Abstract
This study investigated the effect of L-PRF on promoting full-thickness skin grafting for the treatment of diabetic foot ulcer wounds and attempted to characterize the mechanism. In a retrospective study, we centrifugated 10–20 ml of venous blood at 1006.2 g for 20 min. The fibrin clot between the top oligocellular plasma layer and the bottom erythrocyte layer was extracted and directly used, without compression, to cover the wound after debridement. Patients who received L-PRF before skin grafting underwent surgery earlier than patients in the control group. Skin necrosis occurred in 7 patients (28%) in the L-PRF group and 16 (64%) in the control group. The difference was statistically significant, P < .05. The postoperative infection rate in the control group (56%) was significantly higher than that in the L-PRF group (24%), P < .05. During a mean follow-up of 1 year, ulcer recurrence occurred in 9 patients (36%) in the control group compared with 4 patients (16%) in the L-PRF group, P < .05. The final amputation rate was also higher in the control group (48%) than in the L-PRF group (20%). The difference is statistically significant, P < .05. The Maryland scale score and SF-36 score of the two groups of patients after treatment were significantly better than those before treatment, and the difference was statistically significant (P < .05). The L-PRF group (94.80 ± 4.14) had better foot scores at the last follow-up after treatment than the control group (88.84 ± 5.22) (P < .05). The results showed that L-PRF played a positive role in the treatment of Wagner grade 4 ulcer gangrene with free full-thickness skin grafts.
P lain l anguage s ummary
What is the context?
● Diabetic foot is a serious complication in the later stage of the disease course of diabetic patients. The incidence rate is increasing year by year. In severe cases, it can lead to amputation or even death.
● For diabetic ulcer wounds, dressings such as L-PRF or autologous fat are often used in the initial stage to speed up wound healing. For advanced wounds, especially patients with local tissue gangrene, simple wound dressings cannot meet the needs of wounds. People often use skin flaps or different types of skin grafts to treat advanced wounds.
● Flap or skin grafting has been shown to be effective, but because of the patient’s own neurovascular injury and infection, the rate of graft necrosis and ulcer recurrence is extremely high. What is new?
● This study discusses the treatment of advanced wounds in diabetes. After thorough debridement and before skin grafting, we first covered the wound with L-PRF and observed the wound condition. Studies have shown that the use of L-PRF can allow the original poor wound to be reconstructed: the content of growth factors and growth-related cells is increased, blood circulation is improved and granulation tissue growth, bone and tendon exposure is improved, and infection is controlled. What is the impact?
● This study provides evidence that using L-PRF to reconstruct wounds can greatly shorten the preparation time for elective surgery. Reconstructed wounds can better accept free skin grafts, and the incidence of postoperative complications and amputation (particularly, toe amputation) is also lower.
Introduction
Diabetic foot is one of the most serious complications of diabetes and the leading cause of nontraumatic amputation. According to statistics, the total incidence of diabetic foot ulcers in the world is approximately 6.3%, and approximately 25% of patients have ulcer wounds that will not heal for the rest of their lives. At the same time, the probability of ulcer recurrence after ulcer healing is as high as 60%, and the extremely high recurrence rate of ulcers also increases the risk of amputation [Citation1,Citation2]. The gradual subsidence of limb sensation also makes the affected limb vulnerable to trauma but unable to be treated in time, which aggravates foot ulcers; such trauma includes friction from shoes, impact and crushing from objects, scalding, and even burns, which especially affect the toes of the diabetic foot [Citation3,Citation4]. In the most commonly used Wagner classification of diabetic foot ulcers, localized gangrene of the toes, heels or dorsum of the forefoot (grade 4) is second only to gangrene of the affected limb (grade 5) and is an extremely difficult wound to manage. The appearance of localized gangrene marks the destruction of the local blood supply and irreversible necrosis of soft tissue structures, often accompanied by local infection and soft tissue defects (mostly combined with trauma). If not handled properly, this condition can easily develop into larger areas of ulcers and gangrene and eventually lead to amputation (e.g., toe amputation) [Citation1,Citation5].
Skin grafting is an effective way to treat diabetic foot ulcer wounds, especially wounds with exposed bones and tendons. The course of such wounds is prolonged or even aggravated, and simple skin grafting cannot be used for treatment. Although skin grafting can close the wound as soon as possible, the destruction of the nerve and blood supply makes the possibility of wound recovery very small. Infection, destruction of the blood supply to the wound before transplantation, and loss of the effective part of the soft tissue greatly reduce the probability of the skin survival. Therefore, in this study, leukocyte-platelet fibrin (L-PRF) was used first to treat wounds requiring skin grafts after thorough debridement. The efficacy of L-PRF alone in the treatment of early diabetic foot ulcer wounds has been well established in previous studies. Its active role in controlling infection and improving the microenvironment of the wound is an important condition required to improve the survival rate after skin grafting.
Materials and methods
Patient selection and study design
Fifty patients with diagnosed diabetic foot ulcers treated at our hospital from January 2016 to January 2021 were selected as the research subjects. Inclusion criteria were as follows: ①The local gangrene of the wound of the diabetic foot ulcer conformed to Wagner grade 4, and the size of the wound after debridement (length × width) was 5–15 cm2;②The ulcer wound was located in the toe area, and the tendon and bone were exposed after debridement; ③The duration of the diabetic foot ulcer wound was more than 1 week; ④The patient voluntarily accepted L-PRF combined with skin grafting; ⑤There was no serious systemic disease, tumor, anemia or coagulation dysfunction. The exclusion criteria were as follows: ① There was partial amputation of the affected foot; ② The diabetic foot had not yet formed ulcer wounds or the wounds had existed for a short time; ③ The patient could not receive a full-thickness skin graft due to poor pretibial skin or soft-tissue conditions (skin damage, soft tissue contusion, etc.); ④The patient had uncontrolled systemic infectious diseases such as anemia, sepsis or sepsis and ankle-brachial pressure index (ABI) <0.8;⑤ The patient requested or the condition necessitated direct amputation.
After admission, patients were treated with antidiabetic drugs, and all of them underwent thorough debridement before skin grafting. After debridement, the control group was bandaged with sterile Vaseline gauze. In the L-PRF group, 10–20 ml of venous blood was collected according to the size of the patient’s wound after debridement, immediately placed in a centrifuge (Model: Anhui USTC Zonkia Scientific Instruments Co., Ltd., SC-3614) and centrifuged at 1006.2 g for 20 minutes. After standing for 3–5 minutes, the fibrin clot between the top oligocellular plasma layer and the bottom erythrocyte layer was extracted and directly used, without compression, to cover the wound after debridement. Those wounds were observed with dressing changes at intervals of 2 days after surgery; the control group dressings were replaced with Vaseline gauze, and the L-PRF group wounds were treated with L-PRF coverage again until the wounds in the two groups reached the standard for skin grafting.
Skin-graft surgical conditions were as follows: ①The patient’s blood sugar was well controlled; ②There was no infection on the wound surface;③There was no necrotic tissue around the ulcer; ④There were no contraindications to surgery for other systems. Full-thickness anterior tibial skin grafts were used for the treatment of foot ulcer wounds in both groups.
Images of the patient’s wounds were taken during the patient’s treatment, including: before debridement, after debridement, each time the wound was covered with L-PRF, and the final follow-up images of scarring. These images were evaluation by a 3-person group. The team consisted of 1 burn wound surgeon, 1 micromanipulation orthopedic surgeons and 1 trauma orthopedic surgeons. All evaluators were not involved in the treatment grouping process and were blinded to the treatment regime during follow- up and evaluation. Enumeration data and metrology data in experimental data, also obtained and analyzed by group members.
This retrospective study has been approved by the Ethical Committee, Renmin Hospital, Hubei University of Medicine (Shiyan, China) (No. syrmyy2021–026).
Wound care
All patients used drugs to control their blood sugar and were regularly checked for related indicators during hospitalization. The following general data of the patients were assessed: sex, age, wound size, blood sugar control and duration of diabetes. After debridement and skin grafting, antibiotics (first-generation cephalosporins) were used for 3 days to prevent infection, and if infection was diagnosed, sensitive antibiotics were used. After the initial treatment, the dressings were changed regularly to observe the wound condition. The time T1 from debridement to skin grafting was counted in the two groups, and the skin necrosis rate (partial and full), the rate of skin infection within 1 week after skin grafting, the probability of recurrence of ulceration after skin survival and the amputation rate during the follow-up period. Finally, we evaluated the quality of life (The medical outcomes study 36-item short from health survey, SF-36) and foot function (Maryland scale) of the two groups of patients before and after treatment. All follow-up results and assessment data were collected by a 3-person group. The team consisted of 1 burn wound surgeon, 1 micromanipulation orthopedic surgeons and 1 trauma orthopedic surgeons. All evaluators were not involved in the treatment grouping process and were blinded to the treatment regime during follow-up and evaluation.
Statistical analysis
Data regarding the wound size, blood sugar control, time T1 from debridement to skin grafting in the two groups of patients, SF-36 score and Maryland scale score were subjected to t tests, and GraphPad Prism 8 software was used to create graphs. The measurement data, such as the number of infected people, the number of necroses, and the number of follow-up amputations after skin grafting, were expressed as ¯x±s, and the X2 test was performed. The t test and X2 test involved in this study were all analyzed using SPSS 25 software, and the difference was statistically significant at P < .05. We use the Walters normal approximation method to approximate the Fisher exact probability method (α = 0.05, 1-β = 0.90). The results showed that when the sample size of both groups was 19 cases, the total sample size was 38, Power = 0.91 > 0.90.
Results
General information
There were no significant differences in sex, age, wound size or duration of diabetes between the two groups before initial treatment (P > .05), and there was no significant difference in diabetes control during treatment (P > .05) ().
Table I. Comparison of general data of the two groups of patients.
Treatment situation
Debridement is an essential first step prior to any topical wound treatment or dressing of the wound [Citation6,Citation7]. The time from debridement to skin grafting (T1) was significantly different between the two groups (P < .05). The timing of skin grafting was significantly earlier in the L-PRF group than in the control group (). This indicates that L-PRF can accelerate the reconstruction of the skin grafting wound and has a positive effect on preventing wound infection, preventing local necrosis, and promoting the growth of granulation tissue. In terms of the incidence of skin graft complications, the incidence of complications in the L-PRF group was significantly lower than that in the control group (P < .05). The L-PRF group had a lower rate of postoperative necrosis and infection, and patients who had used L-PRF had a lower rate of ulcer recurrence at the skin graft at a mean follow-up of 1 year and thus had a lower rate of amputation (). The Maryland scale score () and SF-36 score () of the two groups of patients after treatment were significantly better than those before treatment, and the difference was statistically significant (P < .05). The L-PRF group (94.80 ± 4.14) had better foot scores at the last follow-up after treatment than the control group (88.84 ± 5.22) (P < .05). This indicates that the transplanted wounds reconstructed by L-PRF have better conditions for receiving skin grafts, such as good infection and necrosis control, coverage of granulation tissue, and reconstruction of new blood vessels. There may also be a positive effect in that the local absorption of L-PRF promotes growth bonding between the grafted skin and the in situ tissue, thereby improving skin survival and reducing the risk of diabetic foot amputation.
Figure 1. Comparison of Maryland scale between two groups of patients before and after treatment. P < .05, the difference is statistically significant. Before treatment: control group 35.36 ± 5.02; L-PRF group 34.52 ± 5.29,t = 0.513,p = .600; After treatment: control group 88.84 ± 5.22; L-PRF group 94.80 ± 4.14,t=−4.499,p<.001.
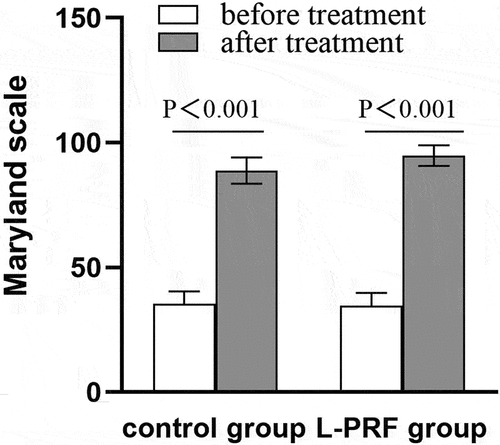
Figure 2. Comparison of SF-36 quality of life scale between two groups of patients before and after treatment.Pf: physical functioning, RP: role- physical, BP: bodily pain, GH: general health, VT: validity, SF: social function, RE: role-emotional, MH: mental health. P < .05, the difference is statistically significant. There were statistically significant differences between the preoperative and postoperative comparisons of each item in the control group. The scores of each item in the L-PRF group after operation were significantly higher than those before operation. P-values are shown in the figure. *: Indicates that there is a statistically significant difference between the scores after treatment and the corresponding scores before treatment. There was no statistical significance in the comparison of SF-36 quality of life scale between the two groups during the same period.
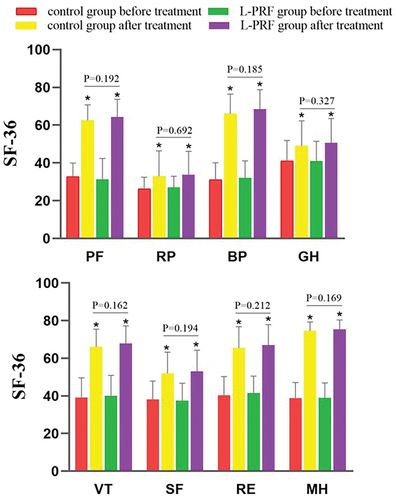
Table II. Treatment conditions and complication rates of the two groups of patients.
Patient wound and treatment images
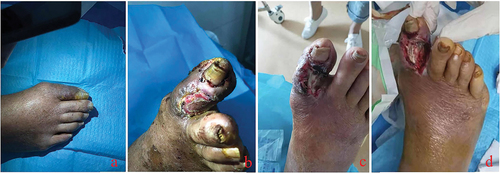
The occurrence and development of diabetic foot ulcer wounds is a process that is usually accompanied by a certain degree of trauma ().
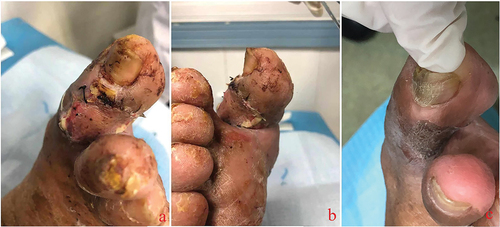
Figure 3. Most diabetic foot wounds start from lesions of the small blood vessels at the distal end of the affected limb. The destruction of the local blood supply impairs survival of the skin and soft tissue of the toe. At this time, the skin of the affected toe is often darkened (a), and mild paresthesia is often combined. Then, local skin necrosis occurs, subcutaneous tissue is exposed, ulcer wounds are formed (b), and the sensory function of the affected toe is impaired. Due to impaired sensory function, the pain is not severe despite the severe wound. The continuous necrosis of the surrounding tissue and the high probability of trauma make the wound develop further, and tendons and bones are gradually exposed (c, d). Local infection can occur at various stages after wound formation.
After repeated use of L-PRF to cover the wound, it was found that most of the reconstructed graft wounds were in excellent transplantation condition (), and the skin had a higher survival rate after skin grafting ().
Figure 4. In the initial treatment of diabetic foot ulcer wounds, sufficient debridement was given to completely remove necrotic tissue. Tendons and bones were exposed after the debridement of most toe wounds (a the first day after debridement). The first day after debridement (b), we used L-PRF to cover the wound. The third day after debridement (c), we again performed wound dressing and covered the wound with L-PRF. The 5th day after debridement (d), after removing the wound dressing we can see that the wound surface was tidy, the wound area was slightly reduced, no necrosis and abnormal secretions were seen.
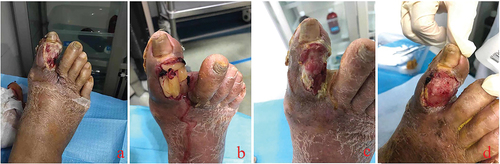
Figure 5. After successful reconstruction of the transplanted wound bed, anterior tibial free skins were used for skin grafting. The L-PRF group had a higher rate of skin graft survival, the well-surviving skin had no necrosis or infection, and the color of the skin was similar to that of normal tissue. (a, b). Well-recovered skin at an average of one-year postoperative follow-up showed good coverage of the ulcer wounds (c). The hyperplasia and skin graft area had good abrasion resistance.
Discussion
Diabetic foot is a common serious complication occurring in the later stages of the development of diabetes. The formation of ulcer wounds is the result of diabetic foot microangiopathy and neuropathy, which is characterized by a prolonged and even lifelong course of disease, and most of these ulcers are complicated by infection [Citation8,Citation9]. The ultimate outcome of wounds that cannot be effectively controlled is amputation. The 5-year mortality rate after foot ulceration is more serious, ranging from 43% to 55%, while the mortality rate for lower limb amputees is as high as 74% [Citation10]. Diabetic foot peripheral neuropathy is the core element of foot ulceration in most patients [Citation11]. Neuropathy causes paresthesia in the feet, deformities and abnormal walking patterns. Loss of sensation, foot deformity, and limited joint mobility can cause abnormal loading and subcutaneous bleeding, and minor trauma can lead to ulceration [Citation12]. Peripheral arterial disease is usually caused by atherosclerosis, which is found in approximately 50% of all patients. It is an important risk factor for impaired wound healing and lower extremity amputations [Citation1,Citation13].
From the microscopic point of view, the mechanism of diabetic foot ulcer formation is very complex, and has not yet been systematically elaborated upon. Oxidative stress plays a key role in the development of diabetic complications [Citation14]. On the one hand, the hyperglycemic state in the body leads to an enhanced nitriding stress response and an increase in the production of nitric oxide (NO). NO promotes to some extent the angiogenesis, migration and proliferation of epithelial and endothelial cells, fibroblasts and keratinocytes. Oxidative stress increases the production of vascular peroxides. These peroxides inactivate nitric oxide, thereby promoting the activation of proinflammatory processes and vascular dysfunction [Citation15,Citation16]. On the other hand, oxidative stress may also increase circulatory diacylglycerol and protein kinase C, leading to vascular dysfunction. Intracellular accumulation of advanced glycation end-products (AGEs) enhances the expression of receptors for advanced glycation end-products (RAGE) and leads to the expression of pro-inflammatory molecules [Citation17,Citation18]. In addition, when the hyperglycemic state persists in the body, it can lead to epithelial cell dysfunction, resulting in reduced proangiogenic signaling and reduced nitric oxide production. The loss of nitric oxide reduces vasodilation and accelerates limb/nerve ischemia. In the course of chronic hyperglycemia, peripheral arterial endothelial cell dysfunction and smooth muscle cell abnormalities lead to decreased endothelial-derived vasodilators, resulting in vasoconstriction and an increased risk of limb ischemia [Citation1,Citation19–21].
In clinical practice, diabetic foot is divided into 6 grades according to the severity of the disease by Wagner classification. Although diabetic foot disease progresses rapidly and is difficult to reverse, a first clinical diagnosis of full foot gangrene is rare, and most of these cases involve localized gangrene (toe, heel, or dorsum of the forefoot), that is, Wagner grade 4. These ulcerated wounds are often coinfected and have tendon-bone exposure and damage, making them extremely difficult to manage, especially in the most distal areas such as the toes [Citation22]. At present, there are few methods to address such wounds, and thorough debridement combined with skin grafting is a common way to treat refractory defect wounds. However, in patients with diabetic foot, the wound microenvironment is extremely poor, and the local blood supply is damaged, which leads to continuous progression of the disease. The survival rate of transplanted skin is low, and ulcers are prone to recurrence [Citation23]. Due to the extremely complex biological process of wound healing, the reduction in local growth factor activity, deficiency in factor quantity, or the loss of regulatory functions of various mechanisms makes wound healing difficult, thus restricting the application of skin grafts to diabetic foot ulcers [Citation24]. Therefore, an increasing number of researchers have begun to explore how to reconstruct these wounds before skin grafting. A good wound microenvironment will greatly improve the success rate of skin grafting. Among the many factors affecting tissue defect repair, growth factors and cytokines have been shown to promote healing [Citation25]. Exogenous cytokines have been used clinically to promote healing. However, due to technical limitations, the most commonly used growth factors or cytokines can only be used alone. This can only promote a certain stage of wound healing. In theory, a combination of cytokines consistent with the biological process of tissue healing can most effectively promote recovery. The in vitro combination technology of multiple functional bioactive molecules is an important research hotspot in the field of clinical application of growth factors [Citation26,Citation27]. We refer to wounds that are considered for skin grafting but are still in preoperative preparation as the “wound bed” for skin grafting.
In previous studies, L-PRF has also been shown to be useful in the treatment of early (Wagner grade 1–2) diabetic foot ulcer wounds. It contains high concentrations and appropriate proportions of bioactive molecules with wound healing functions, such as Transforming growth factor-beta 1 (TGF-b1), Transforming growth factor-beta 2 (TGF-b2), Epidermal growth factor (EGF), Fibroblast growth factor (FGF), Platelet-derived growth factor (PDGF), Vascular endothelial growth factor (VEGF), and Interleukin 1 (IL-1), which can promote the repair of local soft tissue wounds. At the same time, leukocytes, immune cells and immune regulators, such as interleukins Interleukin 6 (IL-6) and Tumor necrosis factor (TNF) in the three-dimensional grid structure of L-PRF, provide immune defense and local anti-inflammatory effects, which can reduce the risk of surgery or a postinflammatory response and can promote tissue repair [Citation28]. The homology of L-PRF is also unmatched by other materials. Because it does not add any exogenous substances during its production and application, this agent is very simple to operate and use, and there is no risk of immune rejection. However, consequently, the shortcomings of L-PRF are also obvious. Since no anticoagulant substances are added, blood must be collected and used in time during the production process, and it cannot be transported and stored for a long time. Blood pressure can be stored for about 5 minutes before centrifugation. And the centrifugation tubes were not coated with a clot activator. The amount of blood collected at a time is limited, and the amount of L-PRF produced is likewise limited, and this amount cannot match the coverage needed for the treatment of large ulcer wounds (such as large-area dorsal wounds).
Various dressings and nursing techniques can be applied to the management of diabetic foot wound beds [Citation29]. Examples include anionic dressings, honey dressings, platelet rich plasma (PRP) [Citation30], negative pressure wound therapy (NPWT) [Citation31], hyperbaric oxygen technology [Citation32], and the use of stem cells [Citation33]. In addition to L-PRF, the honey dressing is also a newly reported biomaterial that can be used to treat diabetic foot. Wang et al [Citation34]. concluded that it can effectively shorten the wound debridement time, wound healing time, and bacterial clearance time; moreover, in the first one to two weeks of use, the wound healing rate and bacterial clearance rate are improved. Medical grade honey has been included in the British National Formulary (BNF) for its antibacterial and anti-inflammatory properties in the treatment of chronic wounds [Citation35]. Despite studies proving its effectiveness, no relevant guidelines have been formed. As a heterologous substance, the use of honey dressings must be extremely cautious, which is also a common problem faced by the other materials mentioned above. In addition to the potential dangers caused by heterogeneity, compared with L-PRF, the currently researched dressings and technologies have disadvantages such as complicated production steps, high prices, and long treatment periods; moreover, even the treatment effect has not been fully confirmed, which makes it difficult to popularize and use L-PRF.
In summary, L-PRF has advantages over other dressings or techniques. The first advantage is the rich content of substances. Monocytes/macrophages and platelets with larger cell structures can help restore a stable wound microenvironment, and they have a certain anti-infection effect [Citation36]. Various growth factors consisting of smaller proteins, including vascular endothelial growth factor, PDGF, TGF-β, epidermal growth factor, Insulin-like growth factor 1 (IGF-1), Hepatocyte growth factor (HGF), IL-1, IL-4, IL-6, and IL-10, etc., supplement the necessary promoting substances for local tissue repair [Citation37]. It is also not negligible that L-PRF can continuously release growth factors for a long time; most growth factors can be released for up to 7 days [Citation38], while others can be released for up to 15 days or even longer [Citation39]. The components of L-PRF and the three-dimensional network structure help prevent the rapid protein breakdown of growth factors, thereby prolonging the secretion time [Citation40]. The long-lasting effect is extremely important for recovery after skin grafting, and the release time of most factors covers the critical period of skin survival (i.e., the early stage when the transplanted skin is most prone to necrosis). This allows the early treatment of L-PRF to not only promote reconstruction of the wound bed but also greatly improve the survival rate of the skin.
Conclusion
Using L-PRF to reconstruct wounds can greatly shorten the preparation time for elective surgery. The reconstructed wounds can better accept free skins, and the incidence of postoperative complications and amputation (toe amputation) rates are lower.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bandyk DF. The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg. 2018;31(2–4):1–7.
- Zha ML, Cai JY, Chen HL. A bibliometric analysis of global research production pertaining to diabetic foot ulcers in the past ten years. J Foot Ankle Surg. 2019;58(2):253–259.
- Arıcan G, Kahraman HÇ, Özmeriç A, İ̇ltar S, Alemdaroğlu KB. Monitoring the prognosis of diabetic foot ulcers: predictive value of neutrophil-to-lymphocyte ratio and red blood cell distribution width. Int J Low Extrem Wounds. 2020;19(4):369–376.
- Engberg S, Kirketerp-Møller K, Ullits Andersen H, Rasmussen A. Incidence and predictors of recurrent and other new diabetic foot ulcers: a retrospective cohort study. Diabet Med. 2019;36(11):1417–1423.
- Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375.
- Gordon KA, Lebrun EA, Tomic-Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Skinmed. 2012;10(1):24–26.
- Game FL, Jeffcoate WJ. Dressing and diabetic foot ulcers: a current review of the evidence. Plast Reconstr Surg. 2016;138(3 Suppl):158S–164S.
- Nather A, Cao S, Chen JLW, Low AY. Prevention of diabetic foot complications. Singapore Med J. 2018;59(6):291–294.
- Noor S, Khan RU, Ahmad J. Understanding diabetic foot infection and its management. Diabetes Metab Syndr. 2017;11(2):149–156.
- Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc. 2008;98(6):489–493.
- Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. 2016;17(6) [Accessed 2016 Jun 10]:[917 p.].
- Nigi L, Fondelli C, de Donato G, Palasciano G, Setacci C, Dotta F. Fighting diabetic foot ulcers-the diabetologist: a king maker of the fight. Semin Vasc Surg. 2018;31(2–4):49–55.
- Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K, International Working Group on the Diabetic Foot (IWGDF)Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diabetes Res Clin Pract. 2017;124:84–92.
- Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: clinical review. J Tissue Viability. 2016;25(4):229–236.
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625.
- Stefano GB, Challenger S, Kream RM. Hyperglycemia-associated alterations in cellular signaling and dysregulated mitochondrial bioenergetics in human metabolic disorders. Eur J Nutr. 2016;55(8):2339–2345.
- Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A. The “Metabolic Memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients. 2017;9(5) [Published 2017 Apr 28]:[437 p.]. doi: 10.3390/nu9050437.
- Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29(6):1420–1432.
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–1551.
- Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5(6):338–349. doi: 10.1038/ncpcardio1211.
- Andrews KL, Houdek MT, Kiemele LJ. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthet Orthot Int. 2015;39(1):29–39.
- Ghotaslou R, Memar MY, Alizadeh N. Classification, microbiology and treatment of diabetic foot infections. J Wound Care. 2018;27(7):434–441.
- Lefrancois T, Mehta K, Sullivan V, Lin S, Glazebrook M. Evidence based review of literature on detriments to healing of diabetic foot ulcers. Foot Ankle Surg. 2017;23(4):215–224.
- Cam B, Bagdas D, Ozyigit MO, Sagdilek E, Buyukcoskun NI, Ozluk K. Effects of adrenomedullin and glucagon-like peptide on distal flap necrosis and vascularity: the role of receptor systems and nitric oxide. Wounds. 2017;29(6):163–167.
- Janis J, Harrison B. Wound healing: part II. Clinical applications. Plast Reconstr Surg. 2014;133(3):383e–392e.
- Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol. 2018;138(4):811–825.
- Jeon YR, Kang EH, Yang CE, Yun IS, Lee WJ, Lew DH. The effect of platelet-rich plasma on composite graft survival. Plast Reconstr Surg. 2014;134(2):239–246.
- Qi M, Zhou Q, Zeng W, Wu L, Zhao S, Chen W, Luo C, Shen M, Zhang J, Tang CE. Growth factors in the pathogenesis of diabetic foot ulcers. Front Biosci (Landmark Ed). 2018;23:310–317.
- Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153–165.
- Gonchar IV, Lipunov AR, Afanasov IM, Larina V, Faller AP, Kibardin AV. Platelet rich plasma and growth factors cocktails for diabetic foot ulcers treatment: state of art developments and future prospects. Diabetes Metab Syndr. 2018;12(2):189–194.
- Guffanti A. Negative pressure wound therapy in the treatment of diabetic foot ulcers: a systematic review of the literature. J Wound Ostomy Continence Nurs. 2014;41(3):233–237.
- Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, van den Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J Vasc Endovasc Surg. 2014;47(6):647–655.
- Blumberg SN, Berger A, Hwang L, Pastar I, Warren SM, Chen W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2012;96(1):1–9.
- Wang C, Guo M, Zhang N, Wang G. Effectiveness of honey dressing in the treatment of diabetic foot ulcers: a systematic review and meta-analysis. Complement Ther Clin Pract. 2019;34:123–131.
- Foster JL, McNaughton A. Honey dressings for diabetic foot ulcers: overview of evidence-based practice for novice researchers. Br J Community Nurs. 2020;25(Sup9):S14–19.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45–50. doi: 10.1016/j.tripleo.2005.07.009.
- Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353–2360.
- Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63–69. doi: 10.1080/08977190802636713.
- He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):707–713.
- Lundquist R, Dziegiel MH, Agren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regen. 2008;16(3):356–363.
