Abstract
C-type lectin-like receptor 2 (CLEC-2) is a platelet-activated receptor expressed on the surface of platelet membranes. Soluble CLEC-2 (sCLEC-2) has been receiving attention as a predictive marker for thrombotic predisposition. The present study examined the relationship between sCLEC-2 level and degree of coagulation disorder in septic patients. Seventy septic patients were divided into the sepsis-induced disseminated intravascular coagulation (DIC) (SID) group (n = 44) and non-SID group (n = 26). The sCLEC-2 levels were compared between the two groups. Because we suspected that the sCLEC-2 level was affected by the platelet count, we calculated the sCLEC-2/platelet count ratio (C2PAC index). We further divided septic patients into four groups using the Japanese Association for Acute Medicine (JAAM) DIC scoring system (DIC scores: 0–1, 2–3, 4–5, and 6–8). The C2PAC index was significantly higher in the SID group (2.6 ± 1.7) compared with the non-SID group (1.2 ± 0.5) (P < .001). The C2PAC indexes in the four JAAM DIC score groups were 0.9 ± 0.3, 1.1 ± 0.3, 1.7 ± 0.7, and 3.6 ± 1.0, respectively, and this index increased significantly as the DIC score increased (P < .001). According to the receiver-operating curve analysis, the area under the curve (AUC) and optimal cutoff value for the diagnosis of SID were 0.8051 and 1.4 (sensitivity, 75.0%; specificity, 76.9%), respectively. When the C2PAC index and D-dimer level, one of the main fibrinolytic markers, were selected as predictive markers for SID diagnosis in stepwise multiple logistic regression analysis, it was possible to diagnose SID with a high probability (AUC, 0.9528; sensitivity, 0.9545; specificity, 0.8846). The C2PAC index is a useful predictor of SID progression and diagnosis in septic patients.
Introduction
Platelets are involved in both hemostasis and immune responses. These mechanisms work together in a complex and synchronous manner, making the contribution of platelets to sepsis of major importance [Citation1]. The traditional roles of platelets in the circulation are to help maintain primary hemostasis and blood flow within vessels by initial clot or thrombus formation when a vascular insult or injury occurs and to cause an occlusive thrombus under pathological conditions, such as arteriosclerosis or dyslipidemia. Thrombus formation is also caused by platelet activation. Therefore, an in vivo biomarker for platelet activation is useful for the prediction or diagnosis of various thrombotic diseases, such as hypertension, atherosclerosis, inflammation, type 2 diabetes mellitus, cardiovascular diseases, and ischemic stroke [Citation2,Citation3].
Recently, another potential role for platelets has been proposed that is independent of hemostasis or thrombosis. This role is related to immunity. Participation of neutrophils, monocytes, and dendritic cells leads to a “thrombosis-related signature” that initiates and propagates fibrin formation and triggers platelet activation during thrombosis. Recent works have mentioned a phenomenon termed “immunothrombosis.” This phenomenon suggests that under certain circumstances, thrombosis is a physiological process that constitutes an effective mechanism within innate immunity in which platelets play an important part [Citation1]. Therefore, to understand the severity of sepsis, it may be important not only to track the changes in the platelet count, but also to determine the platelet activation status.
Several biomarkers have been proposed as useful markers for platelet activation, including P-selectin, platelet factor 4, β-thromboglobulin, soluble glycoprotein VI, and platelet microparticles [Citation2,Citation4]. All of these markers indicate that platelet activation may be an important step in the development and progression of diseases. However, these markers have the disadvantage of being easily released during the minimal platelet activation that occurs during sampling, and thus no efficient markers have been developed to date [Citation5].
C-type lectin-like receptor 2 (CLEC-2) is one of the platelet-activated receptors expressed on the surface of platelet membranes [Citation6–8]. Anticoagulants (EDTA, citrate, and CTAD) have negligible effects on the soluble CLEC-2 (sCLEC-2) enzyme-linked immunosorbent assay (ELISA) [Citation9]. Additionally, the standard blood collection procedures used in daily clinical laboratory tests are sufficient for sCLEC-2 analysis [Citation9]. Recent studies have reported that sCLEC-2 is elevated in patients with cardiovascular diseases [Citation5,Citation10], ischemic stroke [Citation11,Citation12], blunt traumatic brain injury [Citation13], and thrombotic microangiopathy [Citation14,Citation15] compared with healthy volunteers with a mean plasma sCLEC-2 level of 59.1–99.8 pg/mL [Citation5,Citation12,Citation15]. The reported cutoff plasma sCLEC-2 level to identify acute coronary syndrome is 274.6 pg/mL (sensitivity, 0.871; specificity, 0.553) [Citation5], and the median (25th–75th percentile) sCLEC-2 levels of patients with acute cerebral infarction-induced disseminated intravascular coagulation (DIC) and infection-induced DIC are 256 pg/mL (182–340 pg/mL) [Citation12] and 379 pg/mL (275–798 pg/mL) [Citation15], respectively. As the plasma sCLEC-2 concentrations are significantly higher in patients with each of these diseases than in healthy volunteers, these results suggest that the sCLEC-2 level may be a useful predictive marker of the presence or absence of disease.
We previously confirmed that sCLEC-2 was generated by a CLEC-2-stimulating snake venom (rhodocytin), as well as a GPVI agonist [poly(PHG)] and thrombin (Supplementary Figure S1). These findings suggest that sCLEC-2 may be a general platelet activation marker and may be a predictive marker of thrombotic predisposition. Thrombin overproduction during DIC activates platelets, potentially leading to sCLEC-2 generation.
DIC is characterized by systemic and sustained activation of coagulation in the presence of underlying disease, resulting in the intravascular formation of microthrombi. At the same time, the consumption and subsequent depletion of platelets and coagulation factors caused by progressive thrombus formation can induce severe bleeding [Citation16]. DIC occurs in a wide variety of clinical conditions; however, infections, especially sepsis, are one of the most notable underlying diseases associated with DIC [Citation16]. Approximately 30% of patients with sepsis have coagulopathy [Citation17]. The term “sepsis-induced coagulopathy (SIC)” is often used as a synonym for DIC as defined by laboratory criteria [Citation18]. Therefore, SIC and “sepsis-induced DIC (SID)” can be regarded as the same pathological condition. Recently, DIC has been classified into three types depending on the pattern of the fibrinolytic system [Citation19,Citation20], all of which involve substantial activation of coagulation. However, although fibrinolysis is observed alongside coagulation, the degree of fibrinolytic activation varies considerably depending on the underlying disease. SID is defined as suppressed-fibrinolytic-type DIC.
To our knowledge, although some studies have measured sCLEC-2 levels in patients with DIC [Citation14,Citation15], no previous studies have evaluated the relationship between sCLEC-2 level and severity of SID. In the present study, we measured the sCLEC-2 levels in patients with sepsis with or without DIC, and examined the relationship between plasma sCLEC-2 level and degree of coagulation disorder.
Methods
This retrospective single-center observational study was conducted at the Department of Emergency and Critical Care Medicine, Fukuoka University Hospital, Fukuoka, Japan, a 915-bed referral and tertiary hospital, from April 2015 to March 2018. The study was approved by the institutional ethics committees at Fukuoka University Hospital (U19-01-001; registered on 19 January 2019), Yamanashi University Hospital (2289; registered on 17 June 2020), and LSI Medience Corporation (Tokyo, Japan) (Shindan/Narita 19–04; registered on 19 May 2019). We obtained written informed consent for the publication of this study from all patients or their proxy in accordance with the Declaration of Helsinki. Patients aged ≥18 years who were diagnosed with sepsis were enrolled in the study. The exclusion criteria were: post-cardiopulmonary arrest; liver cirrhosis (Child–Pugh grade C or above); chronic hemodialysis; pregnancy; death within 48 hours of hospitalization; continuing antibiotic use; traumatic injury; determination by the doctor in charge that entry was inappropriate. We also excluded patients who lacked any of the biomarkers of coagulation and inflammation under consideration in this study, did not have clinical scores used in predicting the clinical course, did not need hospital resources, lacked sepsis outcome data, or lacked apparent clinical manifestations. Patients were evaluated for the presence of sepsis according to the Sepsis-3 diagnosis criteria [Citation21]. The DIC scoring system of the Japanese Association for Acute Medicine (JAAM) was used for the diagnosis of DIC. The JAAM DIC diagnostic algorithm for scoring DIC includes the following variables: platelet count, prothrombin time (PT), fibrin/fibrinogen degradation product (FDP) level, and systemic inflammatory response syndrome criteria (Supplementary Tables S1 and S2). The details of the algorithm have been published elsewhere [Citation22]. DIC was defined as a score of ≥4. We also evaluated two other major DIC scoring systems: the International Society on Thrombosis and Hemostasis (ISTH) overt DIC criteria (Supplementary Table S1) [Citation23] and the Japanese Ministry of Health and Welfare (JMHW) DIC criteria (Supplementary Table S1) [Citation24]. Illness severity was evaluated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score [Citation25]. The APACHE II score assesses illness severity in critical patients admitted to an intensive care unit (ICU) on the basis of routine physiologic measurements, age, and previous health status; it is used to predict the outcomes of critical illnesses. Organ failure was assessed by the Sequential Organ Failure Assessment (SOFA) score [Citation26]. The SOFA score estimates organ dysfunction related to various disease statuses, especially sepsis, and is calculated using readily available measurements to quantify the dysfunction of the six major organs; it is also useful for evaluating the morbidity and mortality of critical illnesses.
The septic patients were divided based on the presence or absence of DIC at the time of ICU admission into a sepsis-induced DIC (SID) group and a non-SID group. The non-SID group was also divided into a late-SID group that was diagnosed with DIC within 5 days of admission and a non-diagnosed SID group that was not diagnosed with DIC during 5 days from the time of admission. We further divided the septic patients into four groups with a JAAM DIC score on ICU admission of 0–1, 2–3, 4–5, and 6–8. In addition, 37 healthy adult volunteers aged 20–60 years were included as a control group. These healthy volunteers were selected from a previously described cohort [Citation5] after excluding three individuals with incomplete datasets. The data for the healthy volunteers were provided by Yamanashi University Hospital.
Study procedures
Blood samples were collected on admission from septic patients for measurement of various markers. We measured coagulation and fibrinolysis molecular markers such as platelet count, prothrombin time-international normalized ratio (PT-INR), activated partial thromboplastin time (APTT), and levels of FDP, D-dimer, thrombin-antithrombin complex (TAT), plasmin α2-plasmin inhibitor complex (PIC), antithrombin (AT), protein C (PC), thrombomodulin (TM), soluble fibrin (SF), and plasminogen activator inhibitor-1 (PAI-1); we also measured inflammatory molecular markers such as white blood cell (WBC) count, and levels of C-reactive protein (CRP), presepsin (P-SEP), and procalcitonin (PCT). Platelet and WBC counts were measured in whole blood using an XT-1800i (Sysmex Co., Kobe, Japan). PT, APTT, FDP level, D-dimer level, AT activity, PC activity, and SF level were measured in plasma using a CP 3000 (Sekisui Medical, Tokyo, Japan). TM and PAI-1 levels were measured using a STACIA (LSI Medience Co., Tokyo, Japan). CRP concentrations were measured by CRP-LATEX (II) X2 “SEIKEN” (Denka Seiken Co. Ltd., Tokyo, Japan). P-SEP concentrations were measured using a compact automated immunoanalyzer, PATHFAST, based on a chemiluminescent enzyme immunoassay (LSI Medience Co.). PCT concentrations were measured by the Elecsys BRAHMS PCT assay (Roche Diagnostics, Tokyo, Japan). PT-INR was calculated using the following formula: INR = (patient PT/normal PT)ISI, where normal PT represents the average of the mean normal PT range in laboratory results and ISI is the International Sensitivity Index, a correction coefficient for thromboplastin in commercial kits calculated according to international reference samples.
Measurement of plasma sCLEC-2 levels by ELISA
We measured plasma sCLEC-2 levels by ELISA using previously described methods. Briefly, a 96-well F8 Maxisorp plate (Nunc, Roskilde, Denmark) was coated with F(ab’)2 of anti-CLEC-2 monoclonal antibody 11D5 (10 μg/mL) in coating buffer (0.05 M bicarbonate, pH 9.5) overnight at 4°C. The wells were washed six times with 100-μL aliquots of 0.1 M borate-buffered saline pH 8.0 containing 0.1% (v/v) Tween 20 (BBS-T), blocked with 1% (w/v) bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA) in PBS for 1 hour at room temperature, and washed with BBS-T six more times prior to addition of samples. Experiments were performed in duplicate, and each plate routinely included standards consisting of recombinant human CLEC-2 extracellular domain (final concentration, 0–5 ng/mL) in 0.3% BSA, 0.1% sodium octanoate, and 0.14 M NaCl in 25 mM sodium phosphate buffer (pH 7.2; sample diluent buffer). Test samples of plasma were diluted four to eight times with sample diluent buffer. After a 1.5-hour incubation at room temperature, the plates were washed six times with BBS-T, and 100 μL (1 μg/mL) of biotin-labeled F(ab’)2 of anti-CLEC-2 monoclonal antibody 11E6 was added to each well. After 1 hour of incubation and three washes with BBS-T, AMDEX High-Performance Conjugate (RPN4401V; GE Healthcare, Little Chalfont, UK) was added to the plates (100 μL/well; 1:6000 dilution of stock) for 1 hour, followed by another five washes with BBS-T. Next, 100 μL of 3,3ʹ,5,5ʹ-tetramethylbenzidine Liquid Substrate System for ELISA (Sigma-Aldrich) was added to each well and the mixture was incubated for 20–30 minutes at room temperature in the dark. To stop the reaction, 100 μL of 2 N H2S04 was added to each well and the absorbance was measured within 30 minutes at 450 nm following excitation at 630 nm [Citation9].
We previously confirmed that the sCLEC-2 ELISA can detect microparticle-bound CLEC-2 [Citation9]. Briefly, sCLEC-2 concentrations measured by ELISA agree with the data obtained by Western blot analysis, suggesting that this assay is able to detect both shed CLEC-2 and microparticle-bound CLEC-2.
Statistical analysis
Unless otherwise indicated, all data were expressed as mean ± standard deviation. SPSS 15.0 J (SPSS Inc., Chicago, IL, USA) and StatFlex version 7 (Artech Co., Osaka, Japan) were used for statistical analyses. Nonparametric statistical tests were used throughout, as these tests are more appropriate for the analysis of datasets with high variability than traditional parametric tests. Differences in biomarker levels between two patient groups (non-SID and SID) were analyzed using the Mann-Whitney test. A receiver-operating curve (ROC) analysis with the main output being the area under the curve (AUC) was performed to compare the prognostic methods as predictors of SID. The standard error of the ROC analysis was calculated using the formula described by Hanley and McNeil [Citation27]. Correlation was examined by the Spearman’s rank correlation coefficient. A stepwise multiple logistic regression analysis was used to assess the relationship between SID and various molecular markers. The level of statistical significance was set at P < .05.
Results
Population characteristics
Seventy-one septic patients were enrolled during the observation period. Of these, one patient with a platelet count of 733 × 103/µL at the time of ICU admission was excluded. Because it is unexpected that a septic patient would have a platelet count that exceeds the upper limit of the normal range, it is possible that this patient had primary hyperplasia (due to essential thrombocythemia, polycythemia vera, or chronic myelogenous leukemia) or secondary hyperplasia (due to a malignant tumor or chronic inflammatory disease, such as rheumatoid arthritis); therefore, this patient was excluded from this study. We consider that the exclusion of one such patient was unlikely to significantly weaken the predictive value of the C2PAC index. A final total of 70 patients were included in this analysis (). The mean age of the patients (36 men, 34 women) was 67.2 ± 15.6 years (median, 71 years; range, 22–89 years). Among all 70 patients, 26 and 44 patients were classified into the non-SID group and SID group, respectively (). Compared with the non-SID group, the SID group was significantly older and had a significantly higher SOFA score. Although the APACHE II score did not significantly differ between the two groups, the SID group tended to have a higher APACHE II score than the non-SID group (). Overall, these results confirmed that the SID group were in a more serious condition than the non-SID group. The DIC scores calculated using the JAAM DIC, ISTH overt DIC, and JMHW DIC scoring systems were all significantly higher in the SID group compared with the non-SID group (). Furthermore, the DIC positivity rate calculated using the ISTH overt DIC and JMHW DIC scoring systems was significantly higher in the SID group compared with the non-SID group (). The infection focuses in the septic patients with and without DIC are shown in .
Table I. Baseline characteristics of septic patients with and without SID on ICU admission.
Table II. Infection focuses in septic patients.
Coagulation/fibrinolysis and inflammation molecular marker distributions
The data for the coagulation/fibrinolysis molecular markers and inflammatory molecular markers in the non-SID and SID groups are shown in . The non-SID group had results outside the normal ranges for almost all coagulation/fibrinolysis markers (including the PT-INR and levels of fibrinogen, FDP, D-dimer, TAT, PIC, TM, SF, and PAI-1) and inflammatory markers (including the WBC count and levels of CRP, P-SEP, and PCT). In addition, the SID group had a significantly higher PT-INR and levels of FDP, D-dimer, TAT, PIC, SF, P-SEP, and PCT than the non-SID group. In contrast, the SID group had a significantly lower platelet count, AT activity, and PC activity than the non-SID group.
Table III. Various molecular markers in septic patients.
Platelet count, sCLEC-2 level, and C2PAC index
The septic patients had a significantly lower platelet count and significantly higher sCLEC-2 level on ICU admission compared with the healthy volunteers (; P < .01). Further investigations revealed that the SID group also had a significant lower platelet count and significantly higher sCLEC-2 level than the healthy volunteers (P < .01) (). In contrast, the non-SID group had a significantly higher sCLEC-2 level than the healthy volunteers (P < .01), but the platelet count did not differ significantly from that in the healthy volunteers and remained within the normal range (). Among the septic patients, the SID group had a significant lower platelet count than the non-SID group (P < .01), but the sCLEC-2 level did not significantly differ between the two groups ().
Table IV. Platelet count, sCLEC-2 level, and C2PAC index of healthy volunteers and septic patients with or without sepsis-induced DIC on admission.
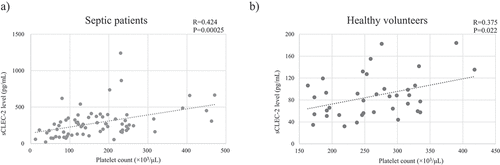
We suspected that the reason why there was no significant difference in the sCLEC-2 levels between the non-SID group and the SID groups was that the platelet count was significantly lower in the SID group than the non-SID group, as we considered that the sCLEC-2 level was likely to be affected by the platelet count. The sCLEC-2 level and platelet count at the time of ICU admission in septic patients showed a weak but significant positive correlation (R = 0.424, P = .00025) (). Incidentally, there was also a weak but significant positive correlation between these two parameters in healthy volunteers (R = 0.375, P = .0022) (). However, when the sCLEC-2 levels in septic patients and healthy volunteers were investigated by platelet count range, sCLEC-2 levels were consistently more than three-fold higher in septic patients than in healthy volunteers. Based on this result, we calculated the C2PAC index in the groups. The C2PAC index of the septic patients on ICU admission was significantly higher than that of the healthy volunteers () (P < .001). Among the septic patients, the C2PAC index was significantly higher in the SID group than the non-SID group () (P < .001).
Figure 1. Correlation between platelet count and sCLEC-2 level assessed using the Spearman’s rank correlation coefficient.
Predictive markers of SID and their diagnostic accuracy
For the univariate logistic regression analysis for the predictors of SID, the explanatory variates were those with a P value of <0.001 for the difference between the non-SID and SID groups. As a result, platelet count, FDP, D-dimer, TAT, PIC, SF, P-SEP, and PCT levels, AT activity, and PC activity were extracted as the explanatory variates (). In addition, the sCLEC-2 level and C2PAC index were selected as explanatory variates. After we performed the univariate logistic regression analysis, we performed a stepwise multiple logistic regression analysis. The explanatory variates in the stepwise multiple logistic regression analysis were the significant SID predictors with an AUC of the ROC of >0.8 in the univariate analysis, which included the C2PAC index, D-dimer, TAT, SF, and P-SEP levels, and PC activity (). The FDP level was excluded as an explanatory variate because it may be a confounder of D-dimer level and is less common than D-dimer level as a global marker.
Table V. Univariate logistic regression model for predictors of sepsis-induced DIC.
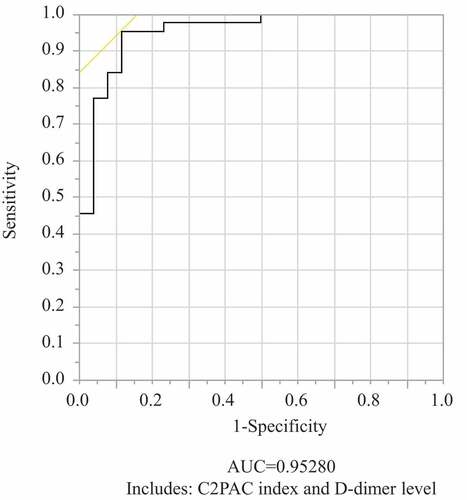
Increases in the D-dimer level and C2PAC index were independent predictors of SID (P < .0001, P = .0228, respectively; ). Using the C2PAC index and D-dimer level as predictive markers enables the diagnosis of SID with a high probability (AUC, 0.9528; sensitivity, 0.9545; specificity, 0.8846) (). According to the ROC analysis, the AUC and optimal cutoff value of the C2PAC index for the diagnosis of SID were 0.8051 and 1.4 (sensitivity, 75.0%; specificity, 76.9%), respectively.
Table VI. Stepwise multiple logistic regression model for predictors of sepsis-induced DIC Explanatory variates*: C2PAC, D-dimer level, TAT level, protein C activity, soluble fibrin level, and presepsin level.
Figure 2. Receiver-operating curves for predictive SID models.
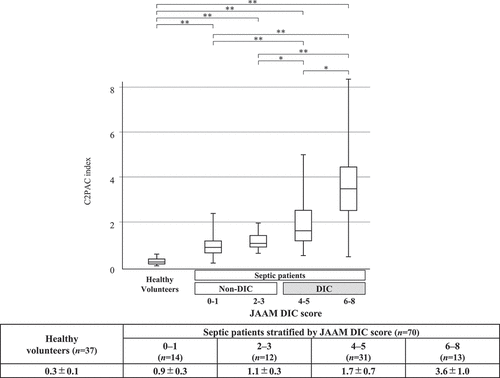
The C2PAC index was 0.3 ± 0.1 in the healthy volunteers and 0.9 ± 0.3, 1.1 ± 0.3, 1.7 ± 0.7, and 3.6 ± 1.0, respectively, in the four JAAM DIC score groups. The C2PAC index increased significantly as the DIC score increased ().
Figure 3. Relationship between the JAAM DIC score and the C2PAC index.
Time courses of platelet count, sCLEC2 level, and C2PAC index in septic patients
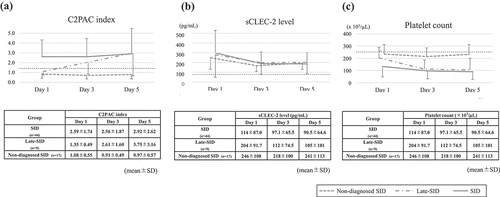
Among all 70 patients, 44, 9, and 17 patients were classified into the SID group, late-SID group, and non-SID group, respectively (). The sCLEC-2 levels in all three groups either decreased or did not significantly change over time. The platelet counts of the SID and non-diagnosed SID groups also decreased or did not significantly change over time. Furthermore, the platelet count in the late-SID group decreased by approximately half from day 1 to day 3, but showed minimal reduction from day 3 to day 5. The C2PAC index in the SID group remained above the DIC cutoff value of 1.4, while the C2PAC index in the non-diagnosed SID group remained below 1.4 throughout the 5 days after admission. In contrast, the C2PAC index in the late-SID group was approximately 1.4 on ICU admission, but gradually and constantly increased over time ().
Figure 4. Time course of the C2PAC index, sCLEC2 level, and platelet count in septic patients.
Discussion
Sepsis, defined as life-threatening organ dysfunction arising from a dysregulated host response to infection [Citation21], is a complex inflammatory syndrome and an important cause of morbidity and mortality in ICU settings worldwide. Sepsis affects between 47 and 50 million people every year and causes at least 11 million deaths, representing one death every 2.8 seconds. Depending on the country involved, sepsis-related mortality varies from 15% to >50% [Citation28]. Sepsis survivors have an increased long-term risk of thromboembolic events, including myocardial infarction and venous thromboembolism [Citation29]. Therefore, many surviving patients suffer from the consequences of sepsis for the rest of their lives [Citation28].
Platelets have received increasing attention for their role in the pathophysiology of infectious diseases, inflammation, and immunity. In sepsis, a low platelet count is a well-known biomarker for disease severity. Recently, attention has been focused on the active role of platelets in the pathogenesis of multiorgan failure. Because of their placement at the crossroads between the immune system, clotting cascade, and endothelial cells, platelets appear to be a central mediator and possible therapeutic target in sepsis [Citation30].
In recent years, increasing numbers of studies have shown that platelets contribute to the pathophysiological processes involved in sepsis and play an important role in organ damage. When pathogens invade the body, activation of the coagulation system at the site of infection and thrombus formation in local capillaries serve as defense mechanisms that limit the infection to the lesions by a process known as immunothrombosis. In sepsis, these local reactions spread to the entire body, and the resulting loss of control of the “inflammation-coagulation” interaction leads to platelet activation, followed by DIC and subsequent multiorgan dysfunction syndrome [Citation31]. Infections lead to decreases in platelet counts through effects on both platelet production and platelet survival [Citation32]. Consequently, early recognition and diagnosis of sepsis is crucial to achieving improved outcomes. Therefore, it is important to measure platelet counts daily and recognize the signs of thrombocytopenia in septic patients admitted to the ICU.
We conducted the present study under the hypothesis that recognizing the stage of platelet activation before the onset of thrombocytopenia could lead to earlier detection of pre-SID or SID. This is the first report to examine the relationship between SID and platelet activation using sCLEC-2 as a marker for platelet activation.
In the present study, almost all coagulation/fibrinolysis markers and inflammatory markers were outside their normal ranges in the non-SID group. These results confirmed that inflammation and coagulation exhibit close crosstalk, and that septic patients simultaneously show not only an elevated inflammatory condition, but also an enhanced abnormal coagulation/fibrinolysis condition [Citation33]. In other words, we suggest that non-SID patients can be considered a pre-SID group. Furthermore, when septic patients developed DIC, both their hyperinflammatory state and abnormal coagulation/fibrinolysis condition became even more pronounced.
The platelet counts in the non-SID group did not differ significantly from that in the healthy volunteers, and remained within the normal range. Meanwhile, the sCLEC-2 level in the non-SID group was approximately three times higher than that in the healthy volunteers, comprising a significant difference. Therefore, we suspect that measurement of sCLEC-2 enhances understanding of pre-SID states in patients who have coagulopathy but have not reached SID. Although the platelet count was significantly lower in the SID group than the non-SID group, there was no difference in the sCLEC-2 levels between the non-SID group and SID group. Furthermore, our study revealed an inconsistent and weak significant positive correlation between the sCLEC-2 level and platelet count in healthy volunteers and in septic patients at the time of ICU admission. Based on these results, we speculated that the sCLEC-2 level was likely to be affected by the platelet count; moreover, we presumed that this level would remain low under the low platelet count condition and would not increase immediately. Therefore, we calculated the C2PAC index to determine the sCLEC2 level per platelet unit and considered this index as an indicator of platelet activation. We confirmed that the C2PAC index was not only significantly higher in septic patients compared with healthy volunteers, but was also significantly higher in the SID group compared with the non-SID group. Therefore, by measuring not only the platelet count but also the sCLEC-2 level and calculating the C2PAC index, it may be possible to recognize coagulation/fibrinolytic abnormalities earlier than by measuring the platelet count alone, and to reduce sepsis-related death by recognizing an increase in sCLEC-2 or C2PAC-index before the platelet count decreases and promptly intervening with antiplatelet [Citation34] or anticoagulation [Citation32] therapy. We consider the C2PAC index to represent the sCLEC-2 level per individual platelet unit and be a true indicator of platelet activation.
The present study confirmed that the C2PAC index and D-dimer level were both good predictors of SID onset. This result appears logical because D-dimer and/or FDP levels are included as diagnostic variables in the main DIC diagnostic criteria, such as the DIC scoring systems proposed by the ISTH [Citation23] and JMHW [Citation24]. Notably, FDP and D-dimer levels are also included in the DIC diagnostic criteria of the JAAM. Thus, there is a clear correlation between the DIC score and the D-dimer level.
The present study also confirmed that the C2PAC index increased significantly as the DIC score increased, and that a C2PAC index of ≥1.4 was likely to result in DIC. These results strongly suggest that the C2PAC index is useful for identifying DIC. Moreover, we investigated the time course of the C2PAC index in septic patients. Interestingly, the C2PAC index in the SID group (who already had DIC at ICU admission) remained above 1.4, while the C2PAC index in the non-diagnosed SID group (who did not have DIC) did not exceed 1.4 over time. Meanwhile, in the late-SID group that developed DIC after admission, the C2PAC index gradually and constantly increased and eventually exceeded 1.4. Furthermore, sCLEC2 levels in all three groups decreased or did not significantly change over time. Additionally, the platelet counts remained high in the SID group and low in the non-diagnosed SID group (both showed minimal changes). The platelet count in the late-SID group decreased by approximately half from day 1 to day 3, while it showed minimal reduction from day 3 to day 5. These results suggest the need to carefully monitor patients with sepsis whose daily C2PAC index exceeds 1.4 and rises over time, because they may be likely to develop DIC.
Some limitations of the present study should be noted. It was a small, retrospective, single-center, observational study, making it difficult to generalize the findings globally. Furthermore, the JAAM DIC scoring system includes platelet count as a diagnostic variable, which affects the relationship between the JAAM DIC score and the C2PAC index. Finally, blood transfusion may affect the platelet count and sCLEC-2 level. However, the measurements of all markers at the time of admission to the ICU were from data collected when no blood transfusion had been performed. Because we conducted most of this study using these measurements, we presume that the results were not affected by blood transfusion. Seven patients in the DIC group and three in the late-DIC group underwent transfusion of blood products within 48 hours after ICU admission. Of these 10 patients, only two in the late-DIC group underwent platelet transfusion. Therefore, we presume that the transfusion did not strongly influence the platelet count, sCLEC-2 level, or C2PAC index in this clinical study. However, the study is being continued, and a prospective multicenter study has been planned.
Conclusions
In patients with sepsis, platelet activation occurred before the platelet count decreased below the lower limit of the normal range and may indicate the preparatory phase for DIC. The findings further suggested that a sustained rise in the C2PAC index may be a predictor of progression to DIC. Therefore, we conclude that sCLEC-2, especially the C2PAC index, is a useful predictive marker for early assessment of coagulopathy and DIC diagnosis in sepsis.
Ethics approval and consent to participate
The following ethics review boards approved the protocol for this study: Fukuoka University Hospital (U19-01-001; registered on 19 January 2019); Yamanashi University Hospital (2289; registered on 17 June 2020); LSI Medience Corporation (Shindan/Narita 19-04; registered on 19 May 2019).
Consent for publication
We obtained written informed consent for the publication of this study from all patients or their proxy in accordance with the Declaration of Helsinki. Copies of the informed consent documents are available from the corresponding author on reasonable request.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
Hiroyasu Ishikura contributed to the study design, statistical analysis, interpretation of the results, drafting of the manuscript, and critical revision of the manuscript for intellectual content. Yuhei Irie and Masahide Kawamura participated in the study design, statistical analysis, and interpretation of the results. Masahide Kawamura is also an employee of LSI Medience Corporation. Kota Hoshino and Yoshihiko Nakamura were involved in data acquisition and performed the statistical analysis. Mariko Mizunuma, Junichi Maruyama and Maiko Nakashio were involved in data acquisition. Katsue Suzuki-Inoue carried out the ELISA assays and participated in the development and methodology. Taisuke Kitamura helped to draft the manuscript. All authors have read and approved the final manuscript.
Authors’ information
Hiroyasu Ishikura is a member of the Scientific Standardization Committee (SSC) for disseminated intravascular coagulation (DIC) of the Japanese Society for Thrombosis and Hemostasis (JSTH).
Supplemental Material
Download PDF (257 KB)Acknowledgements
The authors thank Kelly Zammit, BVSc, and Alison Sherwin, PhD, from Edanz Group (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Disclosure statement
MK is an employee of LSI Medience Corporation. The other authors declare that they have n competing interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Vardon-Bounes F, Ruiz S, Gratacap MP, Garcia C, Payrastre B, Minville V. Platelets are critical key players in sepsis. Int J Mol Sci 2019;20(14):3494. doi:10.3390/ijms20143494
- Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet activation: the mechanisms and potential biomarkers. Biomed Res Int 2016;2016:9060143. doi:10.1155/2016/9060143
- Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol 2018;17(1):121. doi:10.1186/s12933-018-0763-3
- Al-Tamimi M, Grigoriadis G, Tran H, Paul E, Servadei P, Berndt MC, Gardiner EE, Andrews RK. Coagulation-induced shedding of platelet glycoprotein VI mediated by factor Xa. Blood 2011;117(14):3912–3920. doi:10.1182/blood-2010-08-301523
- Inoue O, Osada M, Nakamura J, Kazama F, Shirai T, Tsukiji N, Sasaki T, Yokomichi H, Dohi T, Kaneko M, et al. Soluble CLEC-2 is generated independently of ADAM10 and is increased in plasma in acute coronary syndrome: comparison with soluble GPVI. Int J Hematol 2019;110(3):285–294. doi:10.1007/s12185-019-02680-4
- Suzuki-Inoue K, Tsukiji N, Shirai T, Osada M, Inoue O, Ozaki Y. Platelet CLEC-2: roles beyond hemostasis. Semin Thromb Hemost 2018;44(2):126–134. doi:10.1055/s-0037-1604090
- Meng D, Luo M, Liu B. The role of CLEC-2 and Its Ligands in Thromboinflammation. Front Immunol 2021;12:688643. doi:10.3389/fimmu.2021.688643
- Navarro-Núñez L, Langan SA, Nash GB. Watson SP.The physiological and pathophysiological roles of platelet CLEC-2. Thromb Haemost 2013;109(6):991–998. doi:10.1160/TH13-01-0060
- Kazama F, Nakamura J, Osada M, Inoue O, Oosawa M, Tamura S, Tsukiji N, Aida K, Kawaguchi A, Takizawa S, et al. Measurement of soluble C-type lectin-like receptor 2 in human plasma. Platelets 2015;26(8):711–719. doi:10.3109/09537104.2015.1021319
- Fei M, Xiang L, Chai X, Jin J, You T, Zhao Y, Ruan C, Hao Y, Zhu L. Plasma soluble C-type lectin-like receptor-2 is associated with the risk of coronary artery disease. Front Med 2020;14(1):81–90. doi:10.1007/s11684-019-0692-x
- Zhang X, Zhang W, Wu X, Li H, Zhang C, Huang Z, Shi R, You T, Shi J, Cao Y. Prognostic significance of plasma CLEC-2 (C-type lectin-like receptor 2) in patients with acute ischemic stroke. Stroke 2018;7:STROKEAHA118022563
- Nishigaki A, Ichikawa Y, Ezaki M, Yamamoto A, Suzuki K, Tachibana K, Kamon T, Horie S, Masuda J, Makino K, et al. Soluble C-type lectin-like receptor 2 elevation in patients with acute cerebral infarction. J Clin Med 2021;10(15):3408. doi:10.3390/jcm10153408
- Guo M, Zhang H, Lv QW, Huang HB, Shen LJ. Higher plasma C-type lectin-like receptor 2 concentrations for prediction of higher risk of 30-day mortality in isolated severe blunt traumatic brain injury. Clin Chim Acta 2019;496:1–6. doi:10.1016/j.cca.2019.06.014
- Yamashita Y, Suzuki K, Mastumoto T, Ikejiri M, Ohishi K, Katayama N, Suzuki-Inoue K, Wada H. Elevated plasma levels of soluble C-type lectin-like receptor 2 (CLEC2) in patients with thrombotic microangiopathy. Thromb Res 2019;178:54–58. doi:10.1016/j.thromres.2019.03.018
- Yamamoto A, Wada H, Ichkawa Y, Tanaka M, Tashiro H, Shiraki K, Shimpo H, Yamashita Y, Mastumoto T, Shimaoka M, et al. Soluble C-type lectin-like receptor 2 is a biomarker for disseminated intravascular coagulation. J Clin Med 2021;10(13):2860. doi:10.3390/jcm10132860
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med 1999;341(8):586–592. doi:10.1056/NEJM199908193410807
- Iba T, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology 2020;132(5):1238–1245. doi:10.1097/ALN.0000000000003122
- Saito S, Uchino S, Hayakawa M, Yamakawa K, Kudo D, Iizuka Y, Sanui M, Takimoto K, Mayumi T, Sasabuchi Y. Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study group: epidemiology of disseminated intravascular coagulation in sepsis and validation of scoring systems. J Crit Care 2019;50:23–30. doi:10.1016/j.jcrc.2018.11.009
- Asakura H, Takahashi H, Uchiyama T, Eguchi Y, Kohji Okamoto K, Kawasugi K, Madoiwa S, Wada H. DIC subcommittee of the Japanese Society on Thrombosis and Hemostasis: proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J 2016;14(1):42. doi:10.1186/s12959-016-0117-x
- Asakura H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, Madoiwa S, Wada H. DIC subcommittee of the Japanese Society on Thrombosis and Hemostasis. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care 2014;2:20. doi:10.1186/2052-0492-2-20
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315(8):801–810. doi:10.1001/jama.2016.0287
- Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, Mayumi T, Murata A, Ikeda T, Ishikura H, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med 2006;34(3):625–631. doi:10.1097/01.CCM.0000202209.42491.38
- Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific subcommittee on disseminated intravascular coagulation (DIC) of the international society on thrombosis and haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001;86(11):1327–1330. doi:10.1055/s-0037-1616068
- Kobayashi N, Maegawa K, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the research committee on DIC in Japan. Bibl Haematol 1987;49:265–275
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
- Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998;26(11):1793–1800. doi:10.1097/00003246-199811000-00016
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143(1):29–36. doi:10.1148/radiology.143.1.7063747
- Title of subordinate document. In: Sepsis. Global Sepsis alliance. [Cited 2021 April 25]; Available from: https://www.global-sepsis-alliance.org/sepsis
- Middleton EA, Rowley JW, Campbell RA, Grissom CK, Brown SM, Beesley SJ, Schwertz H, Kosaka Y, Manne BK, Krauel K, et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood 2019;134(12):911–923. doi:10.1182/blood.2019000067
- Greco E, Lupia E, Bosco O, Vizio B, Montrucchio G. Platelets and multi-organ failure in sepsis. Int J Mol Sci 2017;18(10):2200. doi:10.3390/ijms18102200
- Wang Y, Ouyang Y, Liu B, Ma X, Ding R. Platelet activation and antiplatelet therapy in sepsis: a narrative review. Thromb Res 2018;166:28–36. doi:10.1016/j.thromres.2018.04.007
- Parikh F. Infections and thrombocytopenia. J Assoc Physicians India 2016;64(2):11–12
- Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med 2010;38(2 Suppl):S26–34
- Trauer J, Muhi S, McBryde ES, Al Harbi SA, Arabi YM, Boyle AJ, Cartin-Ceba R, Chen W, Chen YT, Falcone M, et al. Quantifying the effects of prior acetyl-salicylic acid on sepsis-related deaths: an individual patient data meta-analysis using propensity matching. Crit Care Med 2017;45(11):1871–1879. doi:10.1097/CCM.0000000000002654
