Abstract
Platelets express P2X1 receptors and our data also show the expression of P2X7 receptors. We studied the role of both receptors in platelet apoptosis by incubation of PRP with P2X agonists, then centrifuged to remove viable platelets, and analyzed the supernatant by flow cytometry to identify a sparse platelet-derived population that stained with MitoTracker dyes and CD41. BzATP, a potent agonist of P2X receptors, and ABT737, an activator of intrinsic apoptosis, produced altered platelets that stained moderately for annexin V and corresponded to an early stage apoptotic platelet (ESAP). Over a range of BzATP concentrations, we observed a dose-dependent formation of ESAPs between 5 and 500 uM BzATP, together with a variable formation of ESAPs at nanomolar ATP or BzATP (50–200 nM). Production of ESAPs occurred with αβ-meATP, while responses with either BzATP or αβ-meATP showed desensitization at a higher agonist concentration. Formation of ESAPs by either 100 nM or 0.5 mM BzATP was inhibited by preincubation of platelets with latrunculin A, an inhibitor of the actin cytoskeleton that prevents apoptosis. ESAP production was totally inhibited by preincubation of platelets with methyl-beta-cyclodextrin, which removes cholesterol from lipid rafts. Our data show that both P2X1 and P2X7 receptors are localized in platelet lipid rafts where P2X-agonists act to produce early stage apoptotic platelets.
Introduction
Extracellular vesicles (EV, also termed microparticles) circulate in human blood in large numbers with about half having a platelet origin. A variety of platelet agonists including thrombin and collagen have been shown to cause formation of EVs from the platelet surface [Citation1,Citation2]. Whether any of the EVs come from the apoptotic death of platelets at the end of their 10 day lifespan is uncertain although blebbing and production of microvesicles is one of the earliest events in apoptosis, which may precede the removal of these cells from the circulation by phagocytosis [Citation3]. There is strong evidence that the platelet lifespan is dictated by an intrinsic platelet pathway regulated by the pro-survival protein Bcl-XL, the degradation of which primes aged platelets for cell death [Citation4–6]. Whether extrinsic signals play an additive role in promoting the platelet apoptotic process remains unclear. However, ion channels are attractive candidates for this role since ionic movements and especially a large K+ efflux is necessary for the apoptotic process [Citation7]. In three nucleated cell types, Cidlowski and colleagues have identified two early stages of apoptosis based on the changes in intracellular cations and cell size and introduced the concept of an “early stage apoptotic” entity [Citation7].
Human platelets are known to express a major channel-forming receptor, the P2X1 receptor that is activated by ATP [Citation8,Citation9], as well as the metabotropic P2Y1 and P2Y12 receptors, both of which are activated by ADP. Addition of ATP alone, the physiological agonist for platelet P2X receptors, does not produce platelet aggregation or activation as defined by P-selectin positivity or PAC-1 staining (Figure S1). Thus, defining the major role for P2X1 receptors in platelets has been elusive [Citation9–12]. The P2X1 receptor channel is highly permeable to calcium ions, and overexpression of this receptor in platelets has been shown to amplify P2Y1 receptor mediated calcium responses and augment hemostatic responses to collagen and ADP [Citation9,Citation13]. However, studies of downstream effects mediated by P2X1 are complicated by the rapid desensitization of this receptor in the continued presence of agonist although some studies have successfully reversed or minimized desensitization by preincubation of cells with apyrase [Citation14]. Human platelets also express the P2X7 receptor [Citation15], although a potential function of P2X7 in this cell type has been little studied. Activated platelets are thought to contribute to inflammation by regulated IL-1beta synthesis although information is lacking on a role of P2X receptors in this process [Citation16,Citation17]. Activation of the P2X7 receptor is also associated with blebbing of the plasma membrane and release of microvesicles, which is independent of the opening of the P2X7 channel/pore [Citation18–21]. Blebbing and production of microvesicles is a consistent effect of ATP when added to a wide range of hemopoietic and non-hemopoietic cell types, and many studies show that this aspect of apoptosis requires a functional actin cytoskeleton that can be inhibited by cytochalasin B or latrunculin A [Citation3,Citation22–24].
We confirm the expression of P2X7 on human platelets and their EVs and show that ATP and BzATP, agonists for both P2X1 and P2X7, stimulate platelets to produce EVs including a subset of larger size that contains a cargo of mitochondria. Similar EVs containing mitochondria are produced by incubating platelets with BH3-mimetic ABT737, a drug that activates the intrinsic pathway of apoptosis [Citation4]. While activation of P2X7 is known to induce apoptosis [Citation24,Citation25], we make the surprising finding that nanomolar BzATP and αβ-meATP, the latter selective for the P2X1 receptor, also trigger formation of the larger mitochondria-containing EVs, which on the basis of size, CD41 expression, annexin V staining and sensitivity to inhibitors point to this particle as an early stage apoptotic platelet (ESAP).
Methods
Materials
2ʹ(3ʹ)-O-(4-benzoylbenzoyl)-ATP (BzATP), ATP, αβ-meATP, ADP, bovine thrombin, methyl-beta-cyclodextrin, ethidium bromide, calcium chloride, bovine serum albumin, glucose, sodium chloride, potassium chloride, latrunculin A and apyrase were bought from Sigma Aldrich (St. Louis, USA). Latrunculin A was kept as a frozen stock solution at 500 μM in DMSO. Cyclodextrin was kept as a frozen stock solution at 200 mM in buffered saline. MRS2365 was from Tocris Bioscience and used at a final concentration of 1μM. MitoTracker Green (MTG) and MitoTracker DeepRed (MTdR) were obtained from Molecular Probes (Oregon, USA). Annexin V antibody was bought from eBioscience. Monoclonal antibody CD41 was from Becton Dickenson and CD61 was from Dako. Mouse anti-human P2X7 receptor monoclonal antibody (clone L4) was conjugated with either FITC or Alexa-647 using protein labeling kits as per manufacturer’s instructions [Citation26]. The isotype control was a monoclonal IgG2b (clone WMD7) conjugated with Alexa-647. Sheep anti-P2X7 polyclonal antibody against a nonhomologous epitope of human P2X7 has been previously described [Citation27]. HEPES-buffered saline contained NaCl 140 mM, HEPES 10 mM, MgCl2 0.1 mM, and pH 7.1.
Study subjects and P2X7 genotyping
Subjects for this study gave written informed consent, and the protocol was approved by the Eastern Health Human Research Ethics Committee (E05/1011), the Melbourne Health Human Research Ethics Committee (2017.354), and the Southern Adelaide Clinical Human Research Ethics Committee (193.06). Subjects were healthy and were not taking regular medication. P2X7 genotyping was performed on purified DNA from subjects in which a total of 12 functional SNPs were genotyped in the P2X7 gene (GenBank accession number Y09561) including rs3751143 (A > C) (which codes for Glu496Ala) using the Sequenom MassARRAY system and iPlex Gold chemistry [Citation28].
Preparation and incubation of platelet-rich plasma (PRP) and platelet poor plasma (PFP)
Blood was venesected from normal donors using light or no tourniquet with a 21 G scalp vein needle following the ISTH recommendations [Citation29]. After discarding the initial 2 mL of blood, we gently withdrew 9 vol blood, which was added to 1 vol 0.13 M sodium citrate (pH 7.0) in a plastic tube followed by gentle inversion (final pH 7.3–7.4). Blood was kept at room temperature with minimum delay before first centrifugation (typically <1 h). For a few studies, peripheral blood was collected via a vacutainer containing ACD-A anticoagulant. Blood was centrifuged at 160x g for 15 min, and the PRP was collected as soon as possible by aspiration of the upper half of the supernatant. Platelet free plasma (PFP) was prepared from PRP by centrifugation at 2500x g for 15 min at 20 °C followed by addition of Krebs substrates: 6 mM succinate, 0.35 mM malate, 6 mM alpha ketoglutarate and 3.5 mM pyruvate. Complete absence of intact platelets in PFP was confirmed by zero platelet counts in a Sysmex XN20 Platelet Counter. Both PRP and PFP were kept at room temperature until use. Incubation of platelets with agonists was at 37 °C and was terminated by transfer of tubes to 18 °C water bath prior to centrifugation.
Separation of platelets by gel-filtration and platelet washing with tyrode’s medium
Platelets were separated from plasma by gel filtration on a Sepharose-2B column as described [Citation30]. The gel-filtered platelets were diluted in Mg-free Tyrode’s medium (1 vol GFP:2 vol Tyrode’s) to reduce magnesium before incubation with agonist. The suspension of gel-filtered platelets is referred to as GFP. All experiments examining the effect of Q-VD-Oph on the formation of ESAPs utilized prostacyclin-washed platelets, as described by Radomski and Moncada [Citation31].
Western blotting of P2X7
PRP was pelleted at 1000x g for 30 min, followed by resuspension of the pellet in buffered medium (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% (w/v) SDS; pH 7.4). Platelet-free plasma was centrifuged at 100,000xg for 70 min at 4 °C and the sediment resuspended as above. Samples were resuspended in sample buffer (250 mM Tris, 40% (v/v) glycerol, 8% (w/v) SDS, 400 mM DTT, and 0.04% (w/v) bromophenol blue; pH 6.8), loaded at 20 uμg per well, and run on gel electrophoresis for 30 min (running buffer made up of 250 mM Tris, 1.92 M glycine, 0.6% (w/v) SDS). Proteins were transferred to a PVDF membrane and incubated with polyclonal sheep antihuman P2X7 antibody (1:1000 dilution) overnight at 4 °C. Blots were then incubated with secondary antibody (1:3000 dilution) for 1 h at room temperature and imaged using chemiluminesence.
Flow cytometry and enumeration of early-stage apoptotic platelets in plasma diluted with tyrode’s medium
PRP or PFP was diluted (1 vol + 4 vol) in Na+ Tyrode’s medium and stained with MTdR 0.5 μM and/or MTG 0.5 μM for 15 min in the dark at 37 °C. Following incubation of PRP with the agonist, tubes were centrifuged at 2,500g for 15 min and the upper half of the supernate was carefully aspirated and stained with CD41. Samples were further diluted 1:20 (v/v) in HEPES-buffered saline immediately before analysis on a CytoFLEX S flow cytometer (Becton Coulter Life Sciences) at a flow rate of 10 μl/min for 3 min. Both diluting media contained Krebs substrates. Acquisition settings for the instrument are listed in the supplementary table [Citation32]. The data were analyzed using FlowJo software (Tree Star, Ashland, Oregon, USA) and corrected for sample dilution. Enumeration of MitoTracker and CD41 positive events was improved by excluding the bulk of smaller EVs using a negative gate with the violet side scatter parameter.
Statistics
Mean and standard deviation are shown, and analyses were carried out using GraphPad Prism 8 (GraphPad Software, La Jolla). One-way ANOVA with Tukey’s multiple comparison post-hoc test was used to analyze data in . A paired t-test was used in addition to confirm significance in .
Table I. Biphasic production of early stage apoptotic platelets (ESAPs) by incubation of PRP with nanomolar (100–500 nM) and millimolar (0.2–0.5 mM) BzATP agonists
Table II. Production of early stage apoptotic platelets (ESAPs) by the P2X1 selective agonist αβ-me ATP
Results
Expression of P2X7 on platelets and their EVs
The expression of the P2X7 receptor on circulating blood platelets and plasma EVs was assessed by equilibrium binding of the P2X7 monoclonal antibody conjugated with Alexa-647 added to PRP or PFP. Flow cytometric assessment of antibody binding reached a plateau at 15 min at 25 °C for both platelets and their EVs in PFP. The binding of antibody to platelets or EVs was greater than either autofluorescence or fluorescence of an isotype monoclonal control () although the mean fluorescence intensity of antibody binding was greater to platelets than to the EVs in plasma.
Figure 1. P2X7 expression on human platelets and circulating EVs. (a and b) Fluorescent histograms of P2X7 expression on human platelets and circulating EVs. GFP and platelet-derived EVs were incubated with AlexaFluor647-labeled P2X7 monoclonal antibody for 15 min at 25 °C and analyzed by flow cytometry. Histograms of isotype control antibody binding and autofluorescence are shown. (c and d) MFI of P2X7 mAb surface binding to platelets and EVs for five subjects; *p < .02 and **p < .005; and 23 subjects; ****p < .001. (e and f) Western analysis of P2X7 expression in platelet and EV lysates. The major P2X7 band migrates at 68–70 kDa with a lower molecular weight band thought to be deglycosylated receptor monomers. Additional lower MW bands present in PLT2, WBC2, and MP1 probably represent a splice isoform. Loading controls are not shown as there are no comparisons of relative amounts between cells and EVs. In (f), lanes designated MP (microparticle) are synonomous with EV. (g) Both P2X and P2Y agonists release EVs from platelets with maximum release by thrombin. Gel filtered platelets were incubated in K+ Tyrodes for 15 min with thrombin (1 U/mL), ADP (10 μM), MRS2365 (10 μM) and BzATP (0.5 mM). Released EVs were enumerated by flow cytometry. Mean and standard deviation shown for three experiments on separate donors, *p < .02, **p < .005, and ****p < .001.
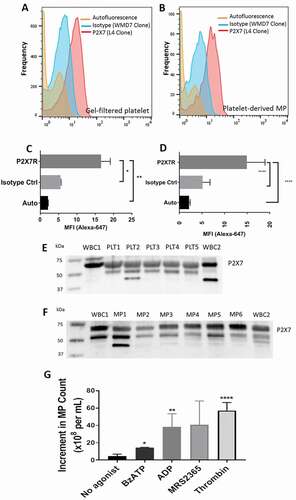
The expression of P2X7 on circulating platelets and plasma EVs of normal subjects was confirmed by western blot analysis of platelet lysates and PFP of the normal donors, which showed an immunoreactive band migrating at 68–70 kDa (). A minor immunoreactive band was consistently present at a slightly lower molecular weight, most likely representing the deglycosylated form of the receptor, and these data show that both platelets and platelet-derived EVs express P2X7 of the expected molecular size [Citation33]. No comparison was made of the relative expression of P2X7 between intact cells and their derived vesicles.
Activation of P2X7 releases extracellular vesicles from platelets
Activation of P2X7 in nucleated cells has been reported to cause blebbing and microvesiculation of the surface of the cell membrane within seconds to minutes [Citation18–21], and we examined this function in fresh human platelet rich plasma (PRP). Since ATP is only a partial agonist for P2X7 [Citation34], BzATP, a more potent and complete agonist for P2X7 was used at 0.5 mM in the following assays. BzATP produced a large number of EVs with a linear time course over 30 min from GFP suspended in Tyrode’s medium. We compared the ability of various platelet agonists to produce EVs from gel-filtered platelets suspended in Tyrode’s medium [Citation1,Citation2]. Thrombin and the P2Y1 agonists, ADP and MRS2365, produced more EVs than BzATP, and the mean number of EVs formed followed a rank order of thrombin > ADP = MRS2365 > BzATP ().
Early stage apoptotic platelets are produced by ABT737 and by BzATP but not by thrombin or ADP
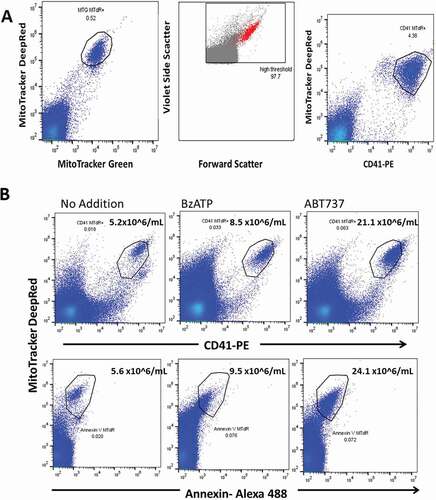
Early apoptotic change involves blebbing, microvesiculation and shrinkage of the cell, although cell shrinkage is not necessary for apoptosis to occur [Citation35]. We noted that short incubation of platelets with BzATP generated a subpopulation of platelets which failed to sediment when PRP was centrifuged at 2,500 g for 15 min (the standard procedure to remove viable platelets and obtain platelet-free plasma). To characterize this population using flow we incubated PRP with mitotracker dyes MTdR (0.5 μM) either with or without MTG (0.5 μM) prior to BzATP treatment and then after centrifugation we stained the supernate plasma with CD41 mAb. This strategy identified a homogeneous MitoTrackerdeepRed+MitoTrackerGreen+ population which included all the MitoTracker positive events () while this population was also strongly positive for platelet integrin marker CD41 which selectively stains this population (). In contrast the platelet marker CD61 stained both MitoTracker positive and MitoTracker negative EVs and CD61 was not studied further (Figure S1A). Backgating the MitoTracker+CD41+ events produced by BzATP showed that this population comprised larger events on the FCS/VSSC plot (). Numerically, the MitoTrackerRed+MitoTrackerGreen+ population did not differ significantly from the MitoTracker+CD41+ population, and this count constituted less than 1% of the total EV count. We confirmed the absence of intact viable platelets from the 2,500g supernate by obtaining a zero impedance count with a Sysmex XN Platelet Analyzer. In occasional fasting donors with high agonist-induced MitoTracker+CD41+ numbers, a trail of cell fragments (apoptosomes) from the major population was observed (Figure S1B). Apoptotic cells are often identified by their positive staining with annexin-V, and we examined this feature in platelets incubated with 0.5 mM BzATP for 60 min as well as with 1 μM ABT737 for the same time. The MitoTracker positive EVs produced by both agonists showed weak to moderate positivity for this membrane marker of apoptosis, suggesting that the MitoTracker+CD41+ events identified an early stage of the apoptotic degradation of the platelet ().
Figure 2. BzATP causes release of EVs containing functional mitochondria. (a) Early stage apoptotic platelets form a homogeneous population when identified with MitoTracker Deep Red and MitoTracker Green staining. Backgating the population within the polygon to the VSSC/FSC plot shows that early stage apoptotic platelets are among the larger of the EVs in size. The platelet integrin marker CD41 is expressed on MitoTracker positive apoptotic platelets but not on MitoTracker negative EVs. PRP was labeled with MitoTracker dyes, then incubated with BzATP (0.5 mM) for 10 min, centrifuged at 2500g for 15 min, and then supernate-labeled with CD41-PE. (b) ESAPs released from platelets by incubation with 100 nanomolar BzATP or by 1 μM ABT737 for 60 min are weakly positive for annexin V staining.
In a recent report, platelets incubated with thrombin were shown to release mitochondria-containing EVs as well as a number of free mitochondria [Citation36]. We examined the release of MitoTracker+CD41+ EVs from gel-filtered platelets incubated without stirring for 10 min with BzATP (0.5 mM) or with the pro-aggregatory agonists thrombin (1.0 U/ml) or ADP (10 uM). BzATP produced significant numbers of MitoTracker+CD41+ EVs, which were not observed with either thrombin or ADP ().
Figure 3. Early stage apoptotic platelets are produced by BzATP and by ABT737 but not by thrombin or by ADP. (a) Gel-filtered platelets were incubated with BzATP (0.5 mM) or with agonists thrombin (1.0 U/mL) or ADP (10 μM). Flow analysis of the cell-free supernate showed thatBzATP released MitoTracker+CD41+ ESAPs, which were not seen after ADP or thrombin incubations. Enumeration of ESAPs is shown above the polygon . (b) Incubation of platelets with ABT737 (1 μM) generates substantial production of MitoTracker + CD41+ ESAPs over 30–90 min incubation.
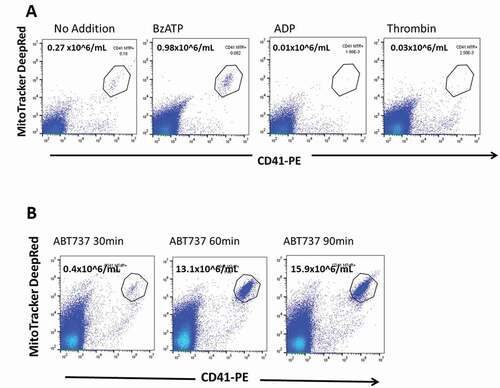
We compared this result to that from the addition of ABT737 to PRP () since the BH3 mimetic ABT737 inhibits the prosurvival molecule Bcl-xL and induces rapid apoptotic changes in a fraction of the platelet population [Citation4,Citation6] in addition to stimulating the release of AnnexinV+ EVs into the medium [Citation37]. Flow cytometric analysis of the PFP supernate after 30–90 min incubation of PRP with ABT737 showed a subpopulation of MitoTracker+CD41+ events similar in staining features, annexin V positivity and size on backgating to that seen following BzATP exposure (. We concluded that the P2X agonist, BzATP, induces a dose dependent formation of early stage apoptotic changes in a small fraction of the platelet population, which is not seen after the treatment of PRP with either ADP or thrombin ().
The agonist BzATP Gives a dose-dependent formation of early-stage apoptotic platelets
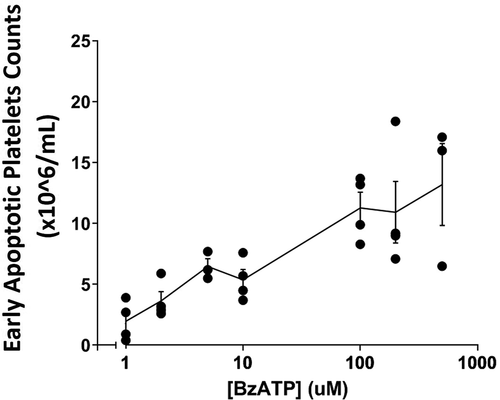
Incubation of platelet-rich plasma with agonists for the P2X7 receptor produces an accumulation of ESAPs in the ambient medium, and we chose an incubation time of 10 min to minimize loss of these particles by continued degradation to apoptosomes. The formation of early stage apoptotic platelets showed a dose-dependent response to increasing BzATP concentration between 5.0 and 500 μM with half maximal effect at values around 50–70 uM (). This value is similar to the half-maximal concentrations of agonist, which have been published for the P2X7 receptor of both native and recombinant origin [Citation38,Citation39].
Figure 4. Release of early stage apoptotic platelets from PRP is BzATP dose-dependent . PRP was labeled with MitoTracker dyes and incubated for 10 min with BzATP at concentrations between 1.0 and 500 µM. Viable platelets were removed by centrifugation and ESAPs in supernate plasma enumerated by flow cytometry. The graph shows the mean ± SD from 3 or 4 normal donors.
Nanomolar concentrations of BzATP also produces early stage apoptotic platelets
We examined the formation of apoptotic platelets in PRP incubated for 10 min with nanomolar concentrations of BzATP. Formation of early stage apoptotic platelets was seen at concentrations of 100, 200 and 500 nM BzATP, which exceeded the levels observed with either no agonist or 1 μM BzATP (). The peak production of ESAPs for each normal donor varied between 100 and 500 nM agonist, and for each subject, we observed a lesser production of apoptotic platelets at 1 μM concentration of the agonist . This anomalous dose-response was confirmed in a total of 6 fasting donors, which showed that the peak nanomolar agonist production (at either 100, 200 or 500 nM BzATP) was significantly greater than the values of apoptotic platelets with no agonist (p = .0086) or with 1 μM BzATP (p = .0202) (). The anomalous dose-response results in are consistent with a biphasic response of two platelet receptors to BzATP; first, P2X1 over the 100 nM–1 μM BzATP range (including P2X1 receptor desensitization at 1 μM) and, second, a P2X7 response at the higher agonist concentrations. also shows that BzATP acting via P2X7 (column D) added to PRP produced significantly more apoptotic platelets than by nanomolar BzATP acting via P2X1 although subject 4 was a notable exception with maximal apoptotic response to the nanomolar agonist.
The physiological agonist ATP is equipotent to bzatp in producing esaps
Incubation of platelets as PRP with ATP, the physiological agonist for P2X receptors, produced early-stage apoptotic platelets in maximal numbers at 50 nM (n = 2) or 100 nM (n = 2) agonist with values of 16.6, 7.6, 39.0 and 14.2 × 106/mL in four separate fasting donors, respectively (). In each experiment, each maximum value was followed by lower ESAP numbers at higher agonist concentration consistent with desensitization, a hallmark of the P2X1 receptor. In parallel with ATP, platelets from each donor were incubated with BzATP as agonist, which yielded maximum values of ESAPs of 24.1, 11.8, 16.8 and 9.3 × 106/mL at 100 or at 200 nM agonist.
Figure 5. The physiological agonist ATP and the P2X1 selective agonist αβ-meATP both release early stage apoptotic platelets from PRP. (a) PRP was incubated with ATP for 10 min at 37 °C and centrifuged, followed by analysis of the supernate for ESAPs. Maximum values of ESAPs are seen at 50 nM ATP. (b and c) PRP was preincubated with apyrase 0.2 U/mL for 60 min and then with αβ-meATP for 10 min at 37 °C .Strong agonist responses are seen at 200 and 500 nM agonists, which almost disappear at 1uM agonist due to receptor desensitization. (c) Agonist responses are blocked by preincubation of PRP with latrunculin A, which blocks subsequent progression to apoptosis. ESAP numbers in the supernate are expressed as number x 106/mL plasma.
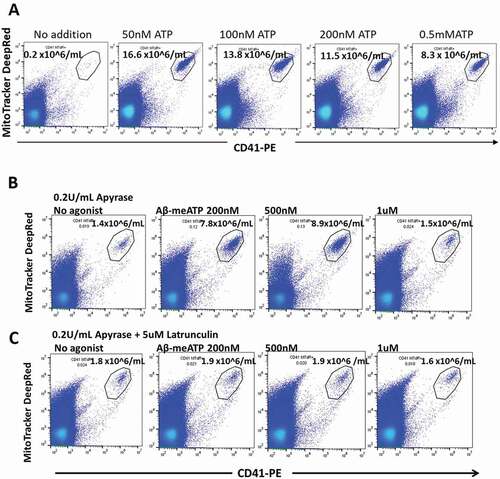
The P2X1 receptor agonist αβ-meATP produces early-stage apoptotic platelets
αβ-meATP is an effective agonist of the P2X1 receptor although with less potency than BzATP [Citation11]. Because αβ-meATP is non-hydrolysable, it is active as an agonist in the presence of apyrase and we preincubated PRP with apyrase to reverse any prexisting desensitization of P2X1. Following this preincubation of PRP with apyrase (0.2 U/mL for 60 min), we further incubated with no agonist or αβ-meATP (200 nM, 500 nM, 1 μM, and 2 μM) for 10 min. A significant increase in ESAP values was observed at 500 nM αβ-meATP compared to the no agonist condition (, p < .02, n = 5). Desensitization of the P2X1 receptor occurs rapidly at 1 μM agonist [Citation11], and our data shows that 1–2 μM agonist gave little or no increase in the production of apoptotic platelets. To ensure that the ESAP production following apyrase/αβ-meATP was P2X1-related, we included control platelets preincubated with apyrase plus latrunculin A to inhibit the apoptotic process. This increase in ESAP production observed with apyrase/αβ-meATP was latrunculin-sensitive, consistent with a previous report that latrunculin blocks P2X1 receptors [Citation40] ().
Genetic loss of P2X7 function does not impair P2X1-mediated platelet apoptosis
We studied platelets from a donor with homozygosity of a polymorphic variant of the P2X7 receptor (Glu496Ala), which results in near total loss of the P2X7 function [Citation28]. As expected, higher concentrations of BzATP (0.5 mM) sufficient to activate wildtype P2X7 failed to generate ESAPs in this subject (Figure S2). Incubation of PRP with BzATP at 100 nM gave a modest production of ESAPs consistent with an intact platelet P2X1 receptor, but this response was abolished at 500 nM BzATP concentration most likely due to receptor desensitization (Figure S2).
Inhibition of the actin cytoskeleton impairs BzATP-mediated platelet apoptosis
Disruption of the actin cytoskeleton by cytochalasin B or latrunculin A prevents the early stage of the apoptotic process [Citation7,Citation41,Citation42] and also inhibits P2X1 receptor currents [Citation40]. We studied the effect of preincubation of platelets with latrunculin A on BzATP-mediated formation of ESAPs and found variable patterns of responses (). Of five donors, three (subjects 1, 2,3) showed predominant ESAP formation with 0.5 mM BzATP, which was largely inhibited by latrunculin A. Subject 4 who was homozygous for a genetic loss of the function variant in the P2X7 receptor [Citation43] gave a major ESAP formation at 100 nM BzATP with few ESAPs from 0.5 mM BzATP. Platelets from subject 5 showed ESAP production at both 100 nM and 0.5 mM BzATP and latrunculin inhibited both ESAP responses to less than 10% of the uninhibited value (). These data show that an intact actin cytoskeleton is necessary for the formation of early stage apoptotic platelets in fresh donor PRP, as previously reported for cultured cell lines [Citation7,Citation41,Citation42]. Our data also highlighted the variability between donor platelets in their response to the potent P2X agonist, BzATP, with variable patterns of the apoptotic responses defined by the latrunculin-sensitive component in platelet ESAP numbers. highlights these different patterns of ESAP production to BzATP and shows that high numbers of ESAPs can be produced either from the nanomolar agonist (from P2X1) or from 0.5 mM agonist (from P2X7) although the contribution of each receptor to ESAP production appears to vary independently of each other.
Table III. Effect of lipid raft inhibitors on the production of ESAPs by brief incubation of PRP with BzATP
Disruption of platelet lipid rafts blocks BzATP-mediated platelet apoptosis
Depletion of plasma membrane cholesterol by preincubation of platelets with methyl-beta-cyclodextrin reduces P2X1-mediated calcium responses by 80% [Citation44], highlighting the major role of lipid rafts in P2X1 channel function. Preincubation of platelets from five donors with methyl-beta-cyclodextrin markedly reduced the major number of ESAPs produced by either 0.5 mM BzATP (subjects 1, 2, 3 and 5) or the major ESAP response observed with 100 nM BzATP (subjects 4 and 5). (). Comparison of the inhibition in ESAP numbers due to latrunculin with the reduction in ESAPs due to methyl-beta-cyclodextrin showed that both antagonists exerted an equal inhibition on the P2X mediated release of ESAPs, albeit by different mechanisms. The data of subject 5 is striking in that BzATP produces large numbers of ESAPs at both 100 nM and at 0.5 mM agonists and, at both concentrations, the ESAPs produced come from P2X receptors colocated within the lipid rafts of blood platelets.
Discussion
Early-stage apoptotic platelets are produced by ABT737 and by P2X7 receptor agonists
Our data has identified an early-stage apoptotic platelet with features similar to those described in a study of apoptosis induced in cultured cell lines by FasL and UV irradiation [Citation7,Citation35]. This early stage of platelet apoptosis constituted a homogeneous flow population of altered platelets containing mitochondria and expressing the platelet integrin CD41 but which fail to sediment when intact platelets were removed by centrifugation. These MitoTracker+ CD41+ EVs were less than 1% of the total EVs present in the supernate but contained all of the mitochondria present in the cell-free plasma. Notably, the MitoTracker+ CD41+ EVs were absent from plasma after incubation of PRP with agonists thrombin or ADP. Incubation of PRP with the BH3 mimetic ABT737, which activates the intrinsic pathway of platelet apoptosis, generated an EV particle in plasma that was similar to that produced by BzATP (). Both agonists BzATP and ABT737 produced a large EV, which was MitoTracker+ CD41+, and had modest positivity to Annexin V staining. However, as shown by Wei and Harper for the EV particle produced by ABT737, at least 60 min of apoptotic stimulus was required for moderate Annexin V positivity to develop [Citation37] . Our data showing similar features of the MitoTracker+ CD41+ Annexin+ particles produced from incubation of platelets with either ABT737 or with BzATP provides strong evidence on P2X-receptor agonists triggering an extrinsic pathway leading to platelet apoptosis.
Further evidence that ESAPs are an early intermediate particle on a pathway of apoptosis is provided by the inhibitory effect of latrunculin A, which like cytochalasin B [Citation7,Citation41,Citation42] blocks P2X1 receptor currents [Citation40] and suggests a significant role of P2X1 in platelet apoptosis. Furthermore, our preliminary experiments on washed platelets from three subjects with robust production of ESAPs when incubated with 100 or 200 nM BzATP also showed a major reduction of ESAP numbers in paired samples preincubated for 30 min with 10 uM Q-VD-Oph, a pan caspase inhibitor with antiapoptotic properties [Citation45].
Nanomolar BzATP is a selective agonist for P2X1 receptors in producing ESAPs
We investigated the dose-dependence of ESAP formation over a range of BzATP concentrations as this agonist has high potency at both P2X1 and P2X7 receptors [Citation34,Citation46]. While BzATP gave a dose-dependent formation of ESAPs at concentrations consistent with a P2X7 receptor effect, we unexpectedly noted a production of early stage apoptotic platelets in PRP incubated with nanomolar concentrations of BzATP (). This effect appeared to be mediated by the activation of the P2X1 receptor, an ion channel that is known to be highly expressed on the human platelet and is known to respond to nanomolar agonist levels [Citation9,Citation11]. Several observations support the involvement of P2X1 in stimulating early stage platelet apoptosis at nanomolar agonist levels. First, ESAP production was observed when platelets were incubated with αβ-meATP (200–500 nM), an agonist that activates P2X1 but not P2X7 receptors [Citation11]. Second, the formation of ESAPs at 100–500 nM BzATP was significantly higher than ESAP production at 1 μM BzATP levels (), consistent with agonist-induced desensitization, which is a hallmark of P2X1 [Citation33]. Finally, in a patient with loss of P2X7 function (homozygosity for Glu496Ala), we observed robust ESAP production with 100–200 nM BzATP but with far lower ESAP values at 0.5 mM BzATP (Figure S2). Previous studies have commented on the considerable variability in P2X1 responses, which may in part be due to the varying extent of receptor desensitization [Citation9,Citation10]. Our data in also shows wide variation in the extent of ESAP formation when produced either from nanomolar (P2X1) or millimolar (P2X7) agonists. We explored the inhibitory effect of NF449 on the production of ESAPs by 100 nanomolar BzATP [Citation47]. This inhibitor at 100 nM blocked half of the ESAP production but also blocked more than half of the ESAPs produced by 0.5 mM BzATP, suggesting low selectivity for the P2X subtype located in a lipid raft environment (see below).
Raft-associated P2X1 and P2X7 mediate ESAP release
Lipid rafts in the plasma membrane are rich in cholesterol and sphingolipids and are known for their role in facilitating downstream signaling following receptor activation. There is strong evidence that P2X1 receptors are localized to the lipid-rich rafts of the cell membrane and that the function of P2X1 is affected by raft disruption [Citation48]. In platelets, only some 20% of the total platelet P2X1 is co-associated with flotillin-2, a lipid raft marker, although removal of cholesterol by filipin or cyclodextrin reduces P2X1-mediated calcium fluxes by 80% [Citation48,Citation49]. Our data shows that preincubation of platelets with cyclodextrin inhibited the subsequent formation of ESAPs by 64–98% of the maximum formation attained for either 100 nM or 0.5 mM BzATP. This data confirms the signaling role of both P2X1 and P2X7 in early apoptosis and shows that the major fraction of P2X7 and most of functional P2X1 are raft-localized in the platelet. Data from other groups has shown that P2X7 may be localized in lipid rafts in primary macrophages, lung alveolar epithelial cells and submandibular gland epithelial cells [Citation50–52], and our data adds blood platelets to this list. Activation of P2X7 can stimulate phospholipase A2 activity whose enzymatic products arachidonic acid and lysophosphatidylcholine can accumulate in lipid rafts and may increase the potency of P2X7 agonists by 30–100 fold [Citation53,Citation54]. While P2X7 is recognized to induce apoptosis, our data showing the involvement of P2X1 in platelet apoptosis is a new finding although P2X1 has been implicated in a previous study of dexamethasone-induced apoptosis of immature thymocytes [Citation55]. Other work has linked P2X1 with thymic apoptosis by finding an orphan mRNA (termed RP-2) from apoptotic rat thymocytes, which exactly coded the rat P2X1 sequence [Citation56] .
Potential for ESAPs to circulate and cause pathophysiology
Elegant experiments by Dubyak and colleagues using platelets with surface attached firefly luciferase have shown that cell surface ATP may reach transient values over 10 μM following thrombin stimulation of platelets [Citation57]. Thrombin, however, does not produce ESAPs, and it is not known whether extracellular ATP in circulating blood or following blood stasis can reach the nanomolar levels in vivo sufficient to produce ESAPs from platelets. Our data shows that early stage apoptotic platelets produced by the P2X receptor activity contain functional mitochondria with intact membrane potential and we have indirect evidence that the ESAP particle is unstable and fragments readily, releasing free mitochondria. Boilard and colleagues have shown that extracellular mitochondria can interact with neutrophils in vivo, triggering neutrophil adhesion to the endothelial wall where this cell may amplify oxidative damage and increase vascular permeability [Citation36]. Our preliminary data suggests that the magnitude of the P2X-responses in blood platelets may have relevance to multiple sclerosis in which circulating extracellular microparticles (EVs) are increased in number and size during the acute relapse, a clinical event that is associated with increased vascular permeability and inflammatory plaque formation in the brain [Citation58,Citation59].
Supplemental Material
Download PDF (353.7 KB)Acknowledgements
The authors thank Dr. Vanta Jameson for technical assistance, Dr Leesa Challis for comments on the manuscript and the nursing staff of the Day Treatment Centre, Box Hill Hospital, for some blood collections.
Disclosure statement
The authors declare no competing financial interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Gilbert GE, Sims PJ, Wiedmer T, Furie B, Furie BC, Shattil SJ. Platelet-derived microparticles express high affinity receptors for factor VIII. J Biol Chem 1991;266:17261–17268. Epub 1991/ 09/15.
- Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J Biol Chem 1989;264:17049–17057. Epub 1989/ 10/15.
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 2008;9:231–241. Epub 2007/ 12/13.
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, et al. Programmed anuclear cell death delimits platelet life span. Cell 2007;128:1173–1186. Epub 2007/ 03/27.
- Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD, O’Reilly LA, Henley KJ, Ono A, Hsiao S, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 2009;114:663–666. Epub 2009/ 04/24.
- Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, Josefsson EC, Alwis I, Ono A, Willcox A, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 2011;118:1663–1674. Epub 2011/ 06/16.
- Bortner CD, Sifre MI, Cidlowski JA. Cationic gradient reversal and cytoskeleton-independent volume regulatory pathways define an early stage of apoptosis. J Biol Chem 2008;283:7219–7229. Epub 2008/ 01/12.
- Clifford EE, Parker K, Humphreys BD, Kertesy SB, Dubyak GR. The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood 1998;91:3172–3181.
- Mahaut-Smith MP, Jones S, Evans RJ. The P2X1 receptor and platelet function. Purinergic Signal 2011;7:341–356. Epub 2011/ 04/13.
- MacKenzie AB, Mahaut-Smith MP, Sage SO. Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J Biol Chem 1996;271:2879–2881.
- North RA. Molecular physiology of P2X receptors. Physiol Rev 2002;82:1013–1067. Epub 2002/ 09/25.
- Leon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett 1997;403:26–30.
- Jones S, Evans RJ, Mahaut-Smith MP. Ca2+ influx through P2X1 receptors amplifies P2Y1 receptor-evoked Ca2+ signaling and ADP-evoked platelet aggregation. Mol Pharmacol 2014;86:243–251. Epub 2014/ 06/14.
- Buell G, Michel AD, Lewis C, Collo G, Humphrey PP, Surprenant A. P2X1 receptor activation in HL60 cells. Blood 1996;87:2659–2664.
- Gu BJ, Zhang WY, Bendall LD, Chessell IP, Buell GN, Wiley JS. Expression of P2X7 purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X7 receptors. Am J Physiol Cell Physiol 2000;279:C1189–C1197.
- Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol 2001;154:485–490. Epub 2001/ 08/08.
- Brown GT, Narayanan P, Li W, Silverstein RL, McIntyre TM. Lipopolysaccharide stimulates platelets through an IL-1β autocrine loop. J Immunol 2013;191:5196–5203. Epub 2013/ 10/02.
- MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 2001;15:825–835.
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Astrocyte-derived VC. ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 2005;174:7268–7277. Epub 2005/ 05/21.
- Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, Di Virgilio F. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 2007;109:3856–3864. Epub 2006/ 12/29.
- Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J Immunol 2003;170:5728–5738. Epub 2003/ 05/22.
- Hanley PJ, Kronlage M, Kirschning C, del Rey A, Di Virgilio F, Leipziger J, Chessell IP, Sargin S, Filippov MA, Lindemann O, et al. Transient P2X7 receptor activation triggers macrophage death independent of Toll-like receptors 2 and 4, caspase-1, and pannexin-1 proteins. J Biol Chem 2012;287:10650–10663. Epub 2012/ 01/12.
- Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR. Cytolytic P2X purinoceptors. Cell Death Differ 1998;5:191–199. Epub 1999/ 04/14.
- Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol 1991;112:279–288. Epub 1991/ 01/01.
- Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett 1999;447:71–75. Epub 1999/ 04/28.
- Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, Gretener D, Grahames C, Kaur R, Kosco-Vilbois MH, et al. Blockade of human P2X7 receptor function with a monoclonal antibody Blood;1998:92:3521–3528.
- Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, Fuller SJ, Barden JA, Petrou S, Sluyter R. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem 2003;278:17108–17113. Epub 2003/ 02/15.
- Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, Barden JA, Wiley JS. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem 2001;276:11135–11142. Epub 2001/ 01/21.
- Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F. Standardization of pre-analytical variables in plasma microparticle determination: results of the international society on thrombosis and haemostasis SSC collaborative workshop. J Thromb Haemost 2013. Epub 2013. doi:10.1111/jth.12207.
- Wiley JS, Kuchibhotla J, Shaller CC, Colman RW. Potassium uptake and release by human blood platelets. Blood 1976;48:185–197.
- Radomski M, Moncada S. An improved method for washing of human platelets with prostacyclin. Thromb Res 1983;30:383–389. Epub 1983/ 05/15.
- Brittain GCT, Chen YQ, Martinez E, Tang VA, Renner TM, Langlois MA, Gulnik S. A novel semiconductor-based flow cytometer with enhanced light-scatter sensitivity for the analysis of biological nanoparticles. Sci Rep 2019;9:16039. Epub 2019/ 11/07.
- Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 1998;17:3016–3028.
- Gargett CE, Cornish JE, Wiley JS. ATP, a partial agonist for the P2Z receptor of human lymphocytes. Br J Pharmacol 1997;122:911–917.
- Bortner CD, Cidlowski JA. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J Biol Chem 2003;278:39176–39184. Epub 2003/ 06/25.
- Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, Pare A, Rousseau M, Naika GS, Levesque T, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014;124:2173–2183. Epub 2014/ 08/02.
- Wei H, Harper MT. ABT-737 triggers caspase-dependent inhibition of platelet procoagulant extracellular vesicle release during apoptosis and secondary necrosis in vitro. Thromb Haemost 2019;119:1665–1674. Epub 2019/ 09/08.
- Wiley JS, Chen R, Wiley MJ, Jamieson GP. The ATP4- receptor-operated ion channel of human lymphocytes: inhibition of ion fluxes by amiloride analogs and by extracellular sodium ions. Arch Biochem Biophys 1992;292:411–418.
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996;272:735–738.
- Lalo U, Roberts JA, Evans RJ. Identification of human P2X1 receptor-interacting proteins reveals a role of the cytoskeleton in receptor regulation. J Biol Chem 2011;286:30591–30599. Epub 2011/ 07/16.
- Cotter TG, Lennon SV, Glynn JM, Green DR. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res 1992;52:997–1005. Epub 1992/ 02/15.
- Levee MG, Dabrowska MI, Lelli JL Jr., Hinshaw DB. Actin polymerization and depolymerization during apoptosis in HL-60 cells. Am J Physiol 1996;271:C1981–1992. Epub 1996/ 12/11.
- Gu BJ, Field J, Dutertre S, Ou A, Kilpatrick TJ, Lechner-Scott J, Scott R, Lea R, Taylor BV, Stankovich J, et al. A rare P2X7 variant Arg307Gln with absent pore formation function protects against neuroinflammation in multiple sclerosis. Hum Mol Genet 2015;24:5644–5654. Epub 2015/ 07/19.
- Vial C, Evans RJ. Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J Biol Chem 2005;280:30705–30711. Epub 2005/ 07/12.
- Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 2003;8:345–352. Epub 2003/ 06/20.
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol 1999;376:127–138. Epub 1999/ 08/10.
- Hechler B, Magnenat S, Zighetti ML, Kassack MU, Ullmann H, Cazenave JP, Evans R, Cattaneo M, Gachet C. Inhibition of platelet functions and thrombosis through selective or nonselective inhibition of the platelet P2 receptors with increasing doses of NF449 [4,4ʹ,4”,4”‘-(carbonylbis(imino-5,1,3-benzenetriylbis-(carbonylimino)))tetrakis -benzene-1,3-disulfonic acid octasodium salt]. J Pharmacol Exp Ther 2005;314:232–243. Epub 2005/ 03/29.
- Allsopp RC, Lalo U, Evans RJ. Lipid raft association and cholesterol sensitivity of P2X1-4 receptors for ATP: chimeras and point mutants identify intracellular amino-terminal residues involved in lipid regulation of P2X1 receptors. J Biol Chem 2010;285:32770–32777. Epub 2010/ 08/12.
- Vial C, Fung CY, Goodall AH, Mahaut-Smith MP, Evans RJ. Differential sensitivity of human platelet P2X1 and P2Y1 receptors to disruption of lipid rafts. Biochem Biophys Res Commun 2006;343:415–419. Epub 2006/ 03/21.
- Gonnord P, Delarasse C, Auger R, Benihoud K, Prigent M, Cuif MH, Lamaze C, Kanellopoulos JM. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J 2009;23:795–805. Epub 2008/ 10/31.
- Barth K, Weinhold K, Guenther A, Young MT, Schnittler H, Kasper M. Caveolin-1 influences P2X7 receptor expression and localization in mouse lung alveolar epithelial cells. FEBS J 2007;274:3021–3033. Epub 2007/ 05/15.
- Garcia-Marcos M, Pérez-Andrés E, Tandel S, Fontanils U, Kumps A, Kabré E, Gómez-Muñoz A, Marino A, Dehaye JP, Pochet S. Coupling of two pools of P2X7 receptors to distinct intracellular signaling pathways in rat submandibular gland. J Lipid Res 2006;47:705–714. Epub 2006/ 01/18.
- Michel AD, Fonfria E. Agonist potency at P2X7 receptors is modulated by structurally diverse lipids. Br J Pharmacol 2007;152:523–537.
- Alloisio S, Aiello R, Ferroni S, Nobile M. Potentiation of native and recombinant P2X7-mediated calcium signaling by arachidonic acid in cultured cortical astrocytes and human embryonic kidney 293 cells. Mol Pharmacol 2006;69:1975–1983. Epub 2006/ 03/03.
- Chvatchko Y, Valera S, Aubry JP, Renno T, Buell G, Bonnefoy JY. The involvement of an ATP-gated ion channel, P(2X1), in thymocyte apoptosis. Immunity 1996;5:275–283. Epub 1996/ 09/01.
- Owens GP, Hahn WE, Cohen JJ. Identification of mRNAs associated with programmed cell death in immature thymocytes. Mol Cell Biol 1991;11:4177–4188. Epub 1991/ 08/01.
- Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol 1999;276:C267–278. Epub 1999/ 01/14.
- Sheremata WA, Jy W, Horstman LL, Ahn YS, Alexander JS, Minagar A. Evidence of platelet activation in multiple sclerosis. J Neuroinflammation 2008;5:27. Epub 2008/ 07/01.
- Marcos-Ramiro B, Oliva Nacarino P, Serrano-Pertierra E, Blanco-Gelaz MA, Weksler BB, Romero IA, Couraud PO, Tunon A, Lopez-Larrea C, Millan J, et al. Microparticles in multiple sclerosis and clinically isolated syndrome: effect on endothelial barrier function BMC Neurosci;2014:15:110.
