Abstract
A multi-center prospective cross-sectional and genome-wide association study (GWAS) recruited pregnant women taking low dose aspirin. Objectives were to (i) develop pregnancy-specific 95% reference intervals for a range of laboratory based platelet function tests (PFTs); (ii) select an optimal and acceptable PFT that reflected aspirin’s COX-1 inhibition in women with confirmed aspirin adherence in pregnancy; and (iii) identify genomic variants that may influence pregnant women’s platelet response to aspirin.
The study included two independent cohorts of pregnant women. A range of PFTs and matched phenotyping with urinary 11-dehydrothromboxane B2 (11DTXB2) and nuclear magnetic resonance (NMR) spectroscopy detection of urinary salicyluric acid as a measure of aspirin adherence were performed. Genome-wide data was acquired from the UK Biobank Axiom® (Thermo Fisher Scientific). 11DTXB2 in combination with adherence testing with NMR salicyluric acid was an accurate and acceptable testing strategy for detecting biochemical aspirin responsiveness in pregnant women, with the provision of relevant reference ranges. GWAS meta-analysis found no significant single nucleotide polymorphisms in association with response to aspirin in pregnancy. Further evaluation in relation to effective dosing of aspirin in pregnancy and optimizing the benefits to specific subgroups should now be a priority for future research.
© 2021 The Author(s). Published with license by Taylor & Francis Group, LLC.
Introduction
Preeclampsia is a multi-system endothelial disorder of pregnancy manifesting with hypertension and proteinuria beyond 20-week gestation [Citation1].With an incidence of 1–8%, it is associated with significant maternal and perinatal morbidity and mortality [Citation2]. Low-dose aspirin (acetylsalicylic acid) can reduce the risk of preterm preeclampsia by up to 62%, although it is unclear which women derive most benefit, with subgroup analyses suggesting variable clinical responses [Citation2,Citation3]. A proportion of women will still develop preeclampsia despite prophylactic aspirin, a phenomenon known as relative or absolute aspirin resistance or non-responsiveness, which may be responsive to an increased dosage. This phenomenon has been widely discussed in the cardiovascular literature and may be identified biochemically, with insufficient COX-mediated suppression of platelet activation [Citation4]. Some authors also define aspirin non-responsiveness clinically, as recurrence of adverse events or by combined biochemical definition and adverse clinical events [Citation5]. However, there is no unified definition and a paucity of evidence regarding aspirin non-responsiveness in pregnancy [Citation6]. A robust method of assessing for the presence of aspirin non-responsiveness in pregnancy is required [Citation6].
Acetylsalicylic acid (ASA) irreversibly inhibits the COX-1 enzyme in platelets. Following oxidation of arachidonic acid, COX-1 supplies PGH2 to TXA synthase, with subsequent production of several metabolites of which thromboxane B2 and urinary 11-dehydrothromboxane B2 (11DTXB2) are an example [Citation7]. In the pregnant state, there is increased platelet turnover which is significantly exaggerated in those at risk of preeclampsia, where TXA2 dominance causes utero-placental and systemic vasoconstriction [Citation8,Citation9]. This directly perpetuates the cycle of platelet activation, stimulating further TXA2 release, leading to the clinical manifestations of preeclampsia [Citation10–13]. It is postulated that low dose aspirin both inhibits the aforementioned process and has an anti-inflammatory effect and can thus mitigate the risk of developing preeclampsia, when commenced prior to 16-week gestation [Citation14,Citation15].
A contributory genomic basis for preeclampsia has been suggested through genome wide association (GWAS) and candidate gene studies (CGS) [Citation16], in addition, genomic determinants for the observed variability of aspirin response have been sought, and various polymorphisms described [Citation4]. In the latter group, the phenotypic endpoint of aspirin non-responsiveness tends to be that of clinical outcome as opposed to impact on platelet function [Citation4]. There are a paucity of genomic studies conducted with pregnant cohorts, which assess the variability in response to aspirin, and none which utilize platelet function as an endpoint, or assess populations with differing baseline risks of preeclampsia [Citation17]. Further to this, there are no available reference ranges based upon sufficiently large populations for platelet function tests (PFTs) [Citation6]. Hence the objectives of this study were to (i) develop pregnancy-specific 95% reference intervals for a full range of laboratory based, non-point-of-care PFTs; (ii) select an optimal and acceptable PFT that reflects aspirin’s COX-1 inhibition in women with confirmed aspirin adherence in pregnancy; and (iii) to identify genomic variants that may influence pregnant women’s platelet response to aspirin.
Methods
The program of work included two stages; first, a cross-sectional study to establish reference intervals and select a PFT which is acceptable to women and reflects aspirin’s platelet effects and develop an accurate low dose aspirin (LDA) adherence test (cohort phenotyping). Second, a meta-analysis of genome-wide association studies from two independent cohorts of pregnant women taking LDA to prevent preeclampsia. We included two cohorts of pregnant women, one with and one without risk factors for preeclampsia from University of Liverpool and cohort at low risk from a published randomized controlled trial (RCT) in University College Dublin, respectively. The data presented from the later cohort represent a secondary analysis from this RCT [ISRCTN 15191778] [Citation18]. Gestational hypertension and preeclampsia were defined for both populations in with theInternational Society for the Study of Hypertension in Pregnancy criteria [Citation1].
Reference intervals for platelet function tests in pregnancy
We conducted a prospective time-limited cross-sectional study in Liverpool Women’s Hospital NHS Foundation Trust between October 2012 and April 2014. The study was approved by the Liverpool Research Ethics Committee. Construction of reference intervals are described in supplementary methods. Pregnant women were provided information and recruited between 11 ± 0 and 20 ± 6 weeks, at antenatal clinics. They donated blood and urine samples on one occasion during pregnancy for PFTs and LDA adherence testing. A broad range of PFTs including point-of-care devices and widely used laboratory measures cited in the current literature were assessed [Citation4]. None had established reference intervals or a significant evidence-base in pregnant populations [Citation6]. PFTs included;
Urinary 11-dehydrothromboxane B2 (11DTXB2) (Randox laboratories)
Platelet Solutions ltd test [Citation19,Citation20]
Multiplate™ impedance platelet aggregometry ASPItest (Roche)
Multiplate™ impedance platelet aggregometry ADPtest (Roche)
PFA 100 with collagen/epinephrine test cartridges (Dade Behring)
PFT results from blood and urine produced by each participant with a normal pregnancy outcome were used to calculate reference intervals for each test for women taking aspirin and aspirin naïve women. Pregnancy outcomes were determined from patient records. Platelet function results from women who had adverse pregnancy outcomes including; gestational hypertension or preeclampsia, placental abruption, miscarriage after 12-week gestation of a structurally normal fetus, birthweight <10th customized centile and perinatal death were excluded (composite adverse outcome). Overall, 95% reference intervals were constructed adhering to recommended approaches, using R version 3.3.2 [R Core Team 2018 URL: https://www.R-project.org/]. Where necessary, data were transformed and outliers were removed (Tukey method [Garren 2018 URL: https://CRAN.R-project.org/package=jmuOutlier]). Quantile regression was applied to assess the need for partitioning of the reference population [Koenker 2018 URL: https://CRAN.R-project.org/package=quantreg]. The 2.5th and 97.5th quantiles were referred to, to calculate 95% reference intervals and 90% confidence intervals (CI) for the upper limit of the interval for each test.
Low dose aspirin adherence testing
LDA’s short half-life and rapid clearance hinder detection and quantification of drug levels in maternal body fluids and are impractical for clinical research. Salicyluric acid (SUA) is the stable urinary metabolite of salicylic acid produced during LDA metabolism. Through a collaboration with experts in nuclear magnetic resonance (NMR), we developed an accurate, semi-automated method for detection of SUA in maternal urine samples of LDA-treated pregnant women. We demonstrated no specific sample preparation is required; SUA is stable at room temperature and through successive freeze-thaw cycles. SUA can readily and reproducibly be detected by NMR using ChenomxTM pattern recognition software (Chenomx, Canada) and expert review. This method has since been used for adherence assessment in clinical research [Citation21]. In an attempt to determine patient acceptability with regards investigative methods of aspirin adherence testing, we assessed the findings from an anonymous questionnaire at 20–22 weeks’ gestation from the low-risk Dublin cohort.Citation18
Genome-wide association meta-analysis
Finally, both cohorts underwent matched phenotyping with 11DTXB2 to assess the platelet effects of LDA and NMR detection of urinary SUA as a measure of LDA adherence. Genome-wide data was acquired from the UK Biobank Axiom® (Thermo Fisher Scientific). Full methods for DNA extraction, genome-wide genotyping, data quality control, phasing and imputation are contained in supplementary methods. Frequentist association analysis of the individual cohorts genome-wide data was conducted. This was followed by meta-analysis completed using METAL software [Willer 2010 URL: http://csg.sph.umich.edu/abecasis/publications/pdf/Bioinformatics.vol.26-pp.2190.pdf]. The GWAS datasets generated and analyzed during this study are available in the European Genome-phenome Archive (EGA) repository [URL: https://ega-archive.org/studies/EGAS00001005188].
Results
Reference intervals
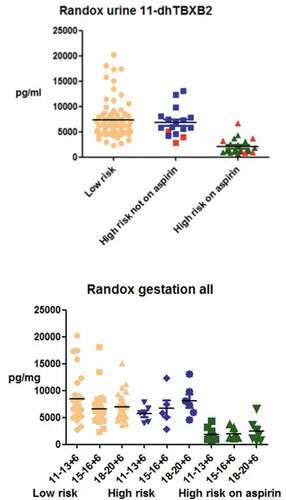
Over a period of 1.5 years, 273 women were recruited in Liverpool (N = 126) and Dublin (N = 147). The final cohort represented those who provided written informed consent to analysis of their blood and urine for the purposes of the study and for whom results are available. Composite adverse outcomes were determined in 29 women considered to have a low-risk pregnancy at enrollment, three in high-risk women who were not taking aspirin and in six women at high risk of preeclampsia who were on aspirin. Reference intervals for platelet activity assays in pregnant women taking LDA, are demonstrated in .
Table I. Reference intervals for platelet activity assays in pregnant women taking low-dose aspirin. (Separate intervals were derived for Randox for pregnant women at high risk of preeclampsia versus those at low risk, from the Liverpool and Dublin cohorts, respectively).
Selection of platelet function test and incorporation of adherence testing for cohort phenotyping
There was considerable variation in the findings from PFTs in our pregnant cohort, overall (. We chose to progress 11DTXB2 forward into cohort phenotyping as it reflects the direct action of aspirin against platelet COX-1 and in our dataset, was the most reliable differentiator between aspirin-treated and naïve platelet function (, Figure S3). This characterization was enhanced by the addition of urinary aspirin metabolite testing to interpret Randox in-light-of a reliable adherence measure. Both Randox and metabolite testing could be accurately performed on small urine samples which are noninvasive and acceptable to pregnant women. Of 406 women who completed an acceptability questionnaire, 1.7% (n = 7) found blood testing inconvenient, while 0.2% (n = 1) found providing a urine sample inconvenient. Both 11DTXB2 and SUA are stable and the tests ease integration into large clinical studies. The reference ranges for the different PFTs are demonstrated in .
GWAS meta-analysis
Meta-analysis of the two cohorts (n = 182 women) did not yield any genome-wide significant findings though several hits reached suggestive threshold p < 1 x 10-5 (). SNP signal identified at chromosome 4 did not map to a gene region and no variant consequence information was available. The next strongest genetic association was identified on chromosome 3 (rs62246396, p = 8.26E-07), an intronic variant of SETD2 (SET domain containing 2, histone lysine methyltransferase) (); Table S6).
Figure 2. GWAS meta-analysis findings of Liverpool and Dublin pregnancy cohorts using METAL software (Willer et al., 2010). a) Manhattan plot urinary 11-dehydrothromboxane B2 measures of aspirin compliant patients (n = 182). The top red threshold indicates genome-wide significance threshold of p < 5 x 10-8, the lower blue threshold indicates suggestive threshold p < 1 x 10-5. R package, qqman, was utilized to produce this Manhattan plot (Turner, 2018). Regional plots generated using LocusZoom (Pruim et al., 2010): b) chromosome 3 (rs62246396, SETD2); c) chromosome 1 (rs1749777, DAB1) indicating multiple SNPs in linkage disequilibrium; d) chromosome 14 (rs7143033, SLC25A21); and e) chromosome 7 (rs10156113, CACNA2D1) with several SNPs in linkage disequilibrium.
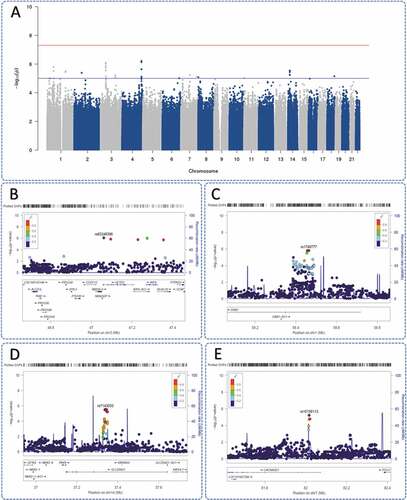
SNP rs1749777 (p = 1.57E-06) was identified in DAB1 (); Table S6). Rs7143033 (p = 2.91E-06) mapped to SLC25A21 (solute carrier family 25 member 21) (); Table S6) and indicated several SNPs in linkage disequilibrium, similar to rs10156113 (p = 5.82E-06) in CACNA2D1 (calcium voltage-gated channel auxiliary subunit alpha 2 delta 1) gene region (); Table S6).
Rs1749777 was found in pre-imputed GWAS data of both cohorts (Figure S8(a); Figure S9(a)). A further two pre-imputed SNPs, rs1408966 and rs7157702, were located in the genetic signal at SLC25A21.
Discussion
This prospective study found that urinary 11DTXB2 in combination with adherence testing with NMR salicyluric acid was an accurate and acceptable objective testing strategy for detecting biochemical aspirin responsiveness in pregnant women, with the provision of relevant reference ranges. GWAS meta-analysis of our carefully phenotyped cohorts found no significant SNPs in association with response to LDA in pregnancy, although we identified potential hits in SETD2, DAB1, CACNA2D1, and SLC25A21.
In non-pregnant populations, it has been historically established that there is limited correlation between findings from different PFTs 22,22,[Citation22, Citation23]. With respect to our own findings in a pregnant population, this was no different. The reasoning behind selection of urinary 11DTXB2 as the optimal PFT in pregnancy is fivefold; (i) it is a direct end product of cyclooxygenase catalyzed metabolism of arachidonic acid, inhibited by LDA; hence as it features within the aspirin pathway, it is biologically plausible to analyze; (ii) it is biologically stable and highly detectable in urine [Citation23]; (iii) urine sampling is a noninvasive and acceptable test for patients; (iv) it correlates significantly with aspirin responsiveness as we have demonstrated; and (v) it has been validated previously as an assessment of aspirin-responsiveness in relation to cardiac events [Citation24].
Previous GWAS and candidate gene studies performed in non-pregnant populations propose only a partial genetic basis for aspirin responsiveness, with identified polymorphisms including those in cyclooxygenase-1, glycoprotein, cytochrome P450, thromboxane, and platelet-receptor genes, such as those of the platelet endothelial aggregation (PEAR-1) and purinergic (P2Y1) receptors [Citation24–28]. However, the phenotypic impact of their presence has been variable [Citation24–28]. The potential clinical impact of knowledge of such polymorphisms is development of genotype-guided aspirin therapy; however, there are considerable gaps in the existing literature. Many studies have not assessed aspirin exclusively and assessment in the pregnant population is scanty, although several candidate genes which reflect platelet response to aspirin have been identified, notably on the PBX1 and MMD genes [Citation17,Citation29]. The findings from our own GWAS concur with existing research in suggesting that aspirin responsiveness has a multifactorial basis within the pregnant population. In assessment of the potential hits identified, the SETD2 domain has been associated with hematopoietic disease, with knockout associated with an increased turnover of platelets phenomenon associated with aspirin non-responsiveness [Citation30]. The DAB1-p38 signaling pathway has been proposed as a novel candidate therapeutic target to decrease angiotensin-II induced podocyte apoptosis in chronic kidney diseases with associated proteinuria and hypertension in rats linked to upregulation of this pathway [Citation31]. Such potential polymorphisms found in our study have not been described previously in relation to aspirin-responsiveness, although the mechanisms of action of SETD2 and DAB1require further exploration.
Qualitative and semi-quantitative methods of assessing LDA adherence, including questionnaires and pill counts have limited reliability [Citation32]. The method of using the more objective assessment of NMR salicyluric acid in combination with urinary 11DTXB2 ensured more robust phenotyping of cases for the GWAS meta-analysis. This method was both novel in relation to both the pregnant and in the non-pregnant aspirin taking population. We believe reliance on an objective endpoint optimized the strength of the GWAS performed. Through assessing patient acceptability around providing urine and blood samples for testing, we robustly demonstrated the feasibility of using our proposed methods to determine aspirin responsiveness and moving forward further studies may utilize this evidence to support such an approach to testing. A further strength of our study was the use of two different pregnant cohorts with genomic similarities but variable baseline clinical risk profiles, which consistently demonstrated reproducibility in findings. This is the first study to our knowledge, which provides reference ranges in relation to aspirin responsiveness for a variety of PFTs in pregnancy.
While GWAS was the most appropriate assessment for our cohort in relation to pharmacotherapeutic effect, it does pose limitations in relation to the high participant numbers required to reach a greater statistical power, which our study has fallen short of. More broadly, mny questions remain unanswered as to the optimal use of LDA in pregnancy, including; the etiology of preeclampsia, which subgroups may derive the greatest benefit, what dosing schedules should be used and the most effective timing of administration [Citation33,Citation34]. While there is some argument for universal LDA provision in pregnancy, this would not be without risk and an associated increased incidence of bleeding [Citation35].
In conclusion, Our GWAS meta-analysis of low-dose aspirin-treated pregnant women, phenotyped with platelet function tests with adherence confirmed by LDA metabolite testing did not identify any genome-wide significant SNPs associated with aspirin responsiveness. Potentially relevant SNPs were identified in four genes with biological plausibility and these may merit further examination. However, our work to date indicates that when LDA’s COX suppression is accurately assessed in a confirmed adherent population, true aspirin non-responsiveness is not demonstrated. This has some implications for research into aspirin non-responsiveness in other specialty areas. Unanswered clinical questions around the most effective dosing in relation to administration of aspirin and optimizing benefits to specific subgroups should be the principal priorities for further research.
Supplementary_results_250821.pdf
Download PDF (1 MB)Supplementary_methods_051121.docx
Download MS Word (51 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Brown MA, Lindheimer MD, de Swiet V, van Assche A, Mountquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001;20:9–143. doi:10.1081/PRG-100104165.
- Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P, Tsatsaris V . Aspirin for prevention of preeclampsia. Drugs 2017;77(17):1819–1831. doi:10.1007/s40265-017-0823-0.
- Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;377(7):613–622. doi:10.1056/NEJMoa1704559.
- Fitzgerald R, Pirohamed M. Aspirin resistance: effect of clinical, biochemical and genetic factors. Pharmacol Ther 2011;130(2):213–225. doi:10.1016/j.pharmthera.2011.01.011.
- Bhala N, Emberson J, Merhi A, Abramson S, Arber, N, Baron, JA, Bombardier, C, Cannon, C, Farkouh, ME, FitzGerald, GA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–779.
- Navaratnam K, Alfirevic A, Alfirevic Z. Low dose aspirin and pregnancy: how important is aspirin resistance?. BJOG 2016;123(9):1481–1487. doi:10.1111/1471-0528.13914.
- Patrono C, Coller B, Dalen JE, Fuster V, Gent M, Harker LA, Hirsh J, Roth G . Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest 1998;114:470s–488s. doi:10.1378/chest.114.5_Supplement.470S.
- Hutt R, Ogunniyi SO, Sullivan MH, Elder MG. Increased platelet volume and aggregation precede the onset of preeclampsia. Obstet Gynecol 1994;83:146–149.
- Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol 1985;152(3):335–340. doi:10.1016/S0002-9378(85)80223-4.
- Schiff E, Peleg E, Goldenberg M, Rosenthal T, Ruppin E, Tamarkin M, Barkai G, Ben-Baruch G, Yahal I, Blankstein J, et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N Eng J Med 1989;321(6):351–356. doi:10.1056/NEJM198908103210603.
- Sibai BM, Mirro R, Chesney CM, Leffler C. Low-dose aspirin in pregnancy. Obstet Gynecol 1989;74:551–557.
- Spitz B, Magness RR, Cox SM, Brown CEL, Rosenfeld CR, Gant NF . Low-dose aspirin. I. Effect on angiotensin II pressor responses and blood prostaglandin concentrations in pregnant women sensitive to angiotensin II. Am J Obstet Gynecol 1988;159(5):1035–1043. doi:10.1016/0002-9378(88)90406-1.
- Thorp JA, Walsh SW, Brath PC. Low-dose aspirin inhibits thromboxane, but not prostacyclin, production by human placental arteries. Am J Obstet Gynecol 1988;159:1381–1384. doi:10.1016/0002-9378(88)90560-1.
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest J-C, Giguère Y . Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 2010;116(2):402–414. doi:10.1097/AOG.0b013e3181e9322a.
- Lindsay D, Levine LD, Holland TL, Kim K, Mumford SL, Schisterman EF . The role of aspirin and inflammation on reproduction: the EAGeR trial. Can J Physiol Pharmacol 2019;97(3):187–192. doi:10.1139/cjpp-2018-0368.
- Giannakou K, Evangelou E, Papathedorou SI. Genetic and non-genetic risk factors for preeclampsia: umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet Gynecol 2018;51:720–730. doi:10.1002/uog.18959.
- Eudy AM, Voora D, Myers RA, Clowse MEB. Effect of aspirin response signature gene expression on preterm birth and preeclampsia among women with lupus: a pilot study. Lupus 2019;28(14):1640–1647. doi:10.1177/0961203319886069.
- Mone F, Mulcahy C, McParland P, Breathnach F, Downey P, McCormack D, Culliton M, Stanton A, Cody F, Morrison JJ, et al. Trial of feasibility and acceptability of routine low-dose aspirin versus Early Screening Test indicated aspirin for preeclampsia prevention (TEST study): a multicentre randomised controlled trial. BMJ Open 2018;8:e022056. doi:10.1136/bmjopen-2018-022056.
- Fox SC, May JA, Shah A, Neubert U, Heptinstall S. Measurement of platelet P-selectin for remote testing of platelet function during treatment with clopidogrel and/or aspirin. Platelets 2009;20:250–259. doi:10.1080/09537100902912451.
- Dovlatova N, May JA, Fox SC. Remote platelet function testing–Significant progress towards widespread testing in clinical practice. Platelets 2015;26:399–401. doi:10.3109/09537104.2015.1043880.
- Navaratnam K, Alfirevic A, Jorgensen A, Alfirevic Z. Aspirin non-responsiveness in pregnant women at high-risk of preeclampsia. Eur J Obstet Gynecol Reprod Biol 2018;221:144–150. doi:10.1016/j.ejogrb.2017.12.052.
- Grove EL, Hvas A-M, Johnsen HL, Hedegaard SS, Pedersen SB, Mortensen J, Kristensen SD . A comparison of platelet function tests and thromboxane metabolites to evaluate aspirin response in healthy individuals and patients with coronary artery disease. Thromb Haemost 2010;103(6):1245–1253. doi:10.1160/TH09-08-0527.
- Cattaneo M. Laboratory detection of ‘aspirin resistance’: what test should we use (if any)?. Eur Heart J 2007;28:1673–1675. doi:10.1093/eurheartj/ehm232.
- Pandey CP, Misra A, Negi MS, Kanuri BN, Chhonker YS, Bhatta R, Narain V, Dikshit M . Aspirin & clopidogrel non-responsiveness & its association with genetic polymorphisms in patients with myocardial infarction. Indian J Med Res 2019;150(1):50–61. doi:10.4103/ijmr.IJMR_782_17.
- Lewis JP, Riaz M, Xie S, Polekhina G, Wolfe R, Nelson M, Tonkin AM, Reid CM, Murray AM, McNeil JJ, et al. Genetic variation in PEAR1, cardiovascular outcomes and effects of aspirin in a healthy elderly population. Clin Pharmacol Ther 2020;108:1289–1298. doi:10.1002/cpt.1959.
- Ferreira M, Freitas-Silva M, Assis J, Pinto R, Nunes JP, Medeiros R . The emergent phenomenon of aspirin resistance: insights from genetic association studies. Pharmacogenomics 2020;21:125–140. doi:10.2217/pgs-2019-0133.
- Liu WW, Wang H, Chen XH, Fu SW, Liu ML. miR-34b-3p may promote antiplatelet efficiency of aspirin by inhibiting thromboxane synthase expression. Thromb Haemost 2019;119:1451–1460. doi:10.1055/s-0039-1692681.
- Massimi I, Alemanno L, Guarino ML, Guerriero R, Mancone M, Ceccacci A, Frati L, Angiolillo D, Pulcinelli F . aspirin-dependent effects on purinergic P2Y1 receptor expression. Thromb Haemost 2019;119(5):726–734. doi:10.1055/s-0039-1678707.
- Lewis JP. Implementation of genotype-guided antiplatelet therapy: feasible but not without obstacles. Circ Genom Precis Med 2018;11:e002118. doi:10.1161/CIRCGEN.118.002118.
- Zhou Y, Yan X, Feng X, Bu J, Dong Y, Lin P, Hayashi Y, Huang R, Olsson A, Andreassen PR, et al. Setd2 regulates quiescence and differentiation of adult hematopoietic stem cells by restricting RNA polymerase II elongation. Haematologica 2018;103(7):1110–1123. doi:10.3324/haematol.2018.187708.
- Gao Z, Chen X, Zhu K, Zeng P, Ding G. Dab1 contributes to angiotensin II-induced apoptosis via p38 signaling pathway in podocytes. Biomed Res Int 2017;2484303. doi:10.1155/2017/2484303.
- Navaratnam K, Alfirevic Z, Pirmohamed M, Alfirevic A. How important is aspirin adherence when evaluating effectiveness of low-dose aspirin?. Eur J Obstet Gynecol Reprod Biol 2017;219:1–9. doi:10.1016/j.ejogrb.2017.10.004.
- Shanmugalingam R, Wang X, Munch G, Fulcher I, Lee G, Chau K, Xu B, Kumar R, Hennessy A, Makris A, et al. A pharmacokinetic assessment of optimal dosing, preparation, and chronotherapy of aspirin in pregnancy. Am J Obstet Gynecol 2019;221:255.e1–255.e9. doi:10.1016/j.ajog.2019.04.027.
- Mone F, Mulcahy C, McParland P, McAuliffe FM. Should we recommend universal aspirin for all pregnant women?. Am J Obstet Gynecol 2017;216:141.e1–141.e5. doi:10.1016/j.ajog.2016.09.086.
- Hastie R, Tong S, Wikstrom A-K, Sandstrom A, Hesselman S, Bergman L. Aspirin use during pregnancy and the risk of bleeding complications: a Swedish population-based cohort study. Am J Obstet Gynecol 2021;224(1):95.e1–95.e12. doi:10.1016/j.ajog.2020.07.023.