Abstract
We carried out a literature search in MEDLINE (PubMed) and EMBASE literature databases to provide a concise review of the role of viscoelastic testing in assessing peri-interventional platelet function and coagulation. The search identified 130 articles that were relevant for the review, covering the basic science of VHA and VHA in clinical settings including cardiac surgery, cardiology, neurology, trauma, non-cardiac surgery, obstetrics, liver disease, and COVID-19. Evidence from these articles is used to describe the important role of VHAs and platelet function testing in various peri-interventional setups. VHAs can help us to comprehensively assess the contribution of platelets and coagulation dynamics to clotting at the site-of-care much faster than standard laboratory measures. In addition to standard coagulation tests, VHAs are beneficial in reducing allogeneic transfusion requirements and bleeding, in predicting ischemic events, and improving outcomes in several peri-interventional care settings. Further focused studies are needed to confirm their utility in the peri-interventional case.
Introduction
A close interplay between platelets and coagulation is essential for the generation of a platelet-fibrin clot during hemostasis. In patients presenting with acute coronary syndromes, stroke, venous thromboembolism in the presence of existing endothelial dysfunction, systemic prothrombotic phenotype (hypercoagulability) results in a strong and occlusive platelet-fibrin clot[Citation1]. Conversely, impaired hemostasis due to low platelet count or low coagulation proteins, and drug-induced inhibition of platelets or coagulation carries an increased risk for spontaneous and peri-interventional bleeding [Citation2–6]. Laboratory tests such as blood count, international normalized ratio/prothrombin time (INR/PTI) and partial thromboplastin time, and Clauss fibrinogen are the current standard methods to assess platelet count and coagulation proteins. However, these tests have a long turnaround time, reflect a single aspect of coagulation, and therefore are suboptimal whenever urgent therapeutic decisions are requested peri-operatively, peri-interventional, or during trauma management [Citation7].
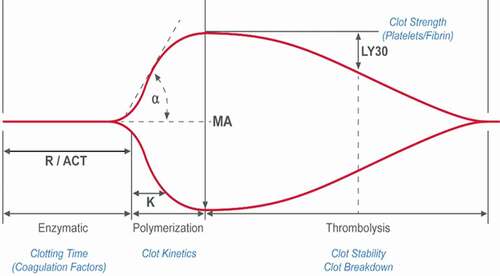
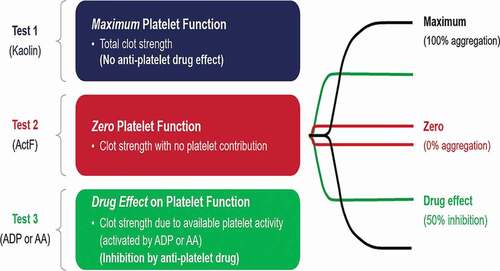
Currently available whole blood testing devices or viscoelastic hemostatic assays (VHA) are Thromboelastography (TEG® analyzer), Rotational thromboelastometry (ROTEM®), Quantra®, and Sonoclot® (). VHAs provide a holistic view of hemostasis with detailed information on dynamic changes in clot characteristics from initiation of clot formation to platelet-fibrin clot generation, stability, and lysis (, ). These characteristics are used to assess the relative contribution of coagulation proteins and platelets to clot formation, fibrinolysis, and to estimate hyper- or hypocoagulability and to assess response to antiplatelet or anticoagulant agents (, ) [Citation8,Citation9]. Briefly, anticoagulants influence clot initiation, clot kinetics and clot strength whereas, fibrinolytics influence clot strength and lysis. In the TEG6s Platelet Mapping assay, ADP- and AA-induced platelet fibrin clot strength is influenced by P2Y12 receptor blockers and aspirin respectively, whereas both ADP- and AA-induced platelet-fibrin clot strength is influenced by glycoprotein (GP)IIB/IIIa inhibitors. Moreover, VHAs can also assess functional fibrinogen levels thereby enabling targeted administration of platelets and fibrinogen in the bleeding patient.
Table 1 Comparison of platelet count and function measures in currently available viscoelastic haemostatic assay analysers
Table 2 Characteristics of viscoelastic haemostatic assay analysers
R/ACT = reaction time/activated clotting time, K = clot kinetics, A = angle, MA- maximum amplitude, LY30 = lysis at 30
ADP = adenosine diphosphate, AA = arachidonic acid
In this literature review, we discuss the currently available evidence on how the assessment of platelet-coagulation interaction and response to antiplatelet agents can help us in guiding the administration of blood products, and antiplatelet treatment in the peri-interventional period in patients including trauma, cardiovascular surgery, cardiology, neurology, obstetrics, and COVID-19 to mitigate the risks of ischemia and bleeding. A systematic literature review was conducted on MEDLINE (PubMed) and EMBASE to identify English language articles of adult humans or human samples published between January 1999 and September 2019. Predefined search terms for platelet inhibition and function (e.g., platelet inhibition, platelet function, antiplatelet, platelet reactivity, platelet mapping, clot-forming, clopidogrel) and thromboelastometry (e.g., thromboelastometry, thromboelastography, TEG, ROTEM, Quantra, Sonoclot, Viscoelastic) were used and articles were screened for inclusion against predefined criteria. Key exclusion criteria included: non-English language articles; pediatric, non-human studies; reviews, editorials, case reports, editorials; and articles with no relevant platelet data or no viscoelastic testing reported. As COVID-19 papers were published after the September 2019 search, additional relevant publications about COVID −19 proposed by the authors were also included.
Viscoelastic testing for hemostasis: current site-of-care analyzers
Currently, TEG® 5000 and TEG® 6s with PlateletMapping® assay, ROTEM®, and Sonoclot® are the main VHA assays being used. The TEG® PlateletMapping® assay has been validated against the current gold-standard light transmission aggregometry and is used assess response to P2Y12 receptor blockers and aspirin by measuring adenosine diphosphate (ADP) - and arachidonic acid (AA) -induced platelet aggregation, respectively () [Citation10,Citation11]. TEG®6s is the new site of-care assay that uses resonance-frequency viscoelasticity measurements and a disposable multi-channel microfluidic cartridge to assess hemostasis and has been validated against the earlier TEG®5000 [Citation12]. Rotational thromboelastometry (ROTEM®) has been shown previously to correlate with conventional coagulation assays, with the newer ROTEM® sigma device showing higher precision compared with the ROTEM® delta [Citation12,Citation13]. ROTEM® is able to assess the intrinsic (INTEM) and extrinsic pathways (EXTEM) of coagulation and functional fibrinogen (FIBTEM), however it is not able to measure drug-induced platelet inhibition. The Sonoclot® coagulation analyzer has been compared with both the TEG® and ROTEM® devices in patients undergoing cardiac surgery [Citation14], with TEG® and ROTEM® devices showing a greater correlation with conventional coagulation tests, including fibrinogen and platelet count [Citation14]. A close correlation between well-established ROTEM parameters including FIBTEM and parameters as assessed by the fully automated Quantra Q Plus system ® has been demonstrated recently [Citation15].
Major limitations of VHAs include labor-intensive methodology (TEG 5000 and ROTEM), and complicated data output. Although the cost is higher, VHAs provide detailed information on clot kinetics and response to antiplatelet agents simultaneously (with TEG6s Platelet Mapping Assay). Moreover, the cost may be recovered by saving of blood products. Other important limitations are the need for significant training to get through the learning curve of the new way of assessing coagulation, and standardization of treatment protocols to provide a consistent methodology of utilizing the information within an institution.”
Clinical applications of VHA: trauma and burns
VHA is becoming more widely used in diagnosis, particularly in the early trauma-induced coagulopathy, to provide a fast and accurate assessment of blood product requirements [Citation16]. VHAs can be effectively used in the resuscitation of trauma patients to reduce the administration of blood products and improve survival [Citation17]. Use of VHA and hemostatic assays to guide fibrinogen concentrate administration during trauma-related surgery have been shown to be successful in improving patient hemostasis [Citation18]. The use of platelet function tests in trauma patients has led to improvements in the current understanding of platelet dysfunction during trauma [Citation19], including the influence of histones on platelets during trauma hemorrhage [Citation20]. VHAs studies also showed that patients with burns are not deprived of growth factor content as previously thought and that both platelet count and growth factor contents are only reduced in the initial period after burn injury [Citation21]. The use of VHAs demonstrated that platelet inhibition is common in both minor and major injuries, and the degree of platelet dysfunction may be proportional to the severity of the injury [Citation22]. VHAs with platelet function assays can in some cases allow physicians to identify patients on antiplatelet therapy admitted for acute trauma treatment [Citation23,Citation24]. However, the current platelet function analysis with VHAs alone is not able to add predictive value for mortality, massive transfusion, or platelet transfusion in either injured patients [Citation25] or patients with septic shock [Citation26]. Since trauma patients are at an elevated risk for venous thromboembolism, VHAs can be used to identify patients at the highest risk for VTE [Citation27,Citation28]. However, further studies are needed to explore the utility of VHAs in these patients to personalize antithrombotic therapy.
The coagulopathy of trauma brain injury (TBI) patients differs from other trauma patients. Drug-induced platelet inhibition assessed by VHAs is a prominent early feature of TBI and is linked to the severity of brain injury in patients with isolated head trauma [Citation18]. Impaired platelet P2Y12 receptor pathway has been associated with increased clot sensitivity, suggesting that P2Y12 receptor pathway inhibition may be an early step in the pathogenesis of systemic hyperfibrinolysis [Citation29,Citation30]. A significant reduction in platelet response to arachidonic acid was also demonstrated in major TBI patients compared to healthy controls [Citation31]. These observations highlight the importance of early identification of coagulopathy and goal-directed treatment in patients with TBI. TBI monitoring and treatment is a challenging area for further studies to explore and validate platelet dysfunction by VHA.
In trauma patients, VHAs can be used to assess primary and secondary fibrinolysis, thrombocytopenia, clotting factor consumption, and hypercoagulability [Citation32]. Serial monitoring of coagulation status in a trauma patient is critical to guide transfusion and the correction of coagulopathy. These observations are incorporated into the current European and American practice guidelines for the management of trauma [Citation33,Citation34]. In the recently reported randomized, international viscoelastic hemostatic assay augmented protocols for major trauma hemorrhage (ITACTIC) trial of 396 trauma patients, there was no difference in outcomes between patients receiving empiric major hemorrhage protocols augmented by either VHAs or conventional coagulation tests (CCTs)-guided interventions. There are numerous important limitations to this study. Nearly 75% of patients were not coagulopathic by prothrombin time ratio at baseline and very few of these patients subsequently developed a prolonged prothrombin time ratio before hemostasis. The reduction in the primary endpoint was 3% as compared to the estimated 13% reduction indicating an inadequate sample size. In the VHA group, 1.8 times more patients received study interventions than the CCT group indicating that the widespread occurrence of coagulation deficits was not detected by the CCTs. Therefore, further studies are warranted to assess the true utility of VHAs in this setting [Citation35].
Cardiac Surgery
The severity of cardiac surgery-related bleeding has been associated with postoperative morbidity and mortality [Citation36,Citation37]. Preventing perioperative blood loss may be more efficacious in improving outcomes than mere reducing allogenic blood components because restrictive transfusion strategy was not superior to liberal transfusion strategy in patients undergoing cardiac surgery in a recent randomized study [Citation3,Citation38]. Current guidelines suggest the implementation of institutional perioperative treatment algorithms based on VHA tests for the bleeding patient to reduce the number of transfusions [Citation2,Citation39,Citation40]. Recent studies indicated that transfusion algorithms including site-of-care tests to measure the interaction of coagulation and platelets, platelet count, and platelet function, reduce allogeneic transfusion and cardiac surgery-associated bleeding without affecting mortality [Citation41–43]. In patients on preoperative dual antiplatelet therapy undergoing cardiac surgery, implementation of preoperative TEG® PlateletMapping® or Multiplate assays in addition to extended intraoperative TEG® assessments allowed targeted post-bypass coagulation management and reduced allogeneic blood transfusion and costs [Citation44]. However, cutoff values of TEG® and ROTEM® used in the RCTs and observational studies included in the meta-analyses differed between studies and ranges rather than specific cutoff values may be more appropriate for guiding transfusion therapy, also considering the multifactorial nature of surgery-related bleeding [Citation45].
Despite conflicting results in individual RCTs targeting fibrinogen administration based on VHA results in heterogeneous cardiac surgical patient populations, a recent meta-analysis demonstrated that intraoperative fibrinogen supplementation reduced the incidence of allogeneic red blood cell transfusion, without however affecting morbidity or mortality [Citation46]. Platelet function tests have been used to schedule elective on-pump and off-pump cardiac surgery in patients on dual antiplatelet therapy, by stratifying preoperative waiting based on the VHA MA-ADP and the Innovance 2Y platelet function test, respectively [Citation47,Citation48]. In the TARGET CABG study, surgery performed within 1 day, 3–5 days, or >5 days using cut off points of MA-ADP >50 mm, 35–50 mm, and <35 mm respectively reduced preoperative waiting by about 50% as compared to the guideline-mandated uniform delay in surgery without any difference in surgery-related bleeding [Citation47].
In order to attenuate the risk of surgery-related bleeding in patients on P2Y12 receptor inhibitors presenting for non-emergent major surgery, current guidelines recommend a “one size fits all” preoperative discontinuation period of at least three to five days for ticagrelor, five days for clopidogrel, and seven days for prasugrel based largely on results of the large scale clinical studies (IIa recommendation). The recommendation for a standardized preoperative waiting period neither accounts for highly variable pharmacodynamic responsiveness nor for variability in platelet reactivity recovery time for clopidogrel, the most widely used P2Y12 receptor blocker. Therefore, an individualized approach using an objective measure of residual preoperative platelet function may serve as an alternative (IIb recommendation) although both the safe cutoff and the optimal device are elusive so far [Citation49,Citation50].
The addition of platelet function assays has improved the predictive power of VHA for perioperative bleeding compared with coagulation factors alone [Citation51–53]. Platelet function measurements allowed accurate prediction of transfusion requirements [Citation44,Citation54,Citation55]. The platelet function assays can also be used to monitor and guide personalized antiplatelet therapy for patients with left ventricular assist devices to reduce bleeding [Citation56,Citation57]. Similarly, guidelines suggest the use of platelet function tests to aid with the timing of surgical procedures, and suggest that further evidence should be gathered on their optimal use [Citation50] (supplemental Table 1)
Non-cardiac surgery
Platelet function tests provide information on antiplatelet response in addition to that provided by hemostatic assays. The extensive use of antiplatelet agents is associated with a higher risk of bleeding during surgical procedures, and VHAs can be used to assess hemostasis, antiplatelet response and monitor antiplatelet therapy. Clopidogrel-induced platelet inhibition levels showed a good correlation with standard VHA [Citation58]. Patients who were currently or had recently received dual antiplatelet therapy presenting for non-cardiac surgery had higher levels of preoperative platelet inhibition compared with patients who had been off therapy for longer. The latter may have a clinical impact on the decisions for antiplatelet treatment before surgery [Citation59]. Two publications exploring the use of platelet function assays to stratify patient bleeding risk in surgical patients demonstrated that assessing ADP-induced platelet aggregation can identify a statistically significant platelet inhibition during antiplatelet therapy (p < .01) [Citation60] and that 35% inhibition of ADP-induced platelet aggregation may be an useful cutoff for proceeding to surgery [Citation61]. However, a recent meta-analysis including four randomized clinical trials with 229 patients undergoing non-cardiac surgery did not provide any concrete evidence to support the use of VHAs in reducing mortality and transfusion needs as compared to standard monitoring [Citation62].
Interventional cardiology
Hemostatic and coagulation assays with VHA have been shown in both prospective and retrospective analyses to provide important information for the treatment of interventional cardiology patients [Citation63,Citation64]. VHAs are useful in monitoring antiplatelet therapy and other cardiology medications (e.g., amlodipine for blood pressure) [Citation65], giving physicians a site-of-care measure of the effectiveness of the therapy [Citation66]. VHA measurements of platelet function and hemostasis factors are demonstrated as risk predictors of ischemia and bleeding in patients undergoing percutaneous coronary intervention (PCI) [Citation67–69], and have shown a correlation with disease severity in patients with cardiovascular diseases [Citation70–73]. The addition of platelet function assays to treatment algorithms in the presence of aspirin or a P2Y12 receptor inhibitor further allows clinicians to monitor antiplatelet therapy and individualize both the medication type and the dose [Citation66,Citation74–85]. VHAs have been used to explore the efficacy and safety of antiplatelet therapies, including aspirin [Citation64], clopidogrel [Citation86], cangrelor [Citation87], and the direct oral anticoagulant such as dabigatran [Citation88]. Dabigatran was found to significantly influence thrombin activity and thrombus generation parameters compared with placebo (p < .001), without affecting clot structure, fibrinolysis or measures of platelet reactivity [Citation88]. Expert consensus statements have suggested that platelet function tests including TEG® be used as tools to guide treatment, e.g., for determining appropriate antiplatelet therapy escalation and de-escalation of antiplatelet therapy and to assess compliance to treatment [Citation89–91] (Supplemental Table 2).
Stroke
Platelet count assays are commonly used in neurology and neurosurgery to monitor hemostasis and assess blood transfusion requirements. The standard global hemostatic VHA showed some predictive value for recurrent ischemic events [Citation92], with coagulation assays able to detect the differences in coagulopathy associated with dual antiplatelet therapy [Citation93]. Platelet function assays have further demonstrated a correlation between platelet dysfunction and subsequent ischemic events following stroke and may be used to predict the risk of future thromboembolic complications [Citation94–97].
As in cardiology patients, platelet function tests are also beneficial in identifying antiplatelet resistance and guiding antiplatelet therapy to improve outcomes in neurological patients [Citation98,Citation99]. The use of site-of-care VHA to assess thrombogenicity in patients with symptoms of acute stroke showed that clotting time could predict acute ischemic stroke [Citation100]. Poor response to clopidogrel therapy was reported in patients sustaining acute ischemic stroke while treated with long-term aspirin [Citation101], and there is evidence that clopidogrel resistance may be associated with an increased risk for ischemic complications in patients undergoing stenting [Citation95]. VHAs with platelet function assays have shown some success in monitoring patients undergoing neurosurgery to guide platelet transfusions for active bleeding, as they can evaluate preoperative coagulation function alongside platelet function [Citation102,Citation103].
Obstetrics
There are fewer publications regarding the use of VHA in obstetrics compared with other clinical indications, this may be because VHA trials in obstetrics are focused on fibrinogen levels rather than platelet function. In total, three papers were found which used VHAs in an obstetric setting [Citation104–106]. The standard VHA hemostatic and fibrinogen assays were shown to be reliable for urgently and emergently assessing patient coagulation status [Citation105] with one suggesting that VHAs should be used to monitor patients for blood transfusion during postpartum hemorrhage [Citation104]. VHAs are also being used to assess the safety of neural analgesia in pregnant patients with possible coagulopathy, such as in thrombocytopenia or preeclampsia [Citation106].
Liver disease/surgery
The literature search identified only two publications using VHA for exploring hemostasis, coagulation factors, and platelet function in patients with cirrhosis [Citation107,Citation108]. Studies have also shown that including platelet function with platelet count assays can reduce the need for blood transfusions in patients undergoing liver surgery and reduces the incidence of bleeding complications [Citation109,Citation110].
Coronavirus Disease −19
COVID-19 is primarily associated with elevated levels of fibrinogen and D-dimer indicating a systemic hypercoagulability state that may contribute to the observed thromboembolic events and to mortality by triggering cardiovascular events [Citation111]. However, studies with mostly limited number of patients have demonstrated abnormalities in hemostasis evaluated by VHAs. Moreover, limited data available exploring the utility of VHAs guided therapy in patients with ARDS, or sepsis that have similar inflammation and hemostasis abnormalities as COVID-19. VHAs based studies in COVID patients reported fibrinolysis shutdown based on the absence of clot lysis, hypercoagulability with highly elevated levels of platelet-fibrin clot strength that can be attributed to high levels of fibrinogen, and an attenuated response to anticoagulant therapy [Citation112–118]. There are opportunities for VHAs in the early identification of COVID-19 patients and management of antithrombotic therapy to improve clinical outcomes[Citation119]. These observations support the guidance published by the United States Food and Drug Administration (FDA) highlighting the importance of measurement of whole blood viscoelastic properties to facilitate management in patients with COVID-19[Citation120].
Major limitations of VHAs include labor-intensive methodology (TEG 5000 and ROTEM delta), and complicated data output. Although the cost is higher, VHAs provide detailed information on clot kinetics and response to antiplatelet agents simultaneously (with TEG 6s PlateletMapping Assay). Moreover, the cost may be recovered by saving of blood products. Other important limitations are the need for significant training to get through the learning curve of the new way of assessing coagulation, and standardization of treatment protocols to provide a consistent methodology of utilizing the information within an institution. Finally, VHAs do not assess the contribution of shear force and endothelial function during clot generation. The latter factors may be a limitation of VHAs to assess some coagulation disorders like Von Willebrand disease.
Discussion
It is vital to monitor platelet function along with coagulation dynamics quickly and efficiently at the site-of-care for optimal management of patients during the peri-operative and peri-intervention period, and trauma management. Evidence from a range of studies shows that currently available VHA correlate well with standard hemostatic tests [Citation121,Citation122]. The TEG® PlateletMapping® assay was shown to correlate well with LTA and compared with other whole blood platelet function tests such as Multiplate® and PFA-100 [Citation123].
Various studies and meta-analyses have shown that monitoring platelet-fibrin clot formation with VHA can improve diagnosis, trigger goal-directed transfusion and antiplatelet therapy, and decrease surgery-related bleeding [Citation42,Citation124,Citation125] In addition, platelet function testing and viscoelastic platelet function assays, when added to standard coagulation assays, demonstrated clinical importance in both surgical settings for assessing coagulopathic bleeding [Citation41,Citation42] cardiology settings for monitoring response to antiplatelet therapy [Citation76], and the prediction of future ischemic events [Citation71]. The addition of the AA- and ADP-based VHA allows platelet function to be assessed alongside coagulation dynamics, so that clinicians can determine response to therapy, and to assess the relative contribution of platelet function and coagulation dynamics, and individually schedule major surgery without increased bleeding risk [Citation47,Citation126]. While TEG® and ROTEM® devices performed similarly for standard platelet count assays, the addition of platelet function assays may enhance the utility of management algorithms in surgical patients [Citation44,Citation92,Citation127].
In other clinical indications such as obstetrics and TBI, while VHAs are commonly used to measure coagulation dynamics [Citation105], platelet function tests are less widely used [Citation26]. Reduced platelet function following ER admission for trauma can be a sign of coagulopathy associated with increased mortality [Citation128], and VHAs have been used to assess platelet dysfunction immediately after isolated blunt TBI [Citation129]. Further data on platelet function tests in these settings may help us to understand the nature of coagulopathy, and to assess patient hemostasis.
Current European and American guidelines suggest implementing an institution-based VHA algorithm in cardiac surgery to decrease surgery-related bleeding [Citation2,Citation40]. Finally, the goal of VHA testing is to optimize transfusion regimes of red cells/platelets, clotting factors, fibrinogen, fresh frozen plasma or cryoprecipitate, and administration of antifibrinolytic drugs. This approach is aimed at improving clinical outcomes and reducing costs.
Acknowledgements
The authors thank Meridian HealthComms, Plumley, UK for librarian support, which was funded by Haemonetics SA, Signy, Switzerland in accordance with Good Publication Practice (GPP3).
Disclosure statement
UST has received honoraria from UpToDate. JH, DM and JD are or were employees of Haemonetics Corporation at the time of the study. MDN has received research support from Haemonetics and from Instrument Laboratories, and an honorarium in return for a speaking engagement for Haemonetics. SA has received research funding and honoraria from Haemonetics, Roche, Pharmacosmos and CSL Behring. HS has received honoraria for participation in advisory board meetings form Bayer Healthcare, Böhringer Ingelheim, Werfen; speakers fees form Haemonetics, Vifor and study grants from CSL Behring. PAG reports consulting fees and/or honoraria from Bayer, Otitopic, Janssen, UpToDate, US WorldMeds, Hikari Dx, and Medicure; institutional research grants from the National Institutes of Health, Haemonetics, Bayer, Medicure, Instrumentation Laboratories, US WorldMeds, Amgen, Idorsia, Otitopic, and Janssen. KPB and EM report no COI.
References
- Hou Y, Carrim N, Wang Y, Gallant RC, Marshall A, Ni H. Platelets in hemostasis and thrombosis: novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J Biomed Res. 2015;29:437–444.
- Boer C, Meesters MI, Milojevic M, Benedetto U, Bolliger D, von Heymann C, Jeppsson A, Koster A, Osnabrugge RL, Ranucci M, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):88–120.
- Biancari F, Mariscalco G, Gherli R, Reichart D, Onorati F, Faggian G, Franzese I, Santarpino G, Fischlein T, Rubino AS, et al. Variation in preoperative antithrombotic strategy, severe bleeding, and use of blood products in coronary artery bypass grafting: results from the multicentre E-CABG registry. Eur Heart J Qual Care Clin Outcomes. 2018;4(4):246–257. doi:10.1093/ehjqcco/qcy027
- Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, Peitzman AB, Moore EE, Cuschieri J, Sperry JL. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013;74(5):1207–1214.
- Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–19. doi:10.1097/TA.0b013e318256deab
- Hansson EC, Jideus L, Aberg B, Bjursten H, Dreifaldt M, Holmgren A, Ivert T, Nozohoor S, Barbu M, Svedjeholm R, et al. Coronary artery bypass grafting-related bleeding complications in patients treated with ticagrelor or clopidogrel: a nationwide study. Eur Heart J. 2016;37(2):189–197. doi:10.1093/eurheartj/ehv381
- Gratz J, Guting H, Thorn S, Brazinova A, Görlinger K, Schäfer N, Schöchl H, Stanworth S, Maegele M. Protocolised thromboelastometric-guided haemostatic management in patients with traumatic brain injury: a pilot study. Anaesthesia. 2019;74(7):883–890. doi:10.1111/anae.14670
- Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015;11:133–148. doi:10.2147/VHRM.S44469
- Peng HT, Nascimento B, Tien H, Callum J, Rizoli S, Rhind SG, Beckett A. A comparative study of viscoelastic hemostatic assays and conventional coagulation tests in trauma patients receiving fibrinogen concentrate. Clin Chim Acta. 2019;495:253–262. doi:10.1016/j.cca.2019.04.066
- Tang XF, Han YL, Zhang JH, Wang J, Zhang Y, Xu B, Gao Z, Qiao S-B, Chen J, Wu Y, et al. Comparing of light transmittance aggregometry and modified thrombelastograph in predicting clinical outcomes in Chinese patients undergoing coronary stenting with clopidogrel. Chin Med J. 2015;128(6):774–779. doi:10.4103/0366-6999.152611
- Gurbel PA, Bliden KP, Navickas IA, Mahla E, Dichiara J, Suarez TA, Antonino MJ, Tantry US, Cohen E. Adenosine diphosphate-induced platelet-fibrin clot strength: a new thrombelastographic indicator of long-term poststenting ischemic events. Am Heart J. 2010;160(2):346–354. doi:10.1016/j.ahj.2010.05.034
- Schenk B, Gorlinger K, Treml B, Tauber H, Fries D, Niederwanger C, Oswald E, Bachler M. A comparison of the new ROTEM® sigma with its predecessor, the ROTEM delta. Anaesthesia. 2019;74(3):348–356. doi:10.1111/anae.14542
- Gillissen A, van den Akker T, Caram-Deelder C, Henriquez DDCA, Bloemenkamp KWM, Eikenboom J, van der Bom JG, de Maat MPM. Comparison of thromboelastometry by ROTEM® Delta and ROTEM® Sigma in women with postpartum haemorrhage. Scand J Clin Lab Invest. 2019;79(1–2):32–38. doi:10.1080/00365513.2019.1571220
- Espinosa A, Stenseth R, Videm V, Pleym H. Comparison of three point-of-care testing devices to detect hemostatic changes in adult elective cardiac surgery: a prospective observational study. BMC Anesthesiol. 2014;14(1):80. doi:10.1186/1471-2253-14-80
- Groves DS, Welsby IJ, Naik BI, Tanaka K, Hauck JN, Greenberg CS, Winegar DA, Viola F. Multicenter evaluation of the quantra qplus system in adult patients undergoing major surgical procedures. Anesth Analg. 2020;130(4):899–909.). doi:10.1213/ANE.0000000000004659
- Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–1059. doi:10.1097/SLA.0000000000001608
- Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: the past, present, and future. J Thromb Haemost. 2019 Jun;17(6):852–862. doi:10.1111/jth.14450
- Schochl H, Posch A, Hanke A, Voelckel W, Solomon C. High-dose fibrinogen concentrate for haemostatic therapy of a major trauma patient with recent clopidogrel and aspirin intake. Scand J Clin Lab Invest. 2010;70(6):453–457. doi:10.3109/00365513.2010.500396
- Vulliamy P, Kornblith LZ, Kutcher ME, Cohen MJ, Brohi K, Neal MD. Alterations in platelet behavior after major trauma: adaptive or maladaptive? Platelets. 2021;32(3):295-304.
- Vulliamy P, Gillespie S, Armstrong PC, Allan HE, Warner TD, Brohi K. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc Nat Acad of Sci. 2019;116(35):17444–17449. doi:10.1073/pnas.1904978116
- Marck RE, van der Bijl I, Korsten H, Lorinser J, de Korte D, Middelkoop E. Activation, function and content of platelets in burn patients. Platelets. 2019;30(3):396–402. doi:10.1080/09537104.2018.1448379
- Sirajuddin S, Valdez C, DePalma L, Maluso P, Singhal R, Schroeder M, Sarani B. Inhibition of platelet function is common following even minor injury. J Trauma Acute Care Surg. 2016;81(2):328–332. doi:10.1097/TA.0000000000001057
- Daley MJ, Trust MD, Peterson EJ, et al. Thromboelastography does not detect preinjury antiplatelet therapy in acute trauma patients. Am Surg. 2016;82(2):175–180. doi:10.1177/000313481608200224
- Connelly CR, Yonge JD, McCully SP, Hart KD, Hilliard TC, Lape DE, Watson JJ, Rick B, Houser B, Deloughery TG, et al. Assessment of three point-of-care platelet function assays in adult trauma patients. J Surg Res. 2017;212:260–269. doi:10.1016/j.jss.2017.01.008
- Stettler GR, Moore EE, Moore HB, Nunns GR, Huebner BR, Einersen P, Ghasabyan A, Silliman CC, Banerjee A, Sauaia A, et al. Platelet adenosine diphosphate receptor inhibition provides no advantage in predicting need for platelet transfusion or massive transfusion. Surgery. 2017;162(6):1286–1294. doi:10.1016/j.surg.2017.07.022
- Russell L, Holst LB, Lange T, Liang X, Ostrowski SR, Perner A. Effects of anemia and blood transfusion on clot formation and platelet function in patients with septic shock: a substudy of the randomized TRISS trial. Transfusion. 2018;58(12):2807–2818. doi:10.1111/trf.14904
- Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256(3):476. doi:10.1097/SLA.0b013e3182658180
- Brill JB, Badiee J, Zander AL, Wallace JD, Lewis PR, Sise MJ, Bansal V, Shackford SR. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J Trauma Acute Care Surg. 2017;83(3):413. doi:10.1097/TA.0000000000001618
- Castellino FJ, Chapman MP, Donahue DL, Thomas S, Moore EE, Wohlauer MV, Fritz B, Yount R, Ploplis V, Davis P, et al. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J Trauma Acute Care Surg. 2014;76(5):1169–1176. doi:10.1097/TA.0000000000000216
- Moore HB, Moore EE, Chapman MP, Gonzalez E, Slaughter AL, Morton AP, D’Alessandro A, Hansen KC, Sauaia A, Banerjee A, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13(10):1878–1887. doi:10.1111/jth.13067
- Nekludov M, Bellander BM, Blombäck M, Wallen HN. Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma. 2007;24(11):1699–1706. doi:10.1089/neu.2007.0322
- Kutcher ME, Cohen MJ Coagulopathy in trauma patients. https://www.uptodate.com/contents/coagulopathy-in-trauma-patients?topicRef=13854&source=see_link. Accessed 11 March 2020.
- Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Nardi G. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100.
- Camazine MN, Hemmila MR, Leonard JC, Jacobs RA, Horst JA, Kozar RA, Bochicchio GV, Nathens AB, Cryer HM, Spinella PC, et al. Massive transfusion policies at trauma centers participating in the American College of Surgeons Trauma Quality Improvement Program. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S48–53. doi:10.1097/TA.0000000000000641
- Baksaas-Aasen K, Gall LS, Stensballe J, Juffermans NP, Curry N, Maegele M, Brooks A, Rourke C, Gillespie S, Murphy J. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med. 2020 Oct;13:1.
- Bartoszko J, Wijeysundera DN, Karkouti K, Callum J, Rao V, Crowther M, Grocott HP, Pinto R, Scales DC, Achen B, et al. Transfusion avoidance in cardiac surgery study investigators. Comparison of two major perioperative bleeding scores for cardiac surgery trials: universal definition of perioperative bleeding in cardiac surgery and European coronary artery bypass grafting bleeding severity grade. Anesthesiology. 2018;129:1092–1100.
- Mariscalco G, Gherli R, Ahmed AB, Zanobini M, Maselli D, Dalén M, Piffaretti G, Cappabianca G, Beghi C, Biancari F, et al. Validation of the European multicenter study on coronary artery bypass grafting (E-CABG) bleeding severity definition. Ann Thorac Surg. 2016;101(5):1782–1788. doi:10.1016/j.athoracsur.2015.10.028
- Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, Reeves BC.TITRe2 investigators. Liberal or Restrictive Transfusion after Cardiac Surgery. N Engl J Med. 2015;372:997–1008.
- Mahla E, Tantry US, Schoerghuber M, Gurbel PA. Platelet function testing in patients on antiplatelet therapy before cardiac surgery. Anesthesiology. 2020 Sep 4. doi:10.1097/ALN.0000000000003541
- Raphael J, Mazer CD, Subramani S, Schroeder A, Abdalla M, Ferreira R, Roman PE, Patel N, Welsby I, Greilich PE, et al. Society of cardiovascular anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2019;33(11):2887–2899. doi:10.1053/j.jvca.2019.04.003
- Karkouti K, Callum J, Wijeysundera DN, Rao V, Crowther M, Grocott HP, Pinto R, Scales DC, Achen B, Brar S, et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation. 2016;134(16):1152–1162. doi:10.1161/CIRCULATIONAHA.116.023956
- Serraino GF, Murphy GJ. Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta-analysis. Br J Anaesth. 2017;118(6):823–833. doi:10.1093/bja/aex100
- Deppe AC, Weber C, Zimmermann J, Kuhn EW, Slottosch I, Liakopoulos OJ, Choi Y-H, Wahlers T. Point-of-care thromboelastography/ thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. J Surg Res. 2016;203(2):424–433. doi:10.1016/j.jss.2016.03.008
- Agarwal S, Johnson RI, Shaw M. Preoperative point-of-care platelet function testing in cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29(2):333–341. doi:10.1053/j.jvca.2014.06.025
- Bolliger D, Tanaka KA. Point-of-care coagulation testing in cardiac surgery. Semin Thromb Hemost. 2017;43:386–396. doi:10.1055/s-0037-1599153
- Li J-Y, Gong J, Zhu F, Moodie J, Newitt A, Uruthiramoorthy L, Cheng D, Martin J. Fibrinogen concentrate in cardiovascular surgery: a meta-analysis of randomized controlled trials. Anesth Analg. 2018;127(3):612–621. doi:10.1213/ANE.0000000000003508
- Mahla E, Suarez TA, Bliden KP, Rehak P, Metzler H, Sequeira AJ, Cho P, Sell J, Fan J, Antonino MJ, et al. Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: the timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ Cardiovasc Interv. 2012;5(2):261–269.
- Mannacio V, Meier P, Antignano A, Di Tommaso L, De Amicis V, Vosa C. Individualized strategy for clopidogrel suspension in patients undergoing off-pump coronary surgery for acute coronary syndrome: a case-control study. J Thorac Cardiovasc Surg. 2014;148(4):1299–1306. doi:10.1016/j.jtcvs.2013.12.011
- Ferraris VA, Saha SP, Oestreich JH, Song HK, Rosengart T, Reece TB, Mazer CD, Bridges CR, Despotis GJ, Jointer K, et al., Society of Thoracic S. 2012. Update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann Thorac Surg. 94:1761–1781. doi:10.1016/j.athoracsur.2012.07.086
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–260.
- Poston R, Gu J, Manchio J, Lee A, Brown J, Gammie J, White C, Griffith B. Platelet function tests Predict bleeding and thrombotic events after off-pump coronary bypass grafting. Eur J Cardio-thoracic Surg off J Eur Assoc Cardio-thoracic Surg. 2005;27(4):584–591. doi:10.1016/j.ejcts.2004.12.061
- Weitzel NS, Weitzel LB, Epperson LE, Karimpour-Ford A, Tran ZV, Seres T. Platelet mapping as part of modified thromboelastography (TEG®) in patients undergoing cardiac surgery and cardiopulmonary bypass. Anaesthesia. 2012;67(10):1158–1165. doi:10.1111/j.1365-2044.2012.07231.x
- Carroll RC, Chavez JJ, Snider CC, Meyer DS, Muenchen RA. Correlation of perioperative platelet function and coagulation tests with bleeding after cardiopulmonary bypass surgery. J Lab Clin Med. 2006;147(4):197–204. doi:10.1016/j.lab.2005.12.007
- Chowdhury M, Shore-Lesserson L, Mais AM, Leyvi G. Thromboelastograph with Platelet Mapping(TM) predicts postoperative chest tube drainage in patients undergoing coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2014;28(2):217–223. doi:10.1053/j.jvca.2013.12.010
- Kwak Y-L, Kim J-C, Choi Y-S, Yoo K-J, Song Y, Shim J-K. Clopidogrel responsiveness regardless of the discontinuation date predicts increased blood loss and transfusion requirement after off-pump coronary artery bypass graft surgery. J Am Coll Cardiol. 2010;56(24):1994–2002. doi:10.1016/j.jacc.2010.03.108
- Shah P, Tantry US, Bliden KP, Gurbel PA. Bleeding and thrombosis associated with ventricular assist device therapy. J Heart Lung Transplant. 2017;36(11):1164–1173. doi:10.1016/j.healun.2017.05.008
- Karimi A, Beaver TM, Hess PJ, Martin TD, Staples ED, Schofield RS, Hill JA, Aranda JM, Klodell CT. Close antiplatelet therapy monitoring and adjustment based upon thrombelastography may reduce late-onset bleeding in HeartMate II recipients. Interact Cardiovasc Thorac Surg. 2014;18(4):457–465. doi:10.1093/icvts/ivt546
- Craft RM, Chavez JJ, Snider CC, Muenchen RA, Carroll RC. Comparison of modified Thrombelastograph and Plateletworks whole blood assays to optical platelet aggregation for monitoring reversal of clopidogrel inhibition in elective surgery patients. J Lab Clin Med. 2005;145(6):309–315. doi:10.1016/j.lab.2005.03.010
- Cattano D, Altamirano AV, Kaynak HE, Seitan C, Paniccia R, Chen Z, Huang H, Prisco D, Hagberg CA, Pivalizza EG, et al. Perioperative assessment of platelet function by Thromboelastograph Platelet Mapping in cardiovascular patients undergoing non-cardiac surgery. J Thromb Thrombolysis. 2013;35(1):23–30. doi:10.1007/s11239-012-0788-5
- Collyer TC, Gray DJ, Sandhu R, Berridge J, Lyons G. Assessment of platelet inhibition secondary to clopidogrel and aspirin therapy in preoperative acute surgical patients measured by Thrombelastography Platelet Mapping. Br J Anaesth. 2009;102(4):492–498. doi:10.1093/bja/aep039
- Kasivisvanathan R, Abbassi-Ghadi N, Kumar S, Mackenzie H, Thompson K, James K, Mallett SV. Risk of bleeding and adverse outcomes predicted by thromboelastography platelet mapping in patients taking clopidogrel within 7 days of non-cardiac surgery. Br J Surg. 2014;101(11):1383–1390. doi:10.1002/bjs.9592
- Franchini M, Mengoli C, Cruciani M, Marietta M, Marano G, Vaglio S, Pupella S, Veropalumbo E, Masiello F, Liumbruno GM. The use of viscoelastic haemostatic assays in non-cardiac surgical settings: a systematic review and meta-analysis. Blood Transfus. 2018;16(3):235–243.
- Volod O, Lam LD, Lin G, Kam C, Kolyouthapong K, Mac J, Mirocha J, Ambrose PJ, Czer LSC, Arabia FA, et al. Role of thromboelastography platelet mapping and international normalized ratio in defining “normocoagulability” during anticoagulation for mechanical circulatory support devices: a pilot retrospective study. ASAIO Journal (American Society for Artificial Internal Organs : 1992). 2017;63(1):24–31. doi:10.1097/MAT.0000000000000445
- Qin J, Yao L, Wang J, Yang B. Application of thromboelastography in evaluating effects of aspirin with or without clopidogrel treatment as well as predictive factors for aspirin resistance in patients with coronary artery disease. Int J Clin Exp Med. 2018;11(12):7.
- Koh J-S, Park Y, Ahn J-H, Kang MG, Kim K-H, Bae JS, Park HW, Jang JY, Park JR, Hwang S-J, et al. Influence of amlodipine on haemostatic measurements during clopidogrel treatment in patients with coronary artery disease. Thromb Haemost. 2019;119(2):264–273. doi:10.1055/s-0038-1676795
- Yao Z, Li G, Fu C. Analysis of antiplatelet activity and short-term prognosis of ticagrelor in AMI patients undergoing emergency PCI during perioperative period. Int J Clin Exp Med. 2017;10:9595–9600.
- Kreutz RP, Schmeisser G, Schaffter A, Kanuri S, Owens J, Maatman B, Sinha A, von der Lohe E, Breall J. Prediction of ischemic events after percutaneous coronary intervention: thrombelastography profiles and factor XIIIa activity. TH Open : Companion J Thrombosis Haemostasis. 2018;2(2):e173–e81. doi:10.1055/s-0038-1645876
- Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, Bassi AK, Tantry US. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005;46(10):1820–1826. doi:10.1016/j.jacc.2005.07.041
- Hou X, Han W, Gan Q, Liu Y, Fang W. Relationship between thromboelastography and long-term ischemic events as gauged by the response to clopidogrel in patients undergoing elective percutaneous coronary intervention. Biosci Trends. 2017;11(2):209–213. doi:10.5582/bst.2016.01233
- Tantry US, Bliden KP, Suarez TA, Kreutz RP, Dichiara J, Gurbel PA. Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: results of the Thrombotic RIsk Progression (TRIP) study. Platelets. 2010;21(5):360–367. doi:10.3109/09537100903548903
- Wu H-Y, Zhang C, Zhao X, et al. Residual platelet reactivity is preferred over platelet inhibition rate in monitoring antiplatelet efficacy: insights using thrombelastography. Acta Pharmacol Sin. 2020;41(2):192-197.
- Fu Z, Dong W, Shen M, Xue H, Guo J, Jing J, Han Y, Yang X, Chen Y. Relationship between hyporesponsiveness to clopidogrel measured by thrombelastography and in stent restenosis in patients undergoing percutaneous coronary intervention. Clin Biochem. 2014;47(16–17):197–202. doi:10.1016/j.clinbiochem.2014.08.009
- Jeong Y-H, Bliden KP, Shuldiner AR, Tantry U, Gurbel P. Thrombin-induced platelet-fibrin clot strength: relation to high on-clopidogrel platelet reactivity, genotype, and post-percutaneous coronary intervention outcomes. Thromb Haemost. 2014;111(4):713–724. doi:10.1160/TH13-08-0643
- Zhao L, Xu X, Du F, Chen B. Thromboelastography evaluation of low response to clopidogrel in patients with acute coronary syndrome. Int J Gerontol. 2018;12(1):43–47. doi:10.1016/j.ijge.2017.07.007
- Tang FK, Lin LJ, Hua N, Lu H, Qi Z, Tang XZ. Earlier application of loading doses of aspirin and clopidogrel decreases rate of recurrent cardiovascular ischemic events for patients undergoing percutaneous coronary intervention. Chin Med J. 2012;125(4):631–638.
- Li Y, Chang H, Ni L, Xue P, Li C, Yuan L, Cui H, Yu C. Analysis of thrombelastogram-guided medication in patients with coronary heart disease after percutaneous coronary intervention. Exp Ther Med. 2019;17(4):3047–3052.
- Xu L, Wang L, Yang X, Li K, Sun H, Zhang D, Wang H, Li W, Ni Z, Xia K, et al. Platelet function monitoring guided antiplatelet therapy in patients receiving high-risk coronary interventions. Chin Med J. 2014;127(19):3364–3370.
- Zhao SW, Wang YP, Xu LD, Gang W. The application of thromboelastogram in detection of indexes of antiplatelet therapy for coronary heart disease. J Thorac Dis. 2016 Dec;8(12):3515-3520. doi: 10.21037/jtd.2016.12.77.
- Tantry US, Bliden KP, Gurbel PA. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J Am Coll Cardiol. 2005;46(9):1705–1709. doi:10.1016/j.jacc.2005.05.090
- Dalal JJ, Digrajkar A, Gandhi A. Oral antiplatelet therapy and platelet inhibition: an experience from a tertiary care center. Indian Heart J. 2016;68(5):624–631. doi:10.1016/j.ihj.2015.12.022
- Hobson AR, Petley G, Morton G, Dawkins KD, Curzen NP. Point-of-care platelet function assays demonstrate reduced responsiveness to clopidogrel, but not aspirin, in patients with Drug-Eluting Stent Thrombosis whilst on dual antiplatelet therapy. Thromb J. 2008;6(1):1. doi:10.1186/1477-9560-6-1
- Chen Y, Wang X, Yu Q, Wang F, Wang H, Liu H. The efficacy of antiplatelet therapies as evaluated by thrombo-elastography in retired Chinese officers. Cardiovasc J Afr. 2018;29(6):357–361. doi:10.5830/CVJA-2018-041
- Tang Y-D, Wang W, Yang M, Zhang K, Chen J, Qiao S, Yan H, Wu Y, Huang X, Xu B, et al. Randomized comparisons of double-dose clopidogrel or adjunctive cilostazol versus standard dual antiplatelet in patients with high posttreatment platelet reactivity: results of the creative trial. Circulation. 2018;137(21):2231–2245. doi:10.1161/CIRCULATIONAHA.117.030190
- Yang B, Zheng C, Yu H, Zhang R, Li S, Tan L, Leng M, Cai S. Comparison of ticagrelor and clopidogrel for patients undergoing emergency percutaneous coronary intervention. Iran J Public Health. 2018;47(7):952–957.
- Rao Z, Zheng H, Wang F, Wang A, Liu L, Dong K, Zhao X, Cao Y, Wang Y. High on-treatment platelet reactivity to adenosine diphosphate predicts ischemic events of minor stroke and transient ischemic attack. J Stroke Cerebrovasc Dis. 2017;26(10):2074–2081. doi:10.1016/j.jstrokecerebrovasdis.2017.04.012
- Yao Y, Zhang J-H, Tang X-F, He C, Ma Y-L, Xu -J-J, Song Y, Liu R, Meng X-M, Song L, et al. Head to head comparison of two point-of-care platelet function tests used for assessment of on-clopidogrel platelet reactivity in Chinese acute myocardial infarction patients undergoing percutaneous coronary intervention. Chin Med J. 2016;129(19):2269–2274. doi:10.4103/0366-6999.190664
- Droppa M, Spahn P, Takhgiriev K, Müller KAL, Alboji A, Straub A, Rath D, Jeong YH, Gawaz M, Geisler T. Periprocedural platelet inhibition with cangrelor in P2Y12-inhibitor naive patients with acute coronary syndromes - A matched-control pharmacodynamic comparison in real-world patients. Int J Cardiol. 2016;223:848–851. doi:10.1016/j.ijcard.2016.08.270
- Franchi F, Rollini F, Cho JR, King R, Phoenix F, Bhatti M, DeGroat C, Tello-Montoliu A, Zenni MM, Guzman LA, et al. Effects of dabigatran on the cellular and protein phase of coagulation in patients with coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel. Results from a Prospective, Randomised, Double-blind, Placebo-controlled Study. Thrombosis and Haemostasis. 2016;115(3):622–631.
- Garcia-Rinaldi R, Carro-Pagan C, Schaff HV, et al. Initial experience with dual antiplatelet thromboprophylaxis using clopidogrel and aspirin in patients with mechanical aortic prostheses. J Heart Valve Dis. 2009;18(6):617–625. discussion 26.
- Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2019;12(16):1521–1537. doi:10.1016/j.jcin.2019.03.034
- Tantry US, Bonello L, Aradi D, Price MJ, Jeong Y-H, Angiolillo DJ, Stone GW, Curzen N, Geisler T, ten Berg J. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261–2273. doi:10.1016/j.jacc.2013.07.101
- Zhang L, Li X, Wang D, Lv H, Si X, Li X, Sun Y, Wang D, Chen K, Kang X, et al. Risk factors of recurrent ischemic events after acute noncardiogenic ischemic stroke. Curr Pharm Des. 2019;25(45):4827-4834. doi: 10.2174/1381612825666191029103756.
- McDonald MM, Almaghrabi TS, Saenz DM, Cai C, Rahbar MH, Choi HA, Lee K, Grotta JC, Chang TR. Dual antiplatelet therapy is associated with coagulopathy detectable by thrombelastography in acute stroke. J Intensive Care Med. 2020;35(1):68–73. doi:10.1177/0885066617729644
- Lv -H-H, Wu S, Liu X, Yang X-L, Xu J-F, Guan Y-T, Dong Q, Zheng SL, Jiang J-M, Li S-X, et al. Comparison of VerifyNow P2Y12 and thrombelastography for assessing clopidogrel response in stroke patients in China. Neurol Sci. 2016;37(2):277–282. doi:10.1007/s10072-015-2407-7
- Yang H, Li Y, Jiang Y. Insufficient platelet inhibition and thromboembolic complications in patients with intracranial aneurysms after stent placement. J Neurosurg. 2016;125(2):247–253. doi:10.3171/2015.6.JNS1511
- Rao Z, Zheng H, Wang F, Wang A, Liu L, Dong K, Zhao X, Wang Y, Cao Y. The association between high on-treatment platelet reactivity and early recurrence of ischemic events after minor stroke or TIA. Neurol Res. 2017;39(8):719–726. doi:10.1080/01616412.2017.1312793
- Yang H, Li Y, Jiang Y, Lv X. Thromboelastography for monitoring platelet function in unruptured intracranial aneurysm patients undergoing stent placement. Interv Neuroradiol. 2015;21(1):61–68. doi:10.15274/INR-2014-10094
- Wu Z, Liu A-F, Zhou J, Zhang Y, Wang K, Li C, Qiu H, Jiang W-J. The safety of triple antiplatelet therapy under thromboelastography guidance in patients undergoing stenting for ischemic cerebrovascular disease. J Neurointerv Surg. 2019;11(4):352–356. doi:10.1136/neurintsurg-2018-013987
- McTaggart RA, Choudhri OA, Marcellus ML, Brennan T, Steinberg GK, Dodd RL, Do HM, Marks MP. Use of thromboelastography to tailor dual-antiplatelet therapy in patients undergoing treatment of intracranial aneurysms with the Pipeline embolization device. J Neurointerv Surg. 2015;7(6):425–430. doi:10.1136/neurintsurg-2013-011089
- Bliden KP, Raviv G, Tantry US, Chaudhary R, Cochran JW, Navarese EP, Brannan T, Vyas A, Gurbel PA. “Blueprinting” thrombogenicity and antithrombotic drug response at the bedside in patients presenting emergently with symptoms of acute stroke. J Thromb Thrombolysis. 2019;47(2):192–199. doi:10.1007/s11239-019-01813-0
- Sternberg Z, Ching M, Sawyer RN, Chichelli T, Li F, Janicke D, Radovic V, Mehta B, Farooq O, Munschauer FE, et al. Clopidogrel responsiveness in stroke patients on a chronic aspirin regimen. J Stroke Cerebrovasc Dis. 2013;22(6):725–732. doi:10.1016/j.jstrokecerebrovasdis.2011.11.009
- Lam H, Katyal N, Parker C, Natteru P, Nattanamai P, Newey CR, Kraus CK.Thromboelastography with platelet mapping is not an effective measure of platelet inhibition in patients with spontaneous intracerebral hemorrhage on antiplatelet therapy. Cureus. 2018;10(4):e2515.
- Zhou H, Chen L, He H. Intraoperative and postoperative effects of TEG-guided platelet transfusion on antiplatelet drug-related intracerebral hemorrhage patients. Exp Ther Med. 2019;17(3):2263–2267.
- Beilin Y, Arnold I, Hossain S. Evaluation of the platelet function analyzer (PFA-100) vs The Thromboelastogram (TEG) in the Parturient. Int J Obstetric Anesthesia. 2006;15(1):7–12. doi:10.1016/j.ijoa.2005.04.013
- Antony KM, Mansouri R, Arndt M, Rocky Hui S-K, Jariwala P, Mcmullen V, Teruya J, Aagaard K. Establishing thromboelastography with platelet-function analyzer reference ranges and other measures in healthy term pregnant women. Am J Perinatol. 2015;32(6):545–554. doi:10.1055/s-0034-1396700
- Davies JR, Fernando R, Hallworth SP. Hemostatic function in healthy pregnant and preeclamptic women: an assessment using the platelet function analyzer (PFA-100) and thromboelastograph. Anesth Analg. 2007;104(2):416–420. doi:10.1213/01.ane.0000253510.00213.05
- Rogalski P, Rogalska-Plonska M, Wroblewski E, Kostecka-Roslen I, Dabrowska M, Swidnicka-Siergiejko A, Wasielica-Berger J, Cydzik M, Hirnle T, Dobrzycki S, et al. Blood platelet function abnormalities in cirrhotic patients with esophageal varices in relation to the variceal bleeding history. Scand J Gastroenterol. 2019;54(3):311–318. doi:10.1080/00365521.2019.1578822
- Pihusch R, Rank A, Gohring P, Pihusch M, Hiller E, Beuers U. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol. 2002;37(5):548–555. doi:10.1016/S0168-8278(02)00239-8
- De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B, Gerunda GE, di Benedetto F, Garcia-Tsao G, Villa E, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology (Baltimore, Md). 2016;63(2):566–573. doi:10.1002/hep.28148
- Wang S-C, Shieh J-F, Chang K-Y, Chu Y-C, Liu C-S, Loong -C-C, Chan K-H, Mandell S, Tsou M-Y. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42(7):2590–2593. doi:10.1016/j.transproceed.2010.05.144
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
- Mortus JR, Manek SE, Brubaker LS, Loor M, Cruz MA, Trautner BW, Rosengart TK. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically Ill. JAMA Netw Open. 2020;3(6):e2011192. doi:10.1001/jamanetworkopen.2020.11192
- Maatman TK, Jalali F, Feizpour C, Douglas A 2nd, McGuire SP, Kinnaman G, Hartwell JL, Maatman BT, Kreutz RP, Kapoor R, et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020;48(9):e783–e790;3. doi:10.1097/CCM.0000000000004466
- Yuriditsky E, Horowitz JM, Merchan C, Ahuja T, Brosnahan SB, McVoy L, Berger JS. Thromboelastography profiles of critically ill patients with coronavirus disease 2019. Crit Care Med. 2020;48(1319–1326):4. doi:10.1097/CCM.0000000000004471
- Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2):193–203.e1. doi:10.1016/j.jamcollsurg.2020.05.007
- Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50(2):281–286. doi:10.1007/s11239-020-02130-7
- Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, Navalesi P, Simioni P. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi:10.1055/s-0040-1714350
- Bliden K, Rout A, Chaudhary R, Barnes J, Singh S, Timilsina S, Tantry U, Gurbel P Heightened platelet function: an unrecognized component of the covid hypercoagulability state. In Proceedings of the American Heart Association 2020, Virtual Congress. 13–17 November 2020.
- Hartmann J, Ergang A, Mason D, Dias JD. The role of TEG analysis in patients with COVID-19-associated coagulopathy: a systematic review. Diagnostics (Basel). 2021;11(2):172. doi:10.3390/diagnostics11020172
- Coagulation Systems for Measurement of Viscoelastic Properties: enforcement Policy During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency (Revised) https://www.fda.gov/media/145135/download. Accessed 17 March 2021
- Craft RM, Chavez JJ, Bresee SJ, Wortham DC, Cohen E, Carroll RC. A novel modification of the Thrombelastograph assay, isolating platelet function, correlates with optical platelet aggregation. J Lab Clin Med. 2004;143(5):301–309. doi:10.1016/j.lab.2004.01.011
- Samos M, Stanciakova L, Duraj L, Kovář F, Fedor M, Šimonová R, Bolek T, Galajda P, Staško J, Kubisz P, et al. Monitoring the hemostasis with rotation thromboelastometry in patients with acute STEMI on dual antiplatelet therapy: first experiences. Medicine. 2017;96(6):e6045. doi:10.1097/MD.0000000000006045
- Dias JD, Pottgiesser T, Hartmann J, Duerschmied D, Bode C, Achneck HE. Comparison of three common whole blood platelet function tests for in vitro P2Y12 induced platelet inhibition. J Thromb Thrombolysis. 2020;50(1):135-143.
- Dias JD, Sauaia A, Achneck HE, Hartmann J, Moore EE. Thromboelastography-guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: a systematic review and analysis. J Thromb Haemost. 2019;17(6):984–994. doi:10.1111/jth.14447
- Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products – a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. 2017;25(1):39. doi:10.1186/s13049-017-0378-9
- Ranucci M, Baryshnikova E. Sensitivity of Viscoelastic Tests to Platelet Function. J Clin Med. 2020;9(1):189. doi:10.3390/jcm9010189
- Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ. 2009;18(4):277-88. doi:10.1016/j.hlc.2008.08.016
- Solomon C, Traintinger S, Ziegler B, et al. Platelet function following trauma A Multiple Electrode Aggregometry Study. Thrombosis and Haemostasis. 2011;106(2):322–330.
- Kay AB, Morris DS, Collingridge DS, Majercik S. Platelet dysfunction on thromboelastogram is associated with severity of blunt traumatic brain injury. Am J Surg. 2019;218(6):1134–1137. doi:10.1016/j.amjsurg.2019.09.024