88 If this is the case, this illustrates the promiscuity of charged ligands consistent with the charge playing a dominant role in mediating activation.
Other Receptors
Other surface glycoproteins are also anticipated to bind to charged ligands and may facilitate activation of platelet glycoprotein receptors. Examples include the role of GPIb in supporting activation of mouse platelets by fucoidansCitation59 and the observation that adhesion but not spreading of platelets to fibrin and fibrinogen is retained in the absence of GPVI and GPIIb/IIIa.Citation70 These receptors therefore may act as adhesion receptors which help to increase binding to the tyrosine kinase receptors for rapid charge-mediated signaling.
Relevance of Charge-mediated Platelet Activation?
Platelets are exposed to a variety of charged matrix proteins in both the blood and following lesion to the vessel wall, including at sites of plaque formation. Atherosclerotic plaques are rich in lipid deposits, connective tissue (collagen, fibronectin), proteoglycans and necrotic cell debrisCitation89,Citation90 and form large areas of charge thereby forming a highly prothrombogenic surface.Citation91 Many of the components of an atherosclerotic plaque present a charged surface for platelets to adhere, activate and aggregate. Moreover, lesion atherosclerotic plaques mediate activation through GPVI.Citation92,Citation93
Charged exogenous ligands induce powerful activation of washed platelets, although not all are active in blood due to binding to plasma proteins, such as albumin.Citation29 This is the case for nanoparticles which bind predominantly because of their charge, whereas protein ligands such as snake venom toxins form a conformationally constrained interaction with their receptors and retain their ability to activate platelets in blood. This has important implications for the clinical significance of exposure to DEPs, with the link to cardiovascular disease more likely to be mediated through damage to blood endothelial cells.
A special consideration is needed for the exposure of platelets to foreign charged surfaces in dialysis and with use of extracorporeal membrane oxygenation (ECMO) and left ventricular assist devices (LVADs).Citation94 Hemostatic complications, both bleeding and activation are frequent in the latterCitation94,Citation95 and associated with build of thrombi on the oxygenation membrane and connectors. It is not known if this is the result of a direct interaction of platelets with the surface, or protein immobilized to the surface and/or to other factors such as coagulation initiation or changes in shear stress.Citation72,Citation96
Binding to Microvesicles?
Platelets rapidly shed microvesicles which express the majority of surface receptors other than GPVI, which has shown to be lost as the result of shedding.Citation97 The microvesicles could potentially therefore interact with charged ligands, however the functional significance of this is uncertain.
Are Platelet Glycoproteins Receptors for DAMPS and PAMPs?
Damage-associated molecular patterns (DAMPs) is the umbrella term for cell debris and soluble mediators released after damage based on the theory of “self” driving immune responses.Citation98 DAMPs, like pathogen-associated molecular patterns (PAMPs), bind to pattern recognition receptors (PRRs) to induce pro-inflammatory cytokine production and invoke immune responses, which can be associated with poor patient outcome.Citation99 PRRs can bind multiple DAMPs thereby mediating activation to a wide range of stimuli.
Platelets express many PRRs belonging to multiple families including toll-like receptors (TLRs), NOD-like receptors (NLRs) and C-type lectin-like receptors and are involved in promoting immune responses both in infection and with sterile inflammation.Citation100 Platelet PRRs recognize a wide range of DAMPs including extracellular/mitochondrial DNA, histones and high mobility group box protein (HMGB)-1.Citation101–104 However, most of these receptors are expressed in low level and, for many, platelets lack their downstream signaling components (see below).
Platelets express several TLRs, including surface TLR-1, -2, -4 and -6,Citation105,Citation106 with TLR-3, -7 and -9 being located in platelet endosomes and translocated to the surface after activation.Citation100 TLRs signal via recruitment of Toll/Il-1 receptor (TIR) domain containing adaptors, such as MyD88, TIRAP and TRIF (depending on TLR function), resulting in activation of transcription factors NF-kB, IRFs and MAPKs, increasing pro-inflammatory cytokine production.Citation107 However, platelet lack many of the TLR signaling proteins and lack a nucleus.Citation106 For example, TLR-4 signaling on other cell types requires CD14, which is not present on platelets.Citation108 TLR-4 is a receptor for LPS and has been reported to mediate platelet activation,Citation106,Citation109 but this is controversial with several groups unable to shown activation by LPS.Citation109–111 It has also been proposed that LPS does not alter TLR-4 expression or enhance platelet–leukocyte interactions,Citation109 suggesting TLR-4 independent mechanisms. Other DAMPs such as histones and HMGB-1 have been proposed to mediate platelet activation through TLR-2 and TLR-4.Citation103,Citation104 However, TLR-independent mechanisms cannot be ruled out, with histones activating GPVI signaling proteinsCitation1 and HMGB-1 also activating the receptor for advanced glycation end product (RAGE).Citation112 In summary, platelets express several Toll receptors but for most of these they lack the signaling proteins to mediate responses such as aggregation, secretion and spreading on surfaces. This means that platelets require other classes of PRRs receptors to mediate their primary functional responses.
CLEC-2 is the most common C-type lectin-like receptor on platelets and is an emerging PPR. Platelet CLEC-2 is involved in invoking immune responses to HIV and cooperates with DC-sign in dengue infections.Citation113,Citation114 Heme, released from red blood cells following hemolysis, also activates platelets through CLEC-2 signaling, emphasizing the importance of CLEC-2 as a PRR receptor.Citation115
As summarized above, GPVI, CLEC-2 and PEAR1 and shown in are activated by a wide range of charged ligands suggesting that they should be considered to be PRRs. This could be an evolutionary conserved mechanism to activate platelets at sites of inflammation and vascular damage in a rapid manner. Designing charge agents to disrupt the interaction of the major signaling glycoprotein receptor in platelets with DAMPs could therefore reduce platelet activation in arterial thrombosis and thromboinflammation.
Charge Interactions in Coagulation
Charge interactions are also important in driving coagulation and are thought to present a primitive and evolutionary conserved way to prevent excessive bleeding. FXII contact with negatively charged surfaces on exposed blood vessels or binding of exogenous negatively charged molecules such as polyphosphate, heparin and nucleotide RNA released after damage or in inflammation leads to FXII activation and initiates coagulation through contact activation.Citation116–119 Potentially, this could work in combination with charged-mediated activation of platelets to prevent excessive blood loss and promote inflammation in times of damage and infection. Tailoring nanoparticles to model important protein–charge interactions with key coagulation factors could provide novel therapeutic angles for charge neutralization in times of hypercoagulation and thrombosis.
Conclusions
There is an urgent need for more powerful antiplatelet therapy that also preserves hemostasis. In this Opinion Article, we argue that this could be achieved by considering several of the key platelet signaling receptors as PRRs that are activated by binding to charged ligands, notably GPVI, CLEC-2 and PEAR1. It is of great interest to map the mechanisms behind charge-mediated platelet activation, and to develop blocking therapeutics. Tailoring nanoparticles and dendrimers to have specific surface areas and charge may provide a valuable tool for disrupting platelet-ligand engagement or neutralize charge related membrane disruptions, providing a new approach for development of novel antithrombotics.
Contributions
S.J.M and P.P. performed the literature review, generated figures and wrote the manuscript. E.M.M and A.S discussed research and generated models. L.G.Q., G.P., M.N., D.M. and S.P.W. performed research experiments. K.C. discussed research and contributed intellect to the review. P.M.M. and S.P.W. led the research, planned the review and wrote the manuscript. All authors contributed, read and edited the manuscript, and approved it for publication.
Disclosure Statement
All authors have no conflicts of interest to declare.
Additional information
Funding
References
- Alshehri OM, Montague S, Watson S, Carter P, Sarker N, Manne BK, Miller JL, Herr AB, Pollitt AY, O’Callaghan CA et al. Activation of glycoprotein vi (gpvi) and c-type lectin-like receptor-2 (clec-2) underlies platelet activation by diesel exhaust particles and other charged/hydrophobic ligands. Biochem J. 2015;468(3):459–473. https://doi.org/10.1042/BJ20150192
- Zia F, Kendall M, Watson SP, Mendes PM. Platelet aggregation induced by polystyrene and platinum nanoparticles is dependent on surface area. RSC Adv. 2018;8(66):37789–37794. https://doi.org/10.1039/C8RA07315E
- Damaskinaki F-N, Moran LA, Garcia A, Kellam B, Watson SP. Overcoming challenges in developing small molecule inhibitors for gpvi and clec-2. Platelets. 2021:1–9. https://doi.org/10.1080/09537104.2020.1863939
- Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019;25(1):112. https://doi.org/10.3390/molecules25010112
- Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979. https://doi.org/10.3390/ijms19071979
- Jeyaraj M, Gurunathan S, Qasim M, Kang M-H, Kim J-H. A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials. 2019;9(12):1719. https://doi.org/10.3390/nano9121719
- Burdușel AC, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials. 2018;8(9):681. https://doi.org/10.3390/nano8090681
- Pedone D, Moglianetti M, De Luca E, Bardi G, Pompa PP. Platinum nanoparticles in nanobiomedicine. Chem Soc Rev. 2017;46(16)4951–4975.
- Ajnai G, Chiu A, Kan T, Cheng -C-C, Tsai T-H, Chang J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. J Exp Med. 2014;6(6)172–178.
- Chen Y-H, Tsai C-Y, Huang P-Y, Chang M-Y, Cheng P-C, Chou C-H, Chen D-H, Wang C-R, Shiau A-L, Wu C-L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol Pharm. 2007;4(5):713–722. https://doi.org/10.1021/mp060132k
- Liao Y-J, Shiang Y-C, Huang -C-C, Chang H-T. Molecularly imprinted aptamers of gold nanoparticles for the enzymatic inhibition and detection of thrombin. Langmuir. 2012;28(24):8944–8951. https://doi.org/10.1021/la204651t
- Shabanova EM, Drozdov AS, Fakhardo AF, Dudanov IP, Kovalchuk MS, Vinogradov VV. Thrombin@fe(3)o(4) nanoparticles for use as a hemostatic agent in internal bleeding. Sci Rep. 2018;8(1):233. https://doi.org/10.1038/s41598-017-18665-4
- Neuwelt A, Sidhu N, Hu CA, Mlady G, Eberhardt SC, Sillerud LO. Iron-based superparamagnetic nanoparticle contrast agents for mri of infection and inflammation. AJR Am J Roentgenol. 2015;204(3):302–313. https://doi.org/10.2214/AJR.14.12733
- Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knüchel R, Kiessling F, Lammers T. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev. 2019;138:302–325.
- Hrapovic S, Liu Y, Male KB, Luong JHT. Electrochemical biosensing platforms using platinum nanoparticles and carbon nanotubes. Anal Chem. 2004;76(4):1083–1088. https://doi.org/10.1021/ac035143t
- Claussen JC, Kim SS, Haque AU, Artiles MS, Porterfield DM, Fisher TS. Electrochemical glucose biosensor of platinum nanospheres connected by carbon nanotubes. J Diabetes Sci Technol. 2010;4(2):312–319. https://doi.org/10.1177/193229681000400211
- Chen D, Zhao C, Ye J, Li Q, Liu X, Su M, Jiang H, Amatore C, Selke M, Wang X. In situ biosynthesis of fluorescent platinum nanoclusters: toward self-bioimaging-guided cancer theranostics. ACS Appl Mater Interfaces. 2015;7(32):18163–18169. https://doi.org/10.1021/acsami.5b05805
- Clark A, Zhu A, Sun K, Petty HR. Cerium oxide and platinum nanoparticles protect cells from oxidant-mediated apoptosis. J Nanopart Res. 2011;13(10):5547–5555. https://doi.org/10.1007/s11051-011-0544-3
- Han M, Gao X, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol. 2001;19(7):631–635. https://doi.org/10.1038/90228
- Alshehri R, Ilyas AM, Hasan A, Arnaout A, Ahmed F, Memic A. Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J Med Chem. 2016;59(18):8149–8167. https://doi.org/10.1021/acs.jmedchem.5b01770
- Lunova M, Prokhorov A, Jirsa M, Hof M, Olżyńska A, Jurkiewicz P, Š K, Lunov O, Dejneka A. Nanoparticle core stability and surface functionalization drive the mtor signaling pathway in hepatocellular cell lines. Sci Rep. 2017;7(1):16049. https://doi.org/10.1038/s41598-017-16447-6
- Caminade A-M, Turrin C-O. Dendrimers for drug delivery. J Mater Chem B. 2014;2(26):4055–4066. https://doi.org/10.1039/C4TB00171K
- Cheng J, Meziani MJ, Sun Y-P, Cheng SH. Poly(ethylene glycol)-conjugated multi-walled carbon nanotubes as an efficient drug carrier for overcoming multidrug resistance. Toxicol Appl Pharmacol. 2011;250(2):184–193. https://doi.org/10.1016/j.taap.2010.10.012
- Villa CH, Dao T, Ahearn I, Fehrenbacher N, Casey E, Rey DA, Korontsvit T, Zakhaleva V, Batt CA, Philips MR et al. Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance igg responses to tumor-associated antigens. ACS Nano. 2011;5(7):5300–5311. https://doi.org/10.1021/nn200182x
- Navarro-Tovar G, Palestino G, Rosales-Mendoza S. An overview on the role of silica-based materials in vaccine development. Expert Rev Vaccines. 2016;15(11):1449–1462. https://doi.org/10.1080/14760584.2016.1188009
- Niculescu V-C. Mesoporous silica nanoparticles for bio-applications. Front Mater. 2020;7:36. https://doi.org/10.3389/fmats.2020.00036
- Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6(3):139–150. https://doi.org/10.4103/0975-7406.130965
- Fox LJ, Richardson RM, Briscoe WH. Pamam dendrimer - cell membrane interactions. Adv Colloid Interface Sci. 2018;257:1–18. https://doi.org/10.1016/j.cis.2018.06.005
- Dobrovolskaia MA, Patri AK, Simak J, Hall JB, Semberova J, De Paoli Lacerda SH, McNeil SE. Nanoparticle size and surface charge determine effects of pamam dendrimers on human platelets in vitro. Mol Pharm. 2012;9(3):382–393. https://doi.org/10.1021/mp200463e
- Miller MR, Newby DE. Air pollution and cardiovascular disease: car sick. Cardiovasc Res. 2019;116(2)279–294.
- Ohlwein S, Kappeler R, Kutlar Joss M, Künzli N, Hoffmann B. Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence. Int J Public Health. 2019;64(4):547–559. https://doi.org/10.1007/s00038-019-01202-7
- Franchini M, Guida A, Tufano A, Coppola A. Air pollution, vascular disease and thrombosis: linking clinical data and pathogenic mechanisms. J Thromb Haemost. 2012;10(12):2438–2451. https://doi.org/10.1111/jth.12006
- Alarabi AB, Karim ZA, Ramirez JEM, Hernandez KR, Lozano PA, Rivera JO, Alshbool FZ, Khasawneh FT. Short-term exposure to waterpipe/hookah smoke triggers a hyperactive platelet activation state and increases the risk of thrombogenesis. Arterioscler Thromb Vasc Biol. 2020;40(2):335–349. https://doi.org/10.1161/ATVBAHA.119.313435
- Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112(25):3930–3936. https://doi.org/10.1161/CIRCULATIONAHA.105.588962
- Langrish JP, Lundbäck M, Mills NL, Johnston NR, Webb DJ, Sandström T, Blomberg A, Newby DE. Contribution of endothelin 1 to the vascular effects of diesel exhaust inhalation in humans. Hypertension. 2009;54(4):910–915. https://doi.org/10.1161/HYPERTENSIONAHA.109.135947
- Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere AJF et al. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano. 2017;11(5):4542–4552. https://doi.org/10.1021/acsnano.6b08551
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65(20):1531–1543. https://doi.org/10.1080/00984100290071658
- Solomon A, Smyth E, Mitha N, Pitchford S, Vydyanath A, Luther PK, Thorley AJ, Tetley TD, Emerson M. Induction of platelet aggregation after a direct physical interaction with diesel exhaust particles. J Thromb Haemost. 2013;11(2):325–334. https://doi.org/10.1111/jth.12087
- Lei J, Li Z, Huang X, Li X, Zhang G, Kan H, Chen R, Zhang Y. The acute effect of diesel exhaust particles and different fractions exposure on blood coagulation function in mice. Int J Environ Health Res. 2021;18(8).
- Tabor CM, Shaw CA, Robertson S, Miller MR, Duffin R, Donaldson K, Newby DE, Hadoke PW. Platelet activation independent of pulmonary inflammation contributes to diesel exhaust particulate-induced promotion of arterial thrombosis. Part Fibre Toxicol. 2016;13(6). https://doi.org/10.1186/s12989-016-0116-x.
- Deb S, Patra HK, Lahiri P, Dasgupta AK, Chakrabarti K, Chaudhuri U. Multistability in platelets and their response to gold nanoparticles. Nanomedicine. 2011;7(4):376–384. https://doi.org/10.1016/j.nano.2011.01.007
- Dobrovolskaia MA, Patri AK, Zheng J, Clogston JD, Ayub N, Aggarwal P, Neun BW, Hall JB, McNeil SE. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomed Nanotechnol Biol Med. 2009;5(2):106–117. https://doi.org/10.1016/j.nano.2008.08.001
- Aseychev AV, Azizova OA, Beckman EM, Dudnik LB, Sergienko VI. Effect of gold nanoparticles coated with plasma components on adp-induced platelet aggregation. Bull Exp Biol Med. 2013;155(5):685–688. https://doi.org/10.1007/s10517-013-2226-x
- Ajdari N, Vyas C, Bogan SL, Lwaleed BA, Cousins BG. Gold nanoparticle interactions in human blood: a model evaluation. Nanomed Nanotechnol Biol Med. 2017;13(4):1531–1542. https://doi.org/10.1016/j.nano.2017.01.019
- Huang H, Lai W, Cui M, Liang L, Lin Y, Fang Q, Liu Y, Xie L. An evaluation of blood compatibility of silver nanoparticles. Sci Rep. 2016;6(1):25518. https://doi.org/10.1038/srep25518
- Jun E-A, Lim K-M, Kim K, Bae O-N, Noh J-Y, Chung K-H, Chung J-H. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology. 2011;5(2):157–167. https://doi.org/10.3109/17435390.2010.506250
- Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. 2009;3(6):1357–1364. https://doi.org/10.1021/nn900277t
- Laloy J, Minet V, Alpan L, Mullier F, Beken S, Toussaint O, Lucas S, Dogné J-M. Impact of silver nanoparticles on haemolysis, platelet function and coagulation. Nanobiomedicine. 2014;1(4):4. https://doi.org/10.5772/59346
- Deb S, Raja SO, Dasgupta AK, Sarkar R, Chattopadhyay AP, Chaudhuri U, Guha P, Sardar P. Surface tunability of nanoparticles in modulating platelet functions. Blood Cells Mol Dis. 2012;48(1):36–44. https://doi.org/10.1016/j.bcmd.2011.09.011
- Liu T, Bai R, Zhou H, Wang R, Liu J, Zhao Y, Chen C. The effect of size and surface ligands of iron oxide nanoparticles on blood compatibility. RSC Adv. 2020;10(13):7559–7569. https://doi.org/10.1039/C9RA10969B
- Nemmar A, Hoylaerts MF, Hoet PH, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Crit Care Med. 2002;166(7):998–1004. https://doi.org/10.1164/rccm.200110-026OC
- Smyth E, Solomon A, Vydyanath A, Luther PK, Pitchford S, Tetley TD, Emerson M. Induction and enhancement of platelet aggregation in vitro and in vivo by model polystyrene nanoparticles. Nanotoxicology. 2015;9(3):356–364. https://doi.org/10.3109/17435390.2014.933902
- Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146(6):882–893. https://doi.org/10.1038/sj.bjp.0706386
- Semberova J, De Paoli Lacerda SH, Simakova O, Holada K, Gelderman MP, Simak J. Carbon nanotubes activate blood platelets by inducing extracellular ca2+ influx sensitive to calcium entry inhibitors. Nano Lett. 2009;9(9):3312–3317. https://doi.org/10.1021/nl901603k
- Guidetti GF, Consonni A, Cipolla L, Mustarelli P, Balduini C, Torti M. Nanoparticles induce platelet activation in vitro through stimulation of canonical signalling pathways. Nanomed Nanotechnol Biol Med. 2012;8(8):1329–1336. https://doi.org/10.1016/j.nano.2012.04.001
- Ritz S, Schöttler S, Kotman N, Baier G, Musyanovych A, Kuharev J, Landfester K, Schild H, Jahn O, Tenzer S et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules. 2015;16(4):1311–1321. https://doi.org/10.1021/acs.biomac.5b00108
- Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007;104(7):2050–2055. https://doi.org/10.1073/pnas.0608582104
- Guildford AL, Poletti T, Osbourne LH, Di Cerbo A, Gatti AM, Santin M. Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J R Soc Interface. 2009;6(41):1213–1221. https://doi.org/10.1098/rsif.2009.0021
- Kardeby C, Fälker K, Haining EJ, Criel M, Lindkvist M, Barroso R, Påhlsson P, Ljungberg LU, Tengdelius M, Rainger GE et al. Synthetic glycopolymers and natural fucoidans cause human platelet aggregation via pear1 and gpibα. Blood Adv. 2019;3(3):275–287. https://doi.org/10.1182/bloodadvances.2018024950
- Thanh NTK, Maclean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev. 2014;114(15):7610–7630. https://doi.org/10.1021/cr400544s
- Polte J. Fundamental growth principles of colloidal metal nanoparticles – a new perspective. Cryst Eng Comm. 2015;17(36):6809–6830. https://doi.org/10.1039/C5CE01014D
- Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev. 2012;41(7):2539–2544. https://doi.org/10.1039/c2cs15294k
- Conde J, Dias JT, Grazú V, Moros M, Baptista PV. de la Fuente JM. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front Chem. 2014;2(48).
- Eklund SE, Cliffel DE. Synthesis and catalytic properties of soluble platinum nanoparticles protected by a thiol monolayer. Langmuir. 2004;20(14):6012–6018. https://doi.org/10.1021/la049787n
- Heinz H, Pramanik C, Heinz O, Ding Y, RK M, Marchon D, RJ F, Estrela-Lopis I, Llop J, Moya S, et al. Nanoparticle decoration with surfactants: molecular interactions, assembly, and applications. Surf Sci Rep. 2017;72(1):1–58.
- Durrant TN, Van Den Bosch MT, Hers I. Integrin α(iib)β(3) outside-in signaling. Blood. 2017;130(14):1607–1619. https://doi.org/10.1182/blood-2017-03-773614
- Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors gpvi and clec-2. J Clin Invest. 2019;129(1):12–23. https://doi.org/10.1172/JCI122955
- Mammadova-Bach E, Ollivier V, Loyau S, Schaff M, Dumont B, Favier R, Freyburger G, Latger-Cannard V, Nieswandt B, Gachet C et al. Platelet glycoprotein vi binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126(5):683–691. https://doi.org/10.1182/blood-2015-02-629717
- Alshehri OM, Hughes CE, Montague S, Watson SK, Frampton J, Bender M, Watson SP. Fibrin activates gpvi in human and mouse platelets. Blood. 2015;126(13):1601–1608. https://doi.org/10.1182/blood-2015-04-641654
- Mangin PH, Onselaer MB, Receveur N, Le Lay N, Hardy AT, Wilson C, Sanchez X, Loyau S, Dupuis A, Babar AK et al. Immobilized fibrinogen activates human platelets through glycoprotein vi. Haematologica. 2018;103(5):898–907. https://doi.org/10.3324/haematol.2017.182972
- Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, Stegner D, Remer K, Nurden P, Nurden AT et al. Platelet glycoprotein vi promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135(14):1146–1160. https://doi.org/10.1182/blood.2019002649
- Gardiner EE. Proteolytic processing of platelet receptors. RPTH. 2018;2(2):240–250. https://doi.org/10.1002/rth2.12096
- Krueger I, Gremer L, Mangels L, Klier M, Jurk K, Willbold D, Bock HH, Elvers M. Reelin amplifies glycoprotein vi activation and alphaiib beta3 integrin outside-in signaling via plc gamma 2 and rho gtpases. Arterioscler Thromb Vasc Biol. 2020;40(10):2391–2403. https://doi.org/10.1161/ATVBAHA.120.314902
- Donner L, Toska LM, Krüger I, Gröniger S, Barroso R, Burleigh A, Mezzano D, Pfeiler S, Kelm M, Gerdes N et al. The collagen receptor glycoprotein vi promotes platelet-mediated aggregation of β-amyloid. Sci Signal. 2020;13(643):eaba9872. https://doi.org/10.1126/scisignal.aba9872
- Montague SJ, Hicks SM, Lee CS-M, Coupland LA, Parish CR, Lee WM, Andrews RK, Gardiner EE. Fibrin exposure triggers αiibβ3-independent platelet aggregate formation, adam10 activity and glycoprotein vi shedding in a charge-dependent manner. J Thromb Haemost. 2020;18(6):1447–1458. https://doi.org/10.1111/jth.14797
- Chanzu H, Lykins J, Wigna-Kumar S, Joshi S, Pokrovskaya I, Storrie B, Pejler G, Wood JP, Whiteheart SW. Platelet α-granule cargo packaging and release are affected by the luminal proteoglycan, serglycin. J Thromb Haemost. 2021. https://doi.org/10.1111/jth.15243
- Flierl U, Nero TL, Lim B, Arthur JF, Yao Y, Jung SM, Gitz E, Pollitt AY, Zaldivia MTK, Jandrot-Perrus M et al. Phosphorothioate backbone modifications of nucleotide-based drugs are potent platelet activators. J Exp Med. 2015;212(2):129–137. https://doi.org/10.1084/jem.20140391
- Nagy M, Perrella G, Dalby A, Becerra MF, Garcia Quintanilla L, Pike JA, Morgan NV, Gardiner EE, Heemskerk JWM, Azócar L et al. Flow studies on human gpvi-deficient blood under coagulating and noncoagulating conditions. Blood Adv. 2020;4(13):2953–2961. https://doi.org/10.1182/bloodadvances.2020001761
- Matus V, Valenzuela G, Sáez CG, Hidalgo P, Lagos M, Aranda E, Panes O, Pereira J, Pillois X, Nurden AT et al. An adenine insertion in exon 6 of human GP6 generates a truncated protein associated with a bleeding disorder in four Chilean families. J Thromb Haemost. 2013;11(9):1751–1759. https://doi.org/10.1111/jth.12334
- Andrews RK, Suzuki-Inoue K, Shen Y, Tulasne D, Watson SP, Berndt MC. Interaction of calmodulin with the cytoplasmic domain of platelet glycoprotein vi. Blood. 2002;99(11):4219–4221. https://doi.org/10.1182/blood-2001-11-0008
- Moroi M, Jung SM. Platelet glycoprotein vi: its structure and function. Thromb Res. 2004;114(4):221–233. https://doi.org/10.1016/j.thromres.2004.06.046
- Slater A, Di Y, Clark JC, Jooss NJ, Martin EM, Alenazy F, Thomas MR, Ariëns RAS, Herr AB, Poulter NS et al. Structural characterization of a novel GPVI-nanobody complex reveals a biologically active domain-swapped GPVI dimer. Blood. 2021;137(24):3443–3453. https://doi.org/10.1182/blood.2020009440
- Horii K, Kahn ML, Herr AB. Structural basis for platelet collagen responses by the immune-type receptor glycoprotein vi. Blood. 2006;108(3):936–942. https://doi.org/10.1182/blood-2006-01-010215
- O’Connor MN, Smethurst PA, Farndale RW, Ouwehand WH. Gain- and loss-of-function mutants confirm the importance of apical residues to the primary interaction of human glycoprotein vi with collagen. J Thromb Haemost. 2006;4(4):869–873. https://doi.org/10.1111/j.1538-7836.2005.01764.x
- Martin E, Zuidscherwoude M, Moran L, Di Y, Garcia A, Watson S. The structure of CLEC-2: mechanisms of dimerization and higher-order clustering. Platelets. 2021:1–11. https://doi.org/10.1080/09537104.2021.1906407
- Manne BK, Getz TM, Hughes CE, Alshehri O, Dangelmaier C, Naik UP, Watson SP, Kunapuli SP. Fucoidan is a novel platelet agonist for the c-type lectin-like receptor 2 (clec-2). J Bio Chem 2013;288(11):7717–7726. https://doi.org/10.1074/jbc.M112.424473
- Nagae M, Morita-Matsumoto K, Kato M, Kaneko Mika M, Kato Y, Yamaguchi Y. A Platform of C-type Lectin-like Receptor CLEC-2 for Binding O -Glycosylated Podoplanin and Nonglycosylated Rhodocytin. Structure. 2014;22(12):1711–1721. https://doi.org/10.1016/j.str.2014.09.009
- Dunster JL, Unsworth AJ, Bye AP, Haining EJ, Sowa MA, Di Y, Sage T, Pallini C, Pike JA, Hardy AT et al. Interspecies differences in protein expression do not impact the spatiotemporal regulation of glycoprotein vi mediated activation. J Thromb Haemost. 2020;18(2):485–496. https://doi.org/10.1111/jth.14673
- Wight TN. A role for proteoglycans in vascular disease. Matrix Biol. 2018;71-72:396–420.
- Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–1866. https://doi.org/10.1161/CIRCRESAHA.114.302721
- Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. Journal of Internal Medicine. 2014;276(6):618–632. https://doi.org/10.1111/joim.12296
- Penz S, Reininger AJ, Brandl R, Goyal P, Rabie T, Bernlochner I, Rother E, Goetz C, Engelmann B, Smethurst PA et al. Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein vi. FASEB J. 2005;19(8):898–909. https://doi.org/10.1096/fj.04-2748com
- Schulz C, Penz S, Hoffmann C, Langer H, Gillitzer A, Schneider S, Brandl R, Seidl S, Massberg S, Pichler B et al. Platelet gpvi binds to collagenous structures in the core region of human atheromatous plaque and is critical for atheroprogression in vivo. Basic Res Cardiol. 2008;103(4):356–367. https://doi.org/10.1007/s00395-008-0722-3
- Susen S, Rauch A, Van Belle E, Vincentelli A, Lenting PJ. Circulatory support devices: fundamental aspects and clinical management of bleeding and thrombosis. J Thromb Haemost. 2015;13(10):1757–1767. https://doi.org/10.1111/jth.13120
- Muthiah K, Connor D, Ly K, Gardiner EE, Andrews RK, Qiao J, Rutgers D, Robson D, Low J, Jarvis S et al. Longitudinal changes in hemostatic parameters and reduced pulsatility contribute to non-surgical bleeding in patients with centrifugal continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2016;35(6):743–751. https://doi.org/10.1016/j.healun.2015.12.024
- Chen Z, Mondal NK, Zheng S, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets. 2019;30(1):112–119. https://doi.org/10.1080/09537104.2017.1384542
- Gitz E, Pollitt AY, Gitz-Francois JJ, Alshehri O, Mori J, Montague S, Nash GB, Douglas MR, Gardiner EE, Andrews RK et al. Clec-2 expression is maintained on activated platelets and on platelet microparticles. Blood. 2014;124(14):2262–2270. https://doi.org/10.1182/blood-2014-05-572818
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev of Immunol. 1994;12:991–1045(1). https://doi.org/10.1146/annurev.iy.12.040194.005015
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2)240–273.
- Dib PRB, Quirino‐Teixeira AC, Merij LB, Pinheiro MBM, Rozini SV, Andrade FB, Hottz ED. Innate immune receptors in platelets and platelet-leukocyte interactions.J Leukoc Biol. 2020;108(4):1157–1182. https://doi.org/10.1002/JLB.4MR0620-701R
- Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880–15885. https://doi.org/10.1073/pnas.1005743107
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial damps cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. https://doi.org/10.1038/nature08780
- Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet tlr2 and tlr4. Blood. 2011;118(7):1952–1961. https://doi.org/10.1182/blood-2011-03-343061
- Vogel S, Bodenstein R, Chen Q, Feil S, Feil R, Rheinlaender J, Schäffer TE, Bohn E, Frick J-S, Borst O et al. Platelet-derived hmgb1 is a critical mediator of thrombosis. J Clin Invest. 2015;125(12):4638–4654. https://doi.org/10.1172/JCI81660
- Garraud O, Hamzeh-Cognasse H, Pozzetto B, Cavaillon J-M, Cognasse F. Bench-to-bedside review: platelets and active immune functions - new clues for immunopathology? Crit Care. 2013;17(4):236. https://doi.org/10.1186/cc12716
- Cognasse F. The inflammatory role of platelets via their tlrs and siglec receptors. Front Immunol. 2015;6:83. https://doi.org/10.3389/fimmu.2015.00083
- Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. https://doi.org/10.3389/fimmu.2014.00461
- Montrucchio G, Bosco O, Del Sorbo L, Fascio Pecetto P, Lupia E, Goffi A, Omedè P, Emanuelli G, Camussi G. Mechanisms of the priming effect of low doses of lipopoly-saccharides on leukocyte-dependent platelet aggregation in whole blood. Thromb Haemost. 2003;90(5):872–881. https://doi.org/10.1160/TH03-02-0085
- Andonegui G, Kerfoot SM, McNagny K, Ebbert KVJ, Patel KD, Kubes P. Platelets express functional toll-like receptor-4. Blood. 2005;106(7):2417–2423. https://doi.org/10.1182/blood-2005-03-0916
- Ward JR, Bingle L, Judge HM, Brown SB, Storey RF, Whyte MK, Dower SK, Buttle DJ, Sabroe I. Agonists of toll-like receptor (tlr)2 and tlr4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94(4)831–838.
- de Stoppelaar SF, Claushuis TAM, Schaap MCL, Hou B, van der Poll T, Nieuwland R, Van ‘T Veer C. Toll-like receptor signalling is not involved in platelet response to streptococcus pneumoniae in vitro or in vivo. PLOS ONE. 2016;11(6):e0156977. https://doi.org/10.1371/journal.pone.0156977
- Ahrens I, Chen YC, Topcic D, Bode M, Haenel D, Hagemeyer CE, Seeba H, Duerschmied D, Bassler N, Jandeleit-Dahm KA et al. Hmgb1 binds to activated platelets via the receptor for advanced glycation end products and is present in platelet rich human coronary artery thrombi. Thromb Haemost. 2015;114(5):994–1003. https://doi.org/10.1160/TH14-12-1073
- Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, Geier M, Stewart EA, Eisemann J, Steinkasserer A et al. Dc-sign and clec-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80(18):8951–8960. https://doi.org/10.1128/JVI.00136-06
- Sung P-S, Huang T-F, Hsieh S-L. Extracellular vesicles from clec2-activated platelets enhance dengue virus-induced lethality via clec5a/tlr2. Nat Commun. 2019;10(1):2402. https://doi.org/10.1038/s41467-019-10360-4
- Bourne JH, Colicchia M, Di Y, Martin E, Slater A, Roumenina LT, Dimitrov JD, Watson SP, Rayes J. Heme induces human and mouse platelet activation through c-type-lectin-like receptor-2. Haematologica. 2021;106(2):626–629. https://doi.org/10.3324/haematol.2020.246488
- Maas C, Renné T. Coagulation factor xii in thrombosis and inflammation. Blood. 2018;131(17):1903–1909. https://doi.org/10.1182/blood-2017-04-569111
- Grover Steven P, Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(3):331–338. https://doi.org/10.1161/ATVBAHA.118.312130
- Verhoef JJF, Barendrecht AD, Nickel KF, Dijkxhoorn K, Kenne E, Labberton L, OJT M, Schiffelers R, Heijnen HF, Hendrickx AP et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood. 2017;129(12):1707–1717. https://doi.org/10.1182/blood-2016-08-734988
- Didiasova M, Wujak L, Schaefer L, Wygrecka M. Factor xii in coagulation, inflammation and beyond. Cell Signal. 2018;51:257–265. https://doi.org/10.1016/j.cellsig.2018.08.006
